Abstract
Background
Considering the improvement in adherence and convenience, once-monthly paliperidone palmitate (PP1M) has been increasingly used in the treatment of schizophrenia. However, the outcomes for patients who switch from oral antipsychotics (OAPs) to PP1M have not been reliably assessed. The objective of this systematic review and meta-analysis was to investigate the efficacy and safety of PP1M in the management of patients with schizophrenia with a prior history of OAP use.
Methods
We conducted a systematic search in PubMed, EMBASE, and the Cochrane Library on 19 July 2022 to identify eligible studies. All studies that examined the effectiveness and safety of switching from OAPs to PP1M in patients with schizophrenia were included. The primary outcomes were relapse rate, hospitalisation rate, and the change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score. The secondary outcomes included the changed number of inpatient visits, changed length of stay hospitalisation, change from baseline in the Clinical Global Impressions-Severity (CGI-S) score and the personal and social performance (PSP) total score, response rate, proportion of treatment discontinuation, and adverse events. We included randomised-controlled trials (RCTs), single-arm studies, and observational studies. Case reports, case series, and reviews were excluded. The quality assessment of included studies was performed using the Revised Cochrane risk-of-bias tool for randomised trials (RoB2), the 9-point Newcastle-Ottawa Scale (NOS) instrument for non-randomised studies and cohort studies, and the 12-item National Institutes of Health (NIH) quality assessment tool for before-after (Pre-Post) study without control group. Follow-up times were reported as short- (≤ 13 weeks), medium- (14–26 weeks), and long term (≥ 27 weeks). Data were pooled using meta-analysis.
Results
Fifteen studies with a total of 4740 patients were included. The long-term relapse rates and hospitalisation rates were 12% (95% CI 0.07–0.18) and 18% (95% CI 0.15–0.20), respectively. The short-, medium-, and long-term change in PANSS total score was − 21.69 (95% CI − 30.02 to −13.36), − 14.98 (95% CI − 21.45 to − 8.51) and − 17.88 (95% CI − 31.94 to −3.82), respectively. Approximately 50% of patients reported at least a 30% reduction in the PANSS score at the short-term follow-up. Improvements in CGI-S and PSP score were observed during various periods. There was a reduction in the length of stay hospitalisation and the number of inpatient visits at the medium- and long-term follow-ups. Low discontinuation and adverse event rates were reported.
Conclusion
Based on our findings, this study may support the efficacy and safety of switching from OAPs to PP1M for the treatment of patients with schizophrenia. Future large-scale studies are warranted to confirm our findings.
Similar content being viewed by others
This systematic review indicated that once-monthly paliperidone palmitate (PP1M) may be an effective and tolerable option for managing patients with schizophrenia who were previously on oral antipsychotics (OAPs). |
Our findings suggest PP1M may reduce the severity of disease and symptoms as well as improve functioning states in patients with schizophrenia with OAP history. |
1 Introduction
Schizophrenia is a chronic, serious mental disorder characterised by symptoms such as delusions, hallucinations, disorganised behaviour, emotional withdrawal, and progressive cognitive deficits [1]. It affects approximately 24 million people or 0.32% of the population worldwide [2]. Although schizophrenia is not as common as many other mental disorders, the severity of the disability and the unclear aetiology makes it the 11th leading causes of disability worldwide [3]. Patients with schizophrenia have a 15- to 25-year shorter life expectancy than those without the disorder [4].
Schizophrenia was typically treated with oral antipsychotics (OAPs). The use of second-generation antipsychotics (SGAs) (e.g., paliperidone and risperidone) was more prevalent than first-generation antipsychotics (FGAs) (e.g., chlorpromazine and haloperidol). However, the outcomes associated with OAPs were often suboptimal. For various reasons, approximately one-third of patients with schizophrenia did not adhere to their OAP treatment regimen [5]. To improve adherence, long-acting injectable antipsychotics (LAI) were proposed as an effective tool [6], although they remain underutilised in clinical practice [7, 8]. Several studies suggested LAIs may offer an advantage in increasing patient adherence and reducing relapse and rehospitalisation when compared with OAPs [9,10,11]. Guidelines from the American Psychiatric Association and other organisations have recommended LAIs as a treatment alternative to OAPs for patients with poor or uncertain adherence as well as for patients transitioning from inpatient to outpatient status [12,13,14].
Several studies have demonstrated once-monthly paliperidone palmitate (PP1M), an SGA LAI, is associated with improvements in psychotic symptoms, disease severity, and functional outcomes in patients with newly diagnosed or acute schizophrenia [15, 16]. Although some studies have reported benefits of PP1M—namely improved functioning and symptom severity—for patients who have not responded to prior administration of OAPs [17, 18], there has been a lack of a systematic analysis and synthesis of the data. Thus, we performed a systematic review and meta-analysis to assess the efficacy and safety of PP1M in patients switching from OAP use with the aim of providing evidence that may be used to guide clinical practice.
2 Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA 2020) extension statement [19]. A protocol was registered in the PROSPERO database (CRD42022363726).
2.1 Data Sources and Literature Search
An electronic database search of PubMed, EMBASE, and the Cochrane Library was conducted on 19 July 2022 using the following keywords: ‘oral antipsychotics’, ‘Schizophrenia’, ‘LAI’, ‘Paliperidone Palmitate’, and ‘1 month’. We applied no restrictions on language, date, or publication status. The reference lists of the included studies were searched to identify further eligible studies. The search strategy is provided in Online Resource 1.
2.2 Study Selection
Studies were included if they met the following criteria:
Participants patients with a diagnosis of schizophrenia—as defined by the original study—who had a history of receiving OAPs. For instance, schizophrenia was defined by the International Statistical Classification of Diseases and Related Health Problems-10th revision (ICD-10) [20] or Diagnostic and Statistical Manual of Mental Disorders-Fourth/Fifth Edition (DSM-IV/DSM-5) [21, 22]. There were no restrictions on the age of the patient.
Intervention patients with a prior history of OAP use and who were currently switching to PP1M.
Comparator there were no limitations on comparators as we only pooled data from single arms of included studies.
Outcomes primary outcomes included patient relapse rate, hospitalisation rate, and the change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score. The secondary outcomes included the changed number of inpatient visits, changed length of hospital stays, change from baseline in the Clinical Global Impressions-Severity (CGI-S) score and the Personal and Social Performance (PSP) total score, response rate defined as at least 20%, 30% or 40% reduction in the PANSS score, proportion of treatment discontinuation, total Treatment Emergent Adverse Events (TEAEs), and metabolic-related TEAEs.
Study design randomised controlled trials (RCTs), single-arm interventional trials, and observational studies.
To limit the effect of LAIs, we excluded patients with a history of previous treatment with a LAI that was reported at baseline. For intervention, current treatment with oral antipsychotics was considered ineligible. Case reports, case series, and reviews were excluded. We included studies that reported findings in English only. Two reviewers (XL and MJ) independently screened articles for eligibility. Any disagreements were resolved through discussion with a third reviewer (CY).
2.3 Data Extraction
Two reviewers (XL and CY) extracted the following data from each study into a pre-defined data extraction form: trial characteristics (i.e., country, study design, centre, NCT number, and sample size), participant characteristics (i.e., age, body mass index, sex, prior oral antipsychotics, stages, and ethnicity), baseline data (i.e., number of inpatient visits, PANSS score, CGI-S score, and PSP score), and outcome. Any disagreements were resolved by discussion with a third reviewer (MJ).
2.4 Quality Assessment
The quality of included studies was assessed independently by two reviewers (XL and CY). The Revised Cochrane risk-of-bias tool for randomised trials (RoB2) was employed to assess the risk of bias in RCTs [23]. The 9-point Newcastle-Ottawa Scale (NOS) instrument that contains 8 items was employed for non-randomised studies and cohort studies [24]. For before and after trials, we used the 12-item National Institutes of Health (NIH) quality assessment tool for before-after (Pre-Post) study without control group [25]. Any disagreements were resolved by discussion with a third reviewer (MJ).
2.5 Data Synthesis and Analysis
The R software (version 4.1.0) was used for meta-analyses (two tails with an α = 0.05 unless indicated). For dichotomous outcomes, we extracted the reported rates and calculated data as the proportion and 95% confidence interval (CI) for each study, and then pooled them by meta-analysis. When no event was observed, we added a fixed value (typically 0.5) to the event number of the intervention group following methods described in the Cochrane handbook [26]. For continuous outcomes, we extracted the means and standard deviations (SDs) of the changed score for each study and calculated a pooled changed mean and 95% CI. When the changed SD was not observed, we calculated it from the reported endpoint and baseline value [27]. Also, we used baseline SD when the changed or endpoint SD was not available. For those studies reporting no available data, we imputed SD from another study by considering whether the studies used the same measurement scale, had the same degree of measurement error, had the same time interval between the baseline and post-intervention measurement as well as a similar population [27]. When data were insufficient for meta-analysis, we described the outcome narratively. We divided the follow-up time into short- (≤ 13 weeks), medium (14–26 weeks) and long term (≥ 27 weeks) according to a previously published Cochrane review [28].
A fixed-effects model was used for meta-analyses. Where substantial heterogeneity was identified—defined as I2 ≥ 50% coupled with p < 0.1 from Q test [28, 29]—we explored the sources of heterogeneity from the following aspects: baseline PANSS score, previous concomitant medications, and number of previous hospitalisations. We synthesised data using a random-effects model with restricted maximum likelihood (REML)-based estimation of between-study variance tau2 when heterogeneity was significant, and the source was not identified. A funnel plot used for assessing publication bias was not performed due to the insufficient number of studies for each outcome (i.e., less than 10 studies).
Subgroup analyses were carried out on primary outcomes according to the OAP treatment history, ethnicity (i.e., Asian vs non-Asian), and the stage of schizophrenia at which switching was initiated, where possible. Sensitivity analysis was conducted on meta-analyses including data transformation or imputation to confirm the robustness of the conclusion.
3 Results
3.1 Results of Study Selection
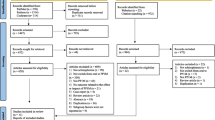
The database search returned 2198 results and provided 1467 unique citations after duplicates were removed. A further 795 articles were excluded at the title-abstract screening stage. Full papers were sought for the remaining 672 articles. Of these, 628 full papers were retrieved and assessed for eligibility according to the inclusion and exclusion criteria. The remaining 44 articles were not successfully retrieved due to unauthorised access. Among the 628 full papers, 602 studies were excluded: 99 studies were excluded due to ineligible patients; 419 studies were excluded due to ineligible intervention; 20 studies were excluded due to ineligible outcomes; 48 studies were excluded due to ineligible study design, and 16 studies were excluded because they were duplicates. Finally, 15 studies with 26 references [17, 18, 20,21,22, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] met full eligibility criteria and were included in the review. No additional eligible studies were identified by manual searching. The study selection process is shown in Fig. 1.
3.2 Characteristics of Included Studies and Participants
The included 15 studies comprised four RCTs (26.7%) [21, 31, 38, 44], 2 cohort studies (13.3%) [33, 50], and 9 pre-post studies (60.0%) [20, 22, 30, 32, 34, 45, 46, 48, 49]. Of these, 3 studies were from the USA [20, 34, 49], 2 from Italy [21, 33], 2 from China [30, 48], 1 from Croatia [32], 1 from Romania [50], and 6 were from multiple countries [22, 31, 38, 44,45,46]. The sample size ranged from 10 to 1169 (Table 1). Across the included studies, a total of 4740 patients were included. The mean age ranged from 28.7 to 52.0 years. Except for 2 studies [21, 50] that did not report sex information, the remaining 13 studies reported that 67.9% of included patients were male. The follow-up period ranged from 1 week to 18 months. The most reported OAPs previously used were risperidone and paliperidone. Others including amisulpride, olanzapine, haloperidol, fluphenazine, ziprasidone, quetiapine, aripiprazole, sertindole, chlorpromazine, penfluridol, perphenazine, and clozapine. Detailed patient characteristics are shown in Table 2.
3.3 Relapse Rate and Hospitalisation Rate
One study with 157 patients reported 19 of 157 patients (12%) had relapsed by the long-term follow-up (95% CI 0.07–0.18) [38]. The long-term pooled hospitalisation rate of two studies was 18% (95% CI 0.15–0.20, I2 = 34%) with a total of 731 patients [20, 22].
3.4 Change in the PANSS Total Score from Baseline
Five studies (N = 1390 patients) reported a change in the PANSS total score at the short-term follow-up that ranged from − 30.87 to − 15.40. The pooled mean change was − 21.69 (95% CI − 30.02 to − 13.36, I2 = 99%, Fig. 2a) [30, 31, 33, 38, 46]. Two studies (N = 1041 patients) reported a change in the total score at the medium-term follow-up ranging from − 18.30 to − 11.70, and the pooled mean change was − 14.98 (95% CI − 21.45 to − 8.51, I2 = 97%, Fig. 2b) [38, 45]. Two studies (N = 533 patients) reported a change in the total score at the long-term follow-up that ranged from − 25.70 to − 11.30. The pooled change was − 17.88 (95% CI − 31.94 to − 3.82, I2 = 91%, Fig. 2c) [22, 33]. Results at Weeks 1, 5, 9, 12 and 13 are provided in Online Resource 2 Figure S1.
3.5 Change in the CGI-S Score from Baseline
Three studies (N = 926 patients) reported a reduction in the CGI-S score at the short-term follow-up that ranged from 0.99 to 1.84 from baseline [30, 31, 46]. The pooled change was − 1.42 (95% CI − 1.89 to − 0.96, I2 = 95%, Fig. 3a). Two studies (N = 618 patients) reported a reduction in the score at the medium-term follow-up that ranged from − 0.74 to − 0.60. The pooled change was − 0.61 (95% CI − 0.69 to − 0.53, I2 = 0%, Fig. 3b) [21, 45]. Another two studies (N = 633 patients) reported a reduction in the score at the long-term follow-up that ranged from − 2.60 to − 0.80. The pooled change was − 1.70 (95% CI − 3.46 to − 0.06, I2 = 100%, Fig. 3c) [22, 32]. Results at Weeks 1, 5, and 9 are provided in Online Resource 2 Figure S2.
3.6 Change in the PSP Score from Baseline
Improvement in PSP score at the short-term follow-up was reported in five studies (N = 1413 patients) and ranged from 5.8 to 19.34 [30, 31, 33, 41, 46]. The pooled change was 13.99 (95% CI 6.94–21.05, I2 = 98%, Fig. 4a). Three studies (N = 1129 patients) reported an improvement of 8.00–12.41 score at the medium-term follow-up [21, 41, 45]. The pooled mean change was 9.27 (95% CI 7.31–11.23, I2 = 73%, Fig. 4b). Another two studies (N = 519 patients) reported an increase in the score at the long-term follow-up that ranged from 10.50 to 13.00 [33, 41]. The pooled result after meta-analysis was 10.69 (95% CI 9.05–12.33, I2 = 0%, Fig. 4c). Results at Week 5, 12, and 13 are provided in Online Resource 2 Fig. S3.
3.7 Response Rate—at Least 20%, 30% or 40% Reduction in the PANSS Score from Baseline
Response rate was defined as at least a 30% and 40% reduction in the PANSS score (Online Resource 2 Figure S4). The pooled response rate at the short-term follow-up was 57% (95% CI 0.32–0.82, I2 = 99%, 3 studies, N = 1341 patients) [22, 30, 46] when defined as at least a 30% reduction and 53% (95% CI 0.46–0.60, one study, N = 210 patients) [46] when defined as at least a 40% reduction in the baseline PANSS score. The medium-term response rate was 51% (95% CI 0.47–0.56, one study, N = 589 patients) [45] and 30% (95% CI 0.27–0.34) [45], respectively. Only one study (N = 521 patients) reported a response rate-defined by at least a 30% reduction in the score of 74% at the long-term follow-up (95% CI 0.70–0.78) [22]. We did not identify studies that reported a response rate defined as at least a 20% reduction in the PANSS score. Results at Weeks 1, 5, 9, 13 are provided in Online Resource 2 Figure S5.
3.8 Changes in Length of Stay Hospitalisation and Number of Inpatient Visits from Baseline
The length of stay hospitalisation and number of inpatient visits at the medium-term follow-up were reported in two studies (N = 846 patients) [34, 49]. The pooled result showed a reduction in hospital stay of 5.45 days (95% CI − 14.77 to 3.87, I2 = 100%). The pooled change in the number of inpatient visits was − 0.79 (95% CI − 1.27 to − 0.31, I2 = 100%). Four studies (N = 558 patients) reported the long-term follow-up results of these two outcomes [20, 32, 48, 49]. The pooled reduction of length of stay was 12.98 days (95% CI − 18.15 to − 5.47, I2 = 97%), and the pooled change in number of inpatients visits was − 0.79 (95% CI − 1.27 to − 0.31, I2 = 98%). See Online Resource 2 Figure S6 for details.
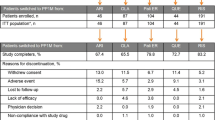
3.9 Discontinuation Rate
Short term, the overall pooled weighted discontinuation rate was 31% (95% CI 0.21–0.41, I2 = 86%, three studies, N = 926 patients) [30, 31, 46] due to all-cause, 4% (95% CI 0.02–0.06, I2 = 54%, three studies, N = 932 patients) [30, 31, 39] due TEAEs, and 7% (95% CI 0.02–0.11, I2 = 84%, three studies, N = 926 patients) [30, 31, 46] due to lack of efficacy. Medium term, meta-analyses showed a 24% discontinuation rate (95% CI 0.00–0.54, I2 = 96%, two studies, N = 173 patients) [21, 44] due to all-cause, 7% (95% CI 0.05–0.09, I2 = 0%, two studies, N = 730 patients) [17, 44] due to TEAEs, and 2% (95% CI 0–0.06, one study, N = 137 patients) due to lack of efficacy [44]. Long term, the discontinuation rate was 14% (95% CI 0.02–0.43) due to non-adherence [33]. See Online Resource 2 Figure S7 for details.
3.10 Adverse Events
The short-term pooled total TEAE rate was 54% (95% CI 0.29–0.79, I2 = 98%, three studies, N = 932 patients) [30, 31, 46], the medium-term was 54% (95% CI 0.43–0.64, I2 = 91%, three studies, N = 1182 patients) [38, 44, 45], and the long-term was 69% (95% CI 0.56–0.82, I2 = 89%, two studies, N = 674 patients) [38, 40]. Results of at least one serious TEAE rate and possibly drug-related TEAE rate are shown in Online Resource 2 Figure S8. The range of long-term abnormal weight gain was 9.8% (one study, N = 521 patients) [22]. The short-term increase in weight ranged from 1.5 to 7.6% (three studies, N = 985 patients) [30, 31, 46] and the long-term increase ranged from 1.5 to 7.5% (three studies, N = 1110 patients [22, 38, 44]. One study (N = 593 patients) reported a 3.2% medium-term increase in weight [45]. In addition, the short-term rate of glucose-related TEAEs was 0.5% (one study, N = 212 patients) [46] and the long-term rate was 0.6% (one study, N = 521 patients) [22]. Results of other metabolic-related TEAEs are shown in Online Resource 2 Table S1. The most reported TEAEs included injection-site pain, weight increase, headache, insomnia, constipation, upper respiratory tract infection, dizziness, nasopharyngitis, somnolence, and extrapyramidal symptom TEAEs (i.e., akathisia and tremor).
3.11 Subgroup and Sensitivity Analysis
The pre-planned subgroup analyses were conducted to explore the source of heterogeneity. However, due to insufficient data, only analyses stratified by OAP history were conducted. There was a significant subgroup difference (p = 0.02) in short-term changes in the PANSS score between patients with a history of olanzapine (mean change − 25.50, 95% CI − 30.94 to − 20.06) and risperidone (mean change − 17.61, 95% CI − 21.42 to − 13.81) use [30, 31, 33]. This indicated that a history of OAP use may be one of the sources of heterogeneity in the short-term change in PANSS score results (Online Resource 3 Table S2). The sensitivity analyses were only available for the change in the PANSS total score. After removing data with imputed SDs, the result did not substantially differ from the result reported with imputed SDs in the review (Online Resource 3 Table S3).
3.12 Quality Assessment of Included Studies
The overall quality assessment result for each study is shown in Table 1. For assessment details pertaining to RoB2, NOS, and NIH tool, see Online Resource 4.
4 Discussion
4.1 Summary of Findings
This review summarises the data supporting the efficacy and safety of switching from OAPs to PP1M in the treatment of patients with schizophrenia as reported in 15 studies with a total of 4740 patients. Our findings indicated the relapse rate was 12% among moderately ill patients over 26 weeks. The long-term rehospitalisation rate was 18%, although the severity and stage of illness was not extractable to provide greater precision. In addition, the PANSS total scores, CGI-S scores, and PSP total scores were improved at short-, medium- and long-term follow-ups. According to subgroup analysis results, there was a greater reduction in the PANSS score among patients who previously used olanzapine than among former risperidone users (− 25.5 vs − 17.61). Compared with previous research, our findings also suggested that a safety profile associated with switching to a PP1M was similar to that observed with OAP use. The sensitivity analyses regarding data imputation did not alter our conclusions about the primary outcomes, although careful interpretation of these results is needed due mainly to limited evidence.
4.2 Change in the PANSS, CGI-S, and PSP Scores from Baseline
The reduction on PANSS and CGI-S scores indicated PP1M clinically reduces the symptoms and severity of schizophrenia in patients with a history of prior OAP use. The short-term (PANSS: − 21.69; CGI-S: − 1.42) and long-term (PANSS: − 17.88; CGI-S: − 1.7) changes in the score met their minimum clinically important difference (MCID) (PANSS MCID: 15 [51]; CGI-S MCID: 1 [52]). The medium-term change was − 14.98 in the PANSS score, which just met the MCID cut-off point, and − 0.61 in the CGI-S score, which was lower than its MCID. However, it might be inappropriate to discredit the benefits of switching to PP1M when the MCID is not met. A previous prospective single-arm study, (N = 546), for example, set a 10-point reduction as a meaningful change on the PANSS score, which indicates MCID should not be used as the only criterion [40]. In addition, the greater reduction in the PANSS score among patients with olanzapine compared with patients with risperidone should be interpreted with caution as the data were very limited [30, 31, 33]. In this review, we did not have a definition of previous OAP users. Although the study providing relevant data mentioned that patients were included when they had been on the same oral antipsychotic given for the treatment of schizophrenia in an adequate therapeutic dose, there was no limitations or restrictions on frequency, duration, and dose of OAPs. Also, patients were not randomly assigned to OAP groups. The difference on relevant clinical characteristics may confound interpretation of our findings. Thus, future studies would be expected to provide more comprehensive and detailed data on this aspect for a better understanding of the effects of switching to a PP1M regimen.
The improvement in the PSP score (approximately 10%) at the short-, medium- and long-term follow-ups demonstrated a clinically meaningful improvement over time in patients' daily function [53]. In addition, the reduced length of stay and inpatient visits at medium- and long-term follow-ups may indicate a clinical benefit of the treatment effectiveness associated with switching to a PP1M. These improvements can be seen as beneficial and important from the perspective of reducing the burden on family caregivers, society, and the health system [36, 54, 55].
Our results demonstrated that over 50% patients showed at least a 30% and 40% reduction from the baseline PANSS total score during various treatment periods. Although a reduction of 50% is recognised as a more meaningful measurement of response rate, few studies set this as a criterion. It should be noted that 50% was considered a cut-off threshold among patients taking OAPs [56]. Thus, along with the change in PANSS and CGI-S scores in our review, setting a lower cut-off point may be more appropriate to reflect clinically meaningful improvement associated with switching patients from OAPs to PP1Ms.
4.3 Comparisons with Previous Research
Compared with the efficacy and safety of PP1M reported in a previous long-term double-blind randomised controlled trial (RCT) with 747 patients [57], our review showed consistent results, such as a low relapse rate (12% vs 18%), reduced PANSS total score (− 17.88 vs − 11.6), and high incidence of total TEAEs (75% vs 76%). Likewise, the short-term change in the PANSS score observed in this review was consistent with a previously unpublished 13-week, double-blind RCT, although the sample size was not retrieved) (− 21.69 vs − 18.6) [58]. Therefore, our findings may support extending the extrapolation of PP1M to patients with history of prior OAP use.
Compared with oral SGAs, switching to PP1M results in a lower relapse rate (12%). This is consistent with results reported in a previous review six RCTs with 525 patients, where the 1-year relapse rate for LAIs (27%) was lower than that of patients who received oral medication (42%) [59]. Patients treated with oral SGAs reported a wide range (29.9–73.7%) of all-cause discontinuation rates at both short- and medium-term follow up in previous studies (a 4-week double-blind RCT with 141 patients, a 12-week single-blind RCT with 72 patients, and a systematic review of 9 included studies with 648 patients) [60,61,62]. Conversely, our results showed a 31% short-term and 24% medium-term discontinuation rate in patients who were switched to a PP1M. For the long-term, we found a much lower discontinuation rate due to non-adherence (14%) when switched to PP1M compared with approximately 30% discontinuation rate reported for oral SGAs in an 18-month RCT with 1493 patients [63]. The lower discontinuation rate associated with switching to a PP1M may be beneficial in terms of greater symptom improvement and a higher response rate [55]. Of note, the time to discontinuation of antipsychotics may influence the inclusion and sequence of administration of other drugs, thereby influencing the switching strategies [64]. Contrarily, a recently published RCT (EULAST trial) with 533 patients with early-phase schizophrenia reported that there was no significant difference in time to all-cause discontinuation between the combined oral and combined LAI treatment groups (71% vs 64%) [65]. The contrary results may be explained by different baseline characteristics. For example, in our review, only patients with previous OAP history and without LAI history were included. Whereas in the EULAST trial, only patients with previous history of investigated drugs were excluded. Different drug history may affect the results.
Despite the fact that a minority of patients prescribed LAIs may still encounter instances of missed or delayed injections for various reasons, such as the COVID-19 pandemic, that may potentially compromise the effectiveness of the treatment regimen, our findings suggested a lower discontinuation rate due to non-adherence in patients switching to PP1M compared to that reported for OAPs in previous research (14% vs 30%). This may provide evidence to support the ability of LAI, including PP1M, to circumvent covert non-adherence commonly associated with oral antipsychotic medications. By eliminating the possibility of undisclosed non-adherence, LAIs offer enhanced assurance regarding treatment adherence, which can positively impact therapeutic outcomes in individuals with schizophrenia [8]. Subsequent investigations are required to obtain direct evidence that substantiates the assumed improvement in adherence among patients who transition to PP1M compared to those receiving OAPs.
Compared to previous study results in a 15-month RCT with 444 patients, the pooled overall TEAE rate for patients switching to PP1M (69%) in this review was much lower than oral FGAs (91.4%) and slightly lower than oral SGAs (77.6%) [66]. At least one serious TEAE rate was similar to that reported for OAPs in a 24-month RCT with 764 patients (8% vs 12.7%) [67]. Consistent with a previous study of SGA-LAIs, the high incidence of injection-site pain and weight gain are findings of concern regarding switching to PP1M [68], indicating the need for careful monitoring in clinical practice. Other commonly reported adverse events such as headache and insomnia were often rated as mild or moderate in intensity [17].
It is worthy of note that no included studies provided a clear definition of discontinuation and adverse events or details on how they assessed these indicators. Different methodologies employed for assessing adverse events may have potential influence on reported results [69]. This indicated the importance of detailing assessment methods for adverse events in future research, in order to enhance the accuracy of safety awareness.
Previous reviews suggested LAIs, including paliperidone palmitate, may be an effective treatment for patients experiencing early psychosis in terms of relapse, hospitalisation reduction, and symptom improvement [70, 71]. Although we were not able to assess the effect of stages of schizophrenia due to insufficient data, two studies that enrolled recent-onset (≤ 5 years) or early-stage patients included in our review reported consistent results with previous reviews. Zhang 2015 reported PP1M significantly improved the PANSS total score from baseline to Month 18 and reduced the percentage of recent-onset patients requiring hospitalisation in the past 12 months [22]. The other study reported that PP1M was associated with significantly improved function status at both short- and long-term follow-ups [41].
4.4 Strengths and Limitations
Our study has some strengths and limitations. To our knowledge, this is the first comprehensive and most current meta-analysis evaluating the efficacy and safety of switching patients with schizophrenia from OAPs to PP1M. The results may guide clinical decisions and fill the evidence gap in the field of PP1M. Moreover, this study was carried out with good quality control as we conducted the review strictly according to the Cochrane and PRISMA standard.
However, there are several limitations. First, of note, although our findings demonstrated several advantages of switching to PP1M in the short-, medium- and long-term time periods, the results should be interpreted with caution. Different follow-up periods should be carefully compared and potential influencing factors taken into consideration. For instance, the smaller absolute change in the PANSS score at the medium-term follow-up should not be interpreted as an indication of a poorer symptom improvement after switching to PP1M at that time period in light of the clear benefits noted at other time points. Furthermore, the pooled results in meta-analysis might be influenced by multiple factors, which may limit the comparability of results between follow-up periods. For example, some research suggests that greater improvement might be due, in part, to a higher baseline PANSS score, shorter duration of illness, greater baseline severity, and a more recent onset phase [40, 72,73,74]. Furthermore, of the 15 included studies, six involved patients who had a baseline PANSS total score exceeding 70 [30, 31, 33, 38, 45, 48]. Additionally, two studies specifically recruited patients in an acute stage [46, 49], implying that these individuals were transitioning from OAPs to a PP1M injection during periods characterised by worsened illness and instability. Consequently, the initial measurements of symptomatology could have been elevated, rendering it plausible that the subsequent improvement observed upon switching to the LAI formulation could be partially ascribed to intensified clinical intervention during periods of illness, as well as a natural alleviation of symptoms over time [75]. Moreover, it is possible that patients diagnosed with schizophrenia had limited clinical monitoring and support while receiving OAPs. However, upon transitioning to PP1M, they would have received monthly visits from a mental health professional responsible for administering the injection. This increased level of clinical monitoring would have allowed for prompt response to reports of side effects and changes in mental state. Therefore, for individuals who experienced an escalation in clinical monitoring, on-going clinical review, and improved communication, any observed benefits subsequent to the switch might be partially attributed to these factors. On the other hand, the increase in clinical care is recognised as the unique benefit of LAIs compared to OAPs in managing schizophrenia [76].
Second, the study design and sample size may limit the generalisability of the results. Unfortunately, many studies did not report sufficient details to enable stratification of the results according to possible influencing factors [20, 34, 36, 38, 49, 50]. Thus, future studies focusing on confounders that might impact the effect of switching to PP1M would be beneficial. Additionally, the treatment effect might also be influenced by the definition of outcomes. Because we included the hospitalisation rate defined by the original studies, the variation in the threshold of hospitalisation may affect the pooled results.
Third, only one study reported the discontinuation rate due to non-adherence, which could compromise the treatment effect [33]. Fourth, regarding the limitations of methodology, because we performed a single arm meta-analysis, the results were pooled from single-arm studies or single arms of a comparative study where the blinding and sample size calculation methods were not reported for most studies. This may lead to an under- or over-estimation of the efficacy and safety of switching to PP1M. The nature of proportional data could lead to important heterogeneity, which was reflected by an I2 over 50% in most of our outcomes. In addition, our findings need to be interpreted carefully and not overly generalised due to the limited evidence included for each outcome and the various follow-up periods. Also, our findings should be carefully generated to patients in early stages of schizophrenia since there is a lack of studies in this area.
4.5 Clinical Implications
Overall, our results provide clinicians with evidence that switching patients with schizophrenia to PP1M may be an effective option for those previously taking OAPs. This approach can reduce relapses and rehospitalisation, and probably improve adherence in the long term [20, 22, 33, 38]. In addition, it can reduce symptoms, disease severity and improve social functioning over the short, medium, and long term [21, 22, 30,31,32,33, 38, 41, 45, 46]. However, the findings should be interpreted and applied with caution as the results were based on limited evidence. The influence of disease stage, ethnicity, and history of OAP use remains unclear. Thus, future high-quality comparative studies are needed to confirm the benefits of switching patients to PP1M. Possible influencing factors should be explored in future studies to generalise the findings more precisely.
5 Conclusions
Our findings indicate switching patients with schizophrenia from OAPs to PP1M is effective and tolerated and is associated with lower relapse and hospitalisation rates, PANSS, CGI-S and PSP scores as well as a comparable safety profile to oral OAPs. Compared with previously published research of OAPs, switching to PP1M may also improve adherence and have a similar safety profile to oral OAPs.
References
Kendler KS. Phenomenology of schizophrenia and the representativeness of modern diagnostic criteria. JAMA Psychiat. 2016;73:1082–92. https://doi.org/10.1001/jamapsychiatry.2016.1976.
WHO. Schizophrenia. 2022. https://www.who.int/news-room/fact-sheets/detail/schizophrenia. Accessed 2022.
Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. https://doi.org/10.1016/S0140-6736(15)60692-4.
Wildgust HJ, Hodgson R, Beary M. The paradox of premature mortality in schizophrenia: new research questions. 2010;24:9–15. https://doi.org/10.1177/1359786810382149.
Correll CU, Citrome L, Haddad PM, Lauriello J, Olfson M, Calloway SM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. https://doi.org/10.4088/JCP.15032su1.
Miron AA, Ifteni PI, Teodorescu A, Petric PS. Long-acting injectable antipsychotics (LAIs) prescribing trends during the COVID-19 Pandemic in Romania. Healthcare (Basel). 2022. https://doi.org/10.3390/healthcare10071265.
Schwartz S, Carilli C, Mian T, Ruekert L, Kumar A. Attitudes and perceptions about the use of long-acting injectable antipsychotics among behavioral health practitioners. Ment Health Clin. 2022;12:232–40. https://doi.org/10.9740/mhc.2022.08.232.
Manchanda R, Chue P, Malla A, Tibbo P, Roy M-A, Williams R, et al. Long-acting injectable antipsychotics: evidence of effectiveness and use. Can J Psychiatry. 2013;58:5–13. https://doi.org/10.1177/088740341305805s02.
Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21:127–34. https://doi.org/10.1080/13696998.2017.1379412.
Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21:754–69. https://doi.org/10.18553/jmcp.2015.21.9.754.
Taipale H, Mehtälä J, Tanskanen A, Tiihonen J. Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia—a nationwide study with 20-year follow-up. Schizophr Bull. 2018;44:1381–7. https://doi.org/10.1093/schbul/sbx176.
Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Focus (Am Psychiatr Publ). 2020;18:493–7. https://doi.org/10.1176/appi.focus.18402.
Keating D, McWilliams S, Schneider I, Hynes C, Cousins G, Strawbridge J, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open. 2017;7: e013881. https://doi.org/10.1136/bmjopen-2016-013881.
Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50:410–72. https://doi.org/10.1177/0004867416641195.
Fu D-J, Turkoz I, Simonson RB, Walling D, Schooler N, Lindenmayer J-P, et al. Paliperidone palmitate once-monthly injectable treatment for acute exacerbations of schizoaffective disorder. J Clin Psychopharmacol. 2016;36:372. https://doi.org/10.1097/JCP.0000000000000535.
Emsley R, Hargarter L, Bergmans P, Uglešić B, Sengül AC, Petralia A, et al. Once-monthly paliperidone palmitate in early stage schizophrenia—a retrospective, non-interventional 1-year study of patients with newly diagnosed schizophrenia. Neuropsychiatr Dis Treat. 2017;13:2261. https://doi.org/10.2147/NDT.S142634.
Hargarter L, Bergmans P, Cherubin P, Keim S, Conca A, Serrano-Blanco A, et al. Once-monthly paliperidone palmitate in recently diagnosed and chronic non-acute patients with schizophrenia. Expert Opin Pharmacother. 2016;17:1043–53. https://doi.org/10.1080/14656566.2016.1174692.
Hargarter L, Cherubin P, Bergmans P, Keim S, Rancans E, Bez Y, et al. Intramuscular long-acting paliperidone palmitate in acute patients with schizophrenia unsuccessfully treated with oral antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:1–7. https://doi.org/10.1016/j.pnpbp.2014.11.006.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:1–11. https://doi.org/10.1186/s13643-021-01626-4.
Mahabaleshwarkar R, Lin D, Fishman J, Blair T, Hetherington T, Palmer P, et al. The impact of once-monthly paliperidone palmitate on healthcare utilization among patients with schizophrenia treated in an integrated healthcare system: a retrospective mirror-image study. Adv Ther. 2021;38:1958–74. https://doi.org/10.1007/s12325-021-01626-9.
Bozzatello P, Bellino S, Mancini I, Sandei L, Zanalda E, Rocca P. Effects on satisfaction and service engagement of paliperidone palmitate compared with oral paliperidone in patients with schizophrenia: an open label randomized controlled trial. Clin Drug Investig. 2019;39:169–78. https://doi.org/10.1007/s40261-018-0734-1.
Zhang F, Si T, Chiou CF, Harris AW, Kim CY, Jahagirdar P, et al. Efficacy, safety, and impact on hospitalizations of paliperidone palmitate in recent-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:657–68. https://doi.org/10.2147/ndt.S77778.
Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. https://www.training.cochrane.org/handbook.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2023
Quality assessment tool for before-after (pre-post) studies with no control group. National Heart, Lung, Blood Institute. 2014.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. New York: Wiley; 2019.
Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane Handb Syst Rev Interv. 2019;143-76.
Nussbaum AM, Stroup TS. Paliperidone palmitate for schizophrenia. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD008296.pub2.
Deeks JJ, Higgins JP, Altman DG, Group CSM. Analysing data and undertaking meta‐analyses. Cochrane Handb Syst Rev Interv. 2019;241-84.
Si T, Fan J, Wang X, Wang C, Xu C, Zhuo J, et al. A subgroup analysis of Chinese patients switched to paliperidone palmitate one-month injectable by prior oral antipsychotic treatment. Pharmacopsychiatry. 2016;49:32–41. https://doi.org/10.1055/s-0035-1565133.
Sliwa JK, Bossie CA, Ma YW, Alphs L. Effects of acute paliperidone palmitate treatment in subjects with schizophrenia recently treated with oral risperidone. Schizophr Res. 2011;132:28–34. https://doi.org/10.1016/j.schres.2011.06.016.
Peitl V, Margetić BA, Vidrih B, Karlović D. The impact of long-acting paliperidone in reducing hospitalizations and clinical severity in recent onset schizophrenia: a mirror-image study in real-world clinical setting. Clin Psychopharmacol Neurosci. 2022;20:118–25. https://doi.org/10.9758/cpn.2022.20.1.118.
Magliocco F, de Filippis R, Aloi M, Staltari FA, Gaetano R, Segura-Garcia C, et al. Second-generation long-acting injections anti-psychotics improve executive functions in patients with schizophrenia: a 12-month real-world study. Int J Psychiatry Clin Pract. 2020;24:201–7. https://doi.org/10.1080/13651501.2020.1737134.
Patel C, Emond B, Lafeuille MH, Côté-Sergent A, Lefebvre P, Tandon N, et al. Real-world analysis of switching patients with schizophrenia from oral risperidone or oral paliperidone to once-monthly paliperidone palmitate. Drugs Real World Outcomes. 2020;7:19–29. https://doi.org/10.1007/s40801-019-00172-9.
Li N, Zhuo JM, Turkoz I, Mathews M, Feng Y, Tan W. A post hoc analysis on hospitalization risk in Asian patients with schizophrenia switching to once-monthly paliperidone palmitate from oral antipsychotics. Expert Opin Pharmacother. 2019;20:2033–9. https://doi.org/10.1080/14656566.2019.1650022.
El Khoury A, Patel C, Huang A, Wang L, Bashyal R. Transitioning from oral risperidone or paliperidone to once-monthly paliperidone palmitate: a real-world analysis among Veterans Health Administration patients with schizophrenia who have had at least one prior hospitalization. Curr Med Res Opin. 2019;35:2159–68. https://doi.org/10.1080/03007995.2019.1651129.
Si T, Zhuo J, Feng Y, Lu H, Hong D, Zhang L. Long-term efficacy and safety of paliperidone palmitate once-monthly in Chinese patients with recent-onset schizophrenia. Neuropsychiatr Dis Treat. 2019;15:1685–94. https://doi.org/10.2147/ndt.S191803.
Mathews M, Pei H, Savitz A, Nuamah I, Hough D, Alphs L, et al. Paliperidone palmitate 3-monthly versus 1-monthly injectable in patients with schizophrenia with or without prior exposure to oral risperidone or paliperidone: a post hoc, subgroup analysis. Clin Drug Investig. 2018;38:695–702. https://doi.org/10.1007/s40261-018-0647-z.
Li H, Li Y, Feng Y, Zhuo J, Turkoz I, Mathews M, et al. Impact of time of initiation of once-monthly paliperidone palmitate in hospitalized Asian patients with acute exacerbation of schizophrenia: a post hoc analysis from the PREVAIL study. Neuropsychiatr Dis Treat. 2018;14:1107–17. https://doi.org/10.2147/ndt.S157399.
Si T, Zhuo J, Turkoz I, Mathews M, Tan W, Feng Y. Once-monthly injection of paliperidone palmitate in patients with recently diagnosed and chronic schizophrenia: a post-hoc comparison of efficacy and safety. Expert Opin Pharmacother. 2017;18:1799–809. https://doi.org/10.1080/14656566.2017.1401608.
Zhang H, Turkoz I, Zhuo J, Mathews M, Tan W, Feng Y. Paliperidone palmitate improves and maintains functioning in Asia-Pacific patients with schizophrenia. Adv Ther. 2017;34:2503–17. https://doi.org/10.1007/s12325-017-0638-0.
Schreiner A, Bergmans P, Cherubin P, Hargarter L. The effect of long-acting paliperidone palmitate once-monthly on negative and depressive symptoms in patients with schizophrenia switched from previous unsuccessful treatment with oral aripiprazole. Ther Adv Psychopharmacol. 2017;7:59–65. https://doi.org/10.1177/2045125316673012.
Schreiner A, Caspi A, Bergmans P, Cherubin P, Keim S, Lara E, et al. Switching from oral atypical antipsychotic monotherapy to paliperidone palmitate once-monthly in non-acute patients with schizophrenia: a prospective, open-label, interventional study. Psychopharmacology. 2017;234:3–13. https://doi.org/10.1007/s00213-016-4445-0.
Naber D, Hansen K, Forray C, Baker RA, Sapin C, Beillat M, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168:498–504. https://doi.org/10.1016/j.schres.2015.07.007.
Schreiner A, Bergmans P, Cherubin P, Keim S, Rancans E, Bez Y, et al. A prospective flexible-dose study of paliperidone palmitate in nonacute but symptomatic patients with schizophrenia previously unsuccessfully treated with oral antipsychotic agents. Clin Ther. 2014;36:1372-88.e1. https://doi.org/10.1016/j.clinthera.2014.08.014.
Li H, Turkoz I, Zhang F. Efficacy and safety of once-monthly injection of paliperidone palmitate in hospitalized Asian patients with acute exacerbated schizophrenia: an open-label, prospective, noncomparative study. Neuropsychiatr Dis Treat. 2016;12:15–24. https://doi.org/10.2147/ndt.S83651.
Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters-Strickland T, et al. Multidimensional assessment of functional outcomes in Schizophrenia: results from QUALIFY, a head-to-head trial of aripiprazole once-monthly and paliperidone palmitate. Int J Neuropsychopharmacol. 2017;20:40–9. https://doi.org/10.1093/ijnp/pyw093.
Liu J, Wang Q, Su L, Yang L, Zou L, Bai L. A health economics study of long-acting injectable once-monthly paliperidone palmitate in schizophrenia: a one-year mirror-image study in China. BMC Psychiatry. 2022;22:95. https://doi.org/10.1186/s12888-022-03728-2.
Patel C, Khoury AE, Huang A, Wang L, Bashyal R. Healthcare resource utilization and costs among patients with schizophrenia switching from oral risperidone/paliperidone to once-monthly paliperidone palmitate: a veterans health administration claims analysis. Curr Ther Res Clin Exp. 2020;92: 100587. https://doi.org/10.1016/j.curtheres.2020.100587.
Enatescu V, Dehelean CA, Alexandra L, Hogea NL, Homorogan C, Enatescu I, et al. Study on tolerability and efficacy of paliperidone palmitate, olanzapine pamoate and risperidone long acting injection in a romanian sample of patients with schizophrenia. Farmacia. 2020;68:242–9. https://doi.org/10.31925/farmacia.2020.2.8.
Hermes ED, Sokoloff DM, Stroup TS, Rosenheck RA. Minimum clinically important difference in the positive and negative syndrome scale using data from the CATIE schizophrenia trial. J Clin Psychiatry. 2012;73:526. https://doi.org/10.4088/JCP.11m07162.
Roux P, Brunet-Gouet E, Ehrminger M, Aouizerate B, Aubin V, Azorin JM, et al. Minimum clinically important differences for the Functioning Assessment Short Test and a battery of neuropsychological tests in bipolar disorders: results from the FACE-BD cohort. Epidemiol Psychiatr Sci. 2020;29:e144. https://doi.org/10.1017/S2045796020000566.
Si T, Shi C, Sun L, Zhang Y, Zhang L. Assessment of the minimum clinically important difference in symptoms and functions of patients with acute schizophrenia: a post hoc analysis of an open-label, single-arm multicenter study. Front Psychiatry. 2021;12: 653916. https://doi.org/10.3389/fpsyt.2021.653916.
Lencer R, Garcia-Portilla MP, Bergmans P, Gopal S, Mathews M, Wooller A, et al. Impact on carer burden when stable patients with schizophrenia transitioned from 1-monthly to 3-monthly paliperidone palmitate. Compr Psychiatry. 2021;107: 152233. https://doi.org/10.1016/j.comppsych.2021.152233.
Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence. 2013;7:1171. https://doi.org/10.2147/PPA.S53795.
Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79:231–8. https://doi.org/10.1016/j.schres.2005.04.008.
Fleischhacker WW, Gopal S, Lane R, Gassmann-Mayer C, Lim P, Hough D, et al. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neuropsychopharmacol. 2012;15:107–18. https://doi.org/10.1017/S1461145711001076.
Sedky K, Nazir R, Lindenmayer J-P, Lippmann S. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Curr Psychiatry. 2010;9:48–50.
Schooler NR. Relapse and rehospitalization: comparing oral and depot antipsychotics. J Clin Psychiatry. 2003;64:14–7.
Berger GE, Proffitt T-M, McConchie M, Kerr M, Markulev C, Yuen HP, et al. Dosing quetiapine in drug-naive first-episode psychosis: a controlled, double-blind, randomized, single-center study investigating efficacy, tolerability, and safety of 200 mg/day vs 400 mg/day of quetiapine fumarate in 141 patients aged 15 to 25 years. J Clin Psychiatry. 2008;69:1702–14.
Gafoor R, Landau S, Craig TK, Elanjithara T, Power P, McGuire P. Esquire trial: efficacy and adverse effects of quetiapine versus risperidone in first-episode schizophrenia. J Clin Psychopharmacol. 2010;30:600–6. https://doi.org/10.1097/JCP.0b013e3181f198da.
Miller BJ, Bodenheimer C, Crittenden K. Second-generation antipsychotic discontinuation in first episode psychosis: an updated review. Clin Psychopharmacol Neurosci. 2011;9:45. https://doi.org/10.9758/cpn.2011.9.2.45.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. https://doi.org/10.1056/NEJMoa051688.
Kroken RA, Kjelby E, Wentzel-Larsen T, Mellesdal LS, Jørgensen HA, Johnsen E. Time to discontinuation of antipsychotic drugs in a schizophrenia cohort: influence of current treatment strategies. Ther Adv Psychopharmacol. 2014;4:228–39. https://doi.org/10.1177/2045125314545614.
Winter-van Rossum I, Weiser M, Galderisi S, Leucht S, Bitter I, Glenthøj B, et al. Efficacy of oral versus long-acting antipsychotic treatment in patients with early-phase schizophrenia in Europe and Israel: a large-scale, open-label, randomised trial (EULAST). Lancet Psychiatry. 2023;10:197–208. https://doi.org/10.1016/s2215-0366(23)00005-6.
Kim E, Correll CU, Mao L, Starr HL, Alphs L. Once-monthly paliperidone palmitate compared with conventional and atypical daily oral antipsychotic treatment in patients with schizophrenia. CNS Spectr. 2016;21:466–77. https://doi.org/10.1017/S1092852916000444.
Schreiner A, Aadamsoo K, Altamura AC, Franco M, Gorwood P, Neznanov NG, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169:393–9. https://doi.org/10.1016/j.schres.2015.08.015.
Gentile S. Safety concerns associated with second-generation antipsychotic long-acting injection treatment. A systematic update. Horm Mol Biol Clin Investig. 2018. https://doi.org/10.1515/hmbci-2017-0004.
Mayo-Wilson E, Fusco N, Li T, Hong H, Canner JK, Dickersin K, et al. Harms are assessed inconsistently and reported inadequately part 1: systematic adverse events. J Clin Epidemiol. 2019;113:20–7. https://doi.org/10.1016/j.jclinepi.2019.04.022.
Lian L, Kim DD, Procyshyn RM, Fredrikson DH, Cázares D, Honer WG, et al. Efficacy of long-acting injectable versus oral antipsychotic drugs in early psychosis: a systematic review and meta-analysis. Early Interv Psychiatry. 2022;16:589–99. https://doi.org/10.1111/eip.13202.
Lian L, Kim DD, Procyshyn RM, Cázares D, Honer WG, Barr AM. Long-acting injectable antipsychotics for early psychosis: a comprehensive systematic review. PLoS One. 2022;17: e0267808. https://doi.org/10.1371/journal.pone.0267808.
Schennach-Wolff R, Meyer S, Seemüller F, Jäger M, Schmauss M, Laux G, et al. Influencing factors and predictors of early improvement in the acute treatment of schizophrenia and schizophrenia spectrum disorder. J Psychiatr Res. 2011;45:1639–47. https://doi.org/10.1016/j.jpsychires.2011.07.014.
Quee PJ, van der Meer L, Bruggeman R, de Haan L, Krabbendam L, Cahn W, et al. Insight in psychosis: relationship with neurocognition, social cognition and clinical symptoms depends on phase of illness. Schizophr Bull. 2011;37:29–37. https://doi.org/10.1093/schbul/sbq133.
Furukawa TA, Levine SZ, Tanaka S, Goldberg Y, Samara M, Davis JM, et al. Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA Psychiat. 2015;72:14–21. https://doi.org/10.1001/jamapsychiatry.2014.2127.
Savill M, Banks C, Khanom H, Priebe S. Do negative symptoms of schizophrenia change over time? A meta-analysis of longitudinal data. Psychol Med. 2015;45:1613–27. https://doi.org/10.1017/s0033291714002712.
Stevens GL, Dawson G, Zummo J. Clinical benefits and impact of early use of long-acting injectable antipsychotics for schizophrenia. Early Interv Psychiatry. 2016;10:365–77. https://doi.org/10.1111/eip.12278.
Acknowledgements
We thank Ms Yang Zhang, Ms Chenchen Xu, and Dr. Sitong Dong from Systematic Review Solutions Ltd. for their assistance in data collection and analysis and Dr Margueritte White for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Xi’an Janssen Pharmaceutical Ltd.
Conflict of interest
Qian Li and Tianmei Si declare that they have no conflict of interest. Xin Li, Chong Ye, and Miaomiao Jia are employees of Xi’an Janssen Pharmaceutical Ltd.
Availability of data and material
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author’s contributions
Qian Li and Tianmei Si conceived the ideas and design of the study. Xin Li, Chong Ye and Miaomiao Jia performed data collection, extraction, and analysis. Qian Li, Xin Li, Chong Ye and Miaomiao Jia drafted the paper. Tianmei Si provided clinical support for drafting the paper. All authors reviewed the final manuscript. All authors have read and approved the final submitted manuscript, and agree to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, Q., Li, X., Ye, C. et al. Effectiveness and Safety of Switching from Oral Antipsychotics to Once-Monthly Paliperidone Palmitate (PP1M) in the Management of Schizophrenia: A Systematic Review and Meta-Analysis. CNS Drugs 37, 695–713 (2023). https://doi.org/10.1007/s40263-023-01028-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01028-1