Abstract
Viral suppression (VS) in children has remained suboptimal compared to that in adults. We evaluated the impact of transitioning children weighing < 20 kg to a pediatric formulation of dolutegravir (pDTG) on VS in Malawi. We analyzed routine retrospective program data from electronic medical record systems pooled across 169 healthcare facilities in Malawi supported by the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF). We included children who weighed < 20 kg and received antiretroviral therapy (ART) between July 2021–June 2022. Using descriptive statistics, we summarized demographic and clinical characteristics, ART regimens, ART adherence, and VS. We used logistic regression to identify factors associated with post-transition VS. A total of 2468 Children Living with HIV (CLHIV) were included, 55.3% of whom were < 60 months old. Most (83.8%) had initiated on non-DTG-based ART; 71.0% of these had a viral load (VL) test result before transitioning to pDTG, and 62.5% had VS. Nearly all (99.9%) CLHIV transitioned to pDTG-based regimens. Six months after the transition, 52.7% had good ART adherence, and 38.6% had routine VL testing results; 81.7% achieved VS. Post-transition VS was associated with good adherence and pre-transition VS: adjusted odds ratios of 2.79 (95% CI 1.65–4.71), p < 0.001 and 5.32 (95% CI 3.23–9.48), p < 0.001, respectively. After transitioning to pDTG, VS was achieved in most children tested within the first 6 months. However, adherence remained suboptimal post-transition and VL testing at 6 months was limited. Interventions to improve VL testing and enhance ART adherence are still needed in CLHIV on pDTG-based regimens.
Similar content being viewed by others
Introduction
An estimated 1.7 million children were living with the Human Immunodeficiency Virus (HIV) globally in 2021. Children living with HIV (CLHIV) (< 15 years) have significantly higher morbidity and mortality than adults [1]. In 2021, while CLHIV represented only approximately 5% of people living with HIV (PLHIV), they contributed to 15% of all HIV-related deaths worldwide [2]. Due to their immature immune systems, young CLHIV, in particular, have a more rapid disease progression than adults without effective treatment [3]. There is a need to hasten the transition of CLHIV to more efficacious antiretroviral therapy (ART) regimens to improve their outcomes [4].
Dolutegravir (DTG), a second-generation integrase strand inhibitor, is the third in its class to be approved for the treatment of HIV in children [5,6,7,8]. DTG is recommended by the World Health Organization (WHO) as part of the first-line treatment of ART-naïve individuals and treatment-experienced patients [9, 10]. It demonstrates a higher genetic barrier to resistance than other drugs in its class with most of its associated mutations likely to reduce HIV replication and integrase enzymatic activity, especially in ART-naïve patients [11]. It also is a safe and effective drug in adults and children, with patients on a DTG-based regimen achieving viral suppression (VS) as early as 28 days from the onset of treatment [7, 8, 12,13,14]. Most DTG-based regimens are once-daily doses, making them easier for clients. Many countries quickly adopted DTG-based regimens in adults[3, 10], but pediatric formulations were limited. Children that weighed 30 kg or more were eligible for the once-daily fixed-dose combination tenofovir/lamivudine/DTG, and children between 20 and 29.9 kg were suitable for the adult 50 mg DTG tablets paired with ABC/3TC [10]. However, children < 20 kg remained on either nevirapine-/efavirenz-based regimens, which are suboptimal due to high rates of HIV drug resistance [15], or lopinavir/ritonavir-based regimens, which are poorly palatable, twice-daily regimens [16].
In 2020, the Food and Drug Administration (FDA) and the WHO approved the use of a pediatric formulation of DTG, a 10 mg dispersible tablet, as part of an antiretroviral regimen in infants and children from 4 weeks weighing at least three kilograms [14].
In Malawi, there were an estimated 58,000 CLHIV in 2021, and CLHIV had lower ART coverage than adults living with HIV (74% vs. 92%). CLHIV have lower rates of VS than adults living with HIV in Malawi (54% VS in children versus 85% VS in adult females and 79% VS in adult males) [1]. In 2018, DTG-based regimens were rolled out in Malawi for children, adolescents, and adults living with HIV who weighed ≥ 20 kg [17]. Due to limited VL testing capacity, PLHIV were transitioned to DTG-based regimens regardless of viral load (VL) testing or suppression status [18]. Once the 10 mg film-coated tablet of pDTG became available in 2021 for children < 20 kg, Malawi rapidly transitioned CLHIV to this new optimal formulation.
Despite the availability of pDTG in sub-Saharan Africa since 2021, there needs to be more data from the region on the impact of the introduction and scale-up of DTG-based ART regimens on VS in CLHIV. The Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) in Malawi has supported the scale-up of transitioning children to pDTG-based regimens. This study evaluated the impact of transitioning CLHIV < 20 kg to pDTG-based regimens on VS in EGPAF-supported facilities in Malawi.
Methods
Study Design
EGPAF-Malawi supports 179 ART facilities in nine districts in the central and southern regions of the country, providing HIV care and treatment services to 304,300 PLHIV in 2021. In July 2021, the foundation introduced pDTG to 169 EGPAF-supported facilities, which allowed initiating and transitioning CLHIV weighing between three and 19.9 kg to pDTG-based regimens as first-line ART. We conducted a cross-sectional study among CLHIV receiving ART in the 169 EGPAF-supported facilities in Malawi that provided pDTG to CLHIV.
Study Population
We included all children weighing less than 20 kg, and receiving ART in EGPAF-supported facilities between July 2021 and June 2022.
Data Collection
We obtained routinely-collected HIV treatment patient-level data for people receiving HIV care in EGPAF-supported ART facilities. We utilized two sources: the Malawi District Health Information Software version 2 (DHIS2) and facility-based ART electronic medical records systems (EMRS). Strategic information and evaluation (SI&E) officers extracted the data using a Microsoft Excel tool.
The outcome variable was VS (defined as < 1000 copies/mL due to varying low detectable limits of VL testing machines), assessed 6 months after initiation or transition to pDTG-based regimens. Independent variables included sociodemographic characteristics (sex, age, guardian type, current district of residence, and the facility location), initial ART regimen, current ART regimen, pre-transition VL results, and adherence to ART which was assessed using pill count. Good adherence was defined as missing ≤ 2 ARV doses/month, whereas poor adherence was defined as missing > 2 ARV doses/month, calculated at the last follow-up visit before the data collection.
Statistical Analysis
We performed data analysis using STATA v16 (Stata SE 16.0, StataCorp, College Station, TX, USA). Frequencies and proportions were used to summarize the distribution of demographic characteristics of patients and the characteristics of virally suppressed patients. We employed the Pearson’s Chi square test to compare adherence to ART and the viral load coverage among pediatric patients weighing less than 20 kg 6 months post-transition to pDTG-based regimens by sociodemographic and clinical characteristics. The statistical significance level was 0.05.
We performed a multivariable logistic regression analysis to determine the factors associated with VS among pediatric patients receiving ART in EGPAF-supported facilities who transitioned to pDTG-based regimens. The covariates included in the crude models were the independent variables listed above. Covariates that were statistically significant in the crude models were included in the adjusted model together with sex and age group.
Ethical Considerations
The Malawi National Health Sciences Research Committee (protocol number 18/09/2130) and Advarra Institutional Review Board (protocol number Pro00032796) in the United States of America (USA) approved the evaluation as part of the protocol titled Evaluation of Outcomes Achieved through Integrated HIV/AIDS and TB Prevention, Care and Treatment Programs in Malawi. We obtained a waiver for informed consent, as only secondary data were utilized for this protocol. This activity was reviewed by the U.S. Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy.
Results
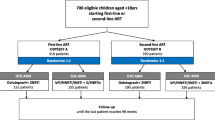
A total of 2,468 CLHIV met the inclusion criteria and were included in the analysis. Over half of the CLHIV (56.0%) were female, and the median age was 48 months (interquartile range (IQR) was 33–72 months)(Table 1). Data on initial ART regimen was available for 93.3% of the children: most (89.7%) had been initiated on non-DTG-based regimens; 77.1% had been initiated on a protease inhibitor (PI)-based regimen. Before the transition to pDTG, 59.4% had a VL result; of these, 62.5% had VS. Nearly all patients (99.9%) on non-DTG regimens transitioned to pDTG-based regimens, with the remaining three remaining on PI-based regimens. There was no change in the nucleoside reverse transcriptase inhibitor (NRTI) backbone for children who transitioned to pDTG.
Six months after the transition to pDTG, almost all CLHIV (97.6%) were alive and in care. Only approximately half of the patients adhered well to ART (52.8%). ART adherence was better among infants (57.8%) than those aged 24–59 months (48.5%), among patients whose guardians were biological parents (54.0%) than others (42.3%), and among those who had been virally suppressed before the transition to dolutegravir-based regimens (57.0%) than those who had been virally non-suppressed (45.9%). There was no difference in adherence between urban and rural facilities (p = 0.79) or in adherence rates between CLHIV who initiated pDTG- or non-pDTG-based regimens (p = 0.09) (Table 2).
Only 38.6% of the patients had VL results. More patients receiving care in rural facilities were missing VL results than those receiving care in urban facilities (62.0% vs. 46.6%, p < 0.001). However, there were no differences in VL coverage by sex or age group (Table 3).
Approximately 81.7% of the patients with post-transition VL results achieved VS. Factors associated with post-transition VS were good adherence (crude odds ratio [cOR] 1.93, 95% confidence interval [CI] 1.37–2.73, p < 0.001) and pre-transition VS (cOR 5.32 95% CI 3.30–8.57, p < 0.001). In a multivariate analysis, good adherence and VS before the transition remained associated with post-transition VS: adjusted odds ratios (aOR) of 2.79 (95% CI 1.65–4.71), p < 0.001 and 5.32 (95% CI 3.23–9.48), p < 0.001, respectively. There were no significant differences in post-transition VS by sex, age group, geographical location, or guardian type (Table 4).
Amongst patients with poor adherence (n = 344), 74.4% achieved VS. Amongst the 254 patients who had been virally non-suppressed before the transition to pDTG-based regimens, 71.2% achieved VS after 6 months of pDTG-based ART. Conversely, only 7.4% of the patients who achieved VS on their initial ART regimens before pDTG introduction were virally non-suppressed after 6 months on pDTG-based regimens (Table 4).
Discussion
Our study demonstrated a successful transition of CLHIV to pDTG-based ART regimens in 169 EGPAF-supported districts in Malawi with improved rates of VS; nearly all CLHIV < 20 kg who had initiated non-DTG-based ART had transitioned to pDTG-based ARV regimens by the end of the study period. This demonstrates the program’s success in transitioning pediatric patients to optimized, pediatric-friendly regimes within the first year of implementation.
Despite improved palatability and once-daily dosing of pDTG-based regimens, ART adherence 6 months post-DTG transition was low, with only about half the patients having good adherence to ARVs, similar to study findings of children on NNRTI- and PI-based regimens from Uganda, Kenya, and Congo [19,20,21]. ART adherence was better in infants than in older children and patients whose guardians were biological parents than others, similar to a study in Ethiopia that found an association between non-biological caretakers and poor adherence among CLHIV [22]. In our study, good adherence to ART was associated with VS, similar to studies in Vietnam, Uganda, and Thailand, which have demonstrated an increased likelihood of viral non-suppression in those with poor adherence [23,24,25]. Poor adherence to ART also leads to drug resistance and mortality [26]. These findings highlight the need to explore interventions that help improve adherence to ART among CLHIV, particularly those not living with their parents, including education of non-parental guardians on how to support child adherence. Further information is needed on why adherence rates in CLHIV did not improve with improved palatability and reduction from twice-daily dosing to once-daily dosing, which has been shown to improve adherence in a study conducted in Zimbabwe [26]. Interventions may focus more on other non-medication-related barriers to adherence, such as nutrition status and psychosocial barriers, and patients may benefit from case management and linkage to community-based services.
There was a reduction in VL coverage from pre-transition to post-transition, with only about a third of the patients who transitioned having VL results. This decline in VL coverage post-transition was not unique to CLHIV, as there was a reported overall drop in VL testing in Malawi in 2021, which was partly attributed to viral load bundle supply interruption due to the COVID-19 pandemic [27]. The low post-transition VL coverage limits our understanding of the impact of pDTG on VS. Efforts are needed to increase VL coverage in CLHIV, including stable commodity supplies for sample collection, transport, and VL reagents, adequate sample transportation, and healthcare workers trained in VL sample collection from children.
Although VL coverage post-transition was low, there was an increase in VS rates among those tested, with rates > 80%. These rates are similar to studies conducted in children and adolescents on DTG-based regimens that reported VS [28, 29] but remain below the 2030 95–95–95 UNAIDS targets [25]. However, the proportion of CLHIV with VS was much higher than the Malawi Population-based HIV Impact Assessment (MPHIA) report from a population-based survey conducted in 2015–16 (54% VS) [30]. These findings are encouraging, as improved VS was observed shortly after transitioning to pDTG-based regimens. Nevertheless, as better VS rates are expected on pDTG-based regimens [12], follow-up studies are needed to assess VS in a similar cohort at 12 months on pDTG-based regimens and with higher rates of VL coverage.
Most of the CLHIV, virally non-suppressed on other regimens, achieved VS within the first 6 months of pDTG-based regimens. However, more than a quarter of the patients who had been virally non-suppressed before the transition remained non-suppressed. It is worth noting that all patients who transitioned to pDTG-based ART maintained the NRTI backbone and had one drug replaced. There may already have been viral resistance to the other ARVs leading to functional monotherapy on the dolutegravir-based regimens and possible resistance [12, 31]. However, most children in our study achieved VS with only a one-drug switch and no change to the NRTI backbone. The high rates of poor adherence also make drug resistance less likely to be the main contributor to viral non-suppression in our study. Therefore, further studies that include resistance testing CLHIV on pDTG-based regimens will be essential to understand the contribution of drug resistance in children who do not achieve VS on pDTG-based regimens.
We recognize the following limitations to our study. First, the analysis was based on a retrospective collection of routinely collected data, resulting in incomplete data. Standard program service delivery disruptions, such as VL testing, also affected the study. Also, we could not evaluate other previously known factors associated with viral non-suppression, including malnutrition and co-morbidities [32, 33], as these data are not routinely collected.
Conclusion
VS was achieved in most CLHIV who received a VL test 6 months after transitioning to pDTG. However, adherence post-transition was suboptimal in this group, and VL testing post-transition was also limited. Interventions that enhance good adherence in children on pDTG-based regimens are required as we progress toward achieving 95–95–95 goals.
Abbreviations
- ART:
-
Antiretroviral therapy
- CDC:
-
Centers for Disease Control and Prevention
- DTG:
-
Dolutegravir
- EGPAF:
-
Elizabeth Glaser Pediatric AIDS Foundation
- VS:
-
Viral suppression
- VL:
-
Viral Load
- CLHIV:
-
Children Living with HIV
- NRTI:
-
Nucleoside Reverse Transcriptase Inhibitor
- NNRTI:
-
Non-nucleoside Reverse Transcriptase Inhibitor
- PI:
-
Protease Inhibitor
- MPHIA:
-
Malawi Population-based HIV Impact Assessment
References
United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS data 2021; 2021. https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf. Accessed 07 Oct 2022.
Joint United Nations Programme on HIV/AIDS (UNAIDS) 2022. In: Danger: UNAIDS Global AIDS Update 2022; 2022. https://www.unaids.org/sites/default/files/media_asset/2022-global-aids-update_en.pdf. Accessed 07 Oct 2022.
World Health Organization (WHO). Consolidated guidelines on using antiretroviral drugs for treating and preventing HIV infection; 2016. https://apps.who.int/iris/rest/bitstreams/925868/retrieve. Accessed 07 Oct 2022.
Penazzato M, et al. A global research agenda for pediatric HIV. J Acquir Immune Defic Syndr. 2018;78(Suppl 1):S10–5. https://doi.org/10.1097/QAI.0000000000001743.
Waalewijn H, et al. Optimizing pediatric dosing recommendations and treatment management of antiretroviral drugs using therapeutic drug monitoring data in children living With HIV. Ther Drug Monit. 2019;41(4):431–43. https://doi.org/10.1097/FTD.0000000000000637.
Archary M, et al. Optimised pediatric antiretroviral treatment to achieve the 95–95–95 goals. S Afr J HIV Med. 2021;22(1):1278. https://doi.org/10.4102/sajhivmed.v22i1.1278.
Gillette MA, et al. Dolutegravir: a new integrase strand transfer inhibitor for the treatment of HIV—an alternative viewpoint. Pharmacotherapy. 2014;34(9):e173–4. https://doi.org/10.1002/phar.1478.
Shah BM, Schafer JJ, Desimone JA Jr. Dolutegravir: a new integrase strand transfer inhibitor for the treatment of HIV. Pharmacotherapy. 2014;34(5):506–20. https://doi.org/10.1002/phar.1386.
World Health Organization (WHO). Application for inclusion of dolutegravir (DTG) 10 mg scored dispersible tablets on the WHO model list of essential medicines for children (EMLc); 2020. https://cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-expert-committee/applications-for-new-formulations-strengths-of-existing-listed-medicines/f.6_dolutegravir.pdf?sfvrsn=a7a18106_4. Accessed 07 Oct 2022.
World Health Organization (WHO). Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. WHO; 2018. https://www.who.int/publications/i/item/WHO-CDS-HIV-18.51. Accessed 07 Oct 2022.
Brenner BG, Wainberg MA. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res. 2016. https://doi.org/10.1016/j.virusres.2016.07.006.
Torres-Fernandez D, et al. Integrase inhibitors in children and adolescents: clinical use and resistance. J Antimicrob Chemother. 2022;77(10):2784–92. https://doi.org/10.1093/jac/dkac259.
Moore CL, et al. ODYSSEY clinical trial design: a randomized, global study to evaluate the efficacy and safety of dolutegravir-based antiretroviral therapy in HIV-positive children, with nested pharmacokinetic sub-studies to evaluate pragmatic WHO-weight-band based dolutegravir dosing. BMC Infect Dis. 2021;21(1):5. https://doi.org/10.1186/s12879-020-05672-6.
Bruzzese E, et al. Dolutegravir-based antiretroviral therapy is effective and safe in HIV-infected pediatric patients. Ital J Pediatr. 2018;44(1):37. https://doi.org/10.1186/s13052-018-0469-x.
Omondi EO, et al. Nevirapine plasma concentration is associated with virologic failure and the emergence of drug-resistant mutations among HIV patients in Kenya: a cross-sectional study. Medicine (Baltimore). 2022;101(50): e32346. https://doi.org/10.1097/MD.0000000000032346.
Frange P, et al. Rilpivirine in HIV-1-positive women initiating pregnancy: to switch or not to switch? J Antimicrob Chemother. 2020;75(5):1324–31. https://doi.org/10.1093/jac/dkaa017.
Department of HIV/AIDS, Ministry of Health and Population. Malawi guidelines for clinical management of HIV in children and adults. 4th ed., vol. 1. Lilongwe: Ministry of Health and Population, Malawi; 2018. p. 1–128. http://dx.doi.org/10.1016/j.eswa.2014.06.009%0Ahttp://dx.doi.org/10.1016/j.procs.2010.12.169%0Ahttp://dx.doi.org/10.1016/j.sbspro.2015.01.1176%0Ahttp://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.683.7048&rep=rep1&type=pdf%0Ahttps://doi.org/10.1016/. Accessed 07 Oct 2022.
Peter T, et al. Scaling up HIV viral load—lessons from the large-scale implementation of HIV early infant diagnosis and CD4 testing. J Int AIDS Soc. 2017. https://doi.org/10.1002/jia2.25008.
Ssanyu JN, Nakafeero M, Nuwaha F. Multi-measure assessment of adherence to antiretroviral therapy among children under five years living with HIV in Jinja, Uganda. BMC Public Health. 2020;20(1):1319. https://doi.org/10.1186/s12889-020-09430-w.
Mabiala Babela JR, et al. Outcome of HIV-positive children and adolescents treated with second-line antiretroviral agents in Congo. Med Sante Trop. 2018;28(4):413–8. https://doi.org/10.1684/mst.2018.0850.
Langat NT, Odero W, Gatongi P. Antiretroviral drug adherence by HIV infected children attending Kericho District Hospital, Kenya. East Afr J Public Health. 2012;9(3):101–4.
Tiruneh CM, et al. Clinical non-adherence and its associated factors among HIV-positive pediatric patients attending HIV care in South Gondar Zone Public Health Facilities, Northwest Ethiopia, 2021. HIV AIDS (Auckl). 2022;14:23–32. https://doi.org/10.2147/HIV.S352386.
Moolasart V, et al. The effect of detectable HIV viral load among HIV-infected children during antiretroviral treatment: a cross-sectional study. Children (Basel). 2018. https://doi.org/10.3390/children5010006.
Rangarajan S, et al. Factors associated with HIV viral load suppression on antiretroviral therapy in Vietnam. J Virus Erad. 2016;2(2):94–101. https://doi.org/10.1016/S2055-6640(20)30466-0.
Church JD, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-Dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198(7):1075–82. https://doi.org/10.1086/591503.
Pasipanodya B, Kuwengwa R, Prust ML, Stewart B, Chakanyuka C, Murimwa T, et al. Assessing the adoption of lopinavir/ritonavir oral pellets for HIV-positive children in Zimbabwe. J Int AIDS Soc. 2018. https://doi.org/10.1002/jia2.25214.
Kalua T, et al. HIV suppression was maintained during the COVID-19 pandemic in Malawi: a program-level cohort study. J Clin Epidemiol. 2022;150:116–25. https://doi.org/10.1016/j.jclinepi.2022.06.019.
Bacha JM, et al. Achieving antiretroviral therapy uptake and viral suppression among children and adolescents living with HIV in the UNAIDS 90–90–90 era across six countries in eastern and southern Africa—lessons from the BIPAI network. J Acquir Immune Defic Syndr. 2022;90(3):300–8. https://doi.org/10.1097/QAI.0000000000002957.
Mutagonda RF, et al. Prevalence and determinants of non-communicable diseases including depression among HIV patients on antiretroviral therapy in Dar es Salaam, Tanzania. Trop Med Int Health. 2022;27(8):742–51. https://doi.org/10.1111/tmi.13790.
Department of HIV/AIDS Ministry of Health. Malawi Population-Based HIV Impact Assessment (MPHIA) 2015–2016: Final Report; 2018. https://phia.icap.columbia.edu/wp-content/uploads/2019/08/MPHIA-Final-Report_web.pdf. Accessed 07 Oct 2022.
Salou M, et al. Challenges of scale-up to dolutegravir-based regimens in sub-Saharan Africa. AIDS. 2020;34(5):783–7. https://doi.org/10.1097/QAD.0000000000002470.
Afrane AKA, et al. HIV virological non-suppression and its associated factors in children on antiretroviral therapy at a major treatment center in Southern Ghana: a cross-sectional study. BMC Infect Dis. 2021;21(1):731. https://doi.org/10.1186/s12879-021-06459-z.
Muenchhoff M, et al. Malnutrition in HIV-infected children is an indicator of severe disease with an impaired response to antiretroviral therapy. AIDS Res Hum Retroviruses. 2018;34(1):46–55. https://doi.org/10.1089/aid.2016.0261.
Acknowledgements
The authors thank the strategic information and evaluation officers in EGPAF Malawi districts who collected the data.
Funding
This research has been supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR) through the United States Centers for Disease Control and Prevention under the terms of NU2GGH002425. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Author information
Authors and Affiliations
Contributions
LM, TM, and LK designed the study. LUK supported the data analysis, and LM, LK, MB, and TM drafted the manuscript. All authors were involved in reviewing and approving the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Ethical Approval
Ethical clearance was obtained from National Health Science Research Committee on September 12th, 2018, approval number 2130, protocol number 18/09/2130. The Advarra Institutional Review Board (IRB) reviewed and approved the study on March 22nd, 2019, Protocol Number Pro00032796.
Consent to Participate
This activity was reviewed by the United States CDC and was conducted consistent with applicable federal law and CDC policy. The protocol was reviewed by the CDC human research protection procedures and was determined to be research. However, CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makonokaya, L., Maida, A., Kalitera, L.U. et al. Early Effects of Scaling Up Dolutegravir-Based ARV Regimens Among Children Living with HIV in Malawi. AIDS Behav (2024). https://doi.org/10.1007/s10461-024-04312-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s10461-024-04312-3