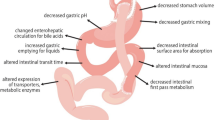
Abstract
Background and Objective
Several drugs on the market are substrates for P-glycoprotein (P-gp), an efflux transporter highly expressed in barrier tissues such as the intestine. Body weight, weight loss, and a Roux-en-Y gastric bypass (RYGB) may influence P-gp expression and activity, leading to variability in the drug response. The objective of this study was therefore to investigate digoxin pharmacokinetics as a measure of the P-gp phenotype in patients with obesity before and after weight loss induced by an RYGB or a strict diet and in normal weight individuals.
Methods
This study included patients with severe obesity preparing for an RYGB (n = 40) or diet-induced weight loss (n = 40) and mainly normal weight individuals scheduled for a cholecystectomy (n = 18). Both weight loss groups underwent a 3-week low-energy diet (<1200 kcal/day) followed by an additional 6 weeks of <800 kcal/day induced by an RYGB (performed at week 3) or a very-low-energy diet. Follow-up time was 2 years, with four digoxin pharmacokinetic investigations at weeks 0, 3, and 9, and year 2. Hepatic and jejunal P-gp levels were determined in biopsies obtained from the patients undergoing surgery.
Results
The RYGB group and the diet group had a comparable weight loss in the first 9 weeks (13 ± 2.3% and 11 ± 3.6%, respectively). During this period, we observed a minor increase (16%) in the digoxin area under the concentration–time curve from zero to infinity in both groups: RYGB: 2.7 µg h/L [95% confidence interval (CI) 0.67, 4.7], diet: 2.5 µg h/L [95% CI 0.49, 4.4]. In the RYGB group, we also observed that the time to reach maximum concentration decreased after surgery: from 1.0 ± 0.33 hours at week 3 to 0.77 ± 0.08 hours at week 9 (−0.26 hours [95% CI −0.47, −0.05]), corresponding to a 25% reduction. Area under the concentration–time curve from zero to infinity did not change long term (week 0 to year 2) in either the RYGB (1.1 µg h/L [−0.94, 3.2]) or the diet group (0.94 µg h/L [−1.2, 3.0]), despite a considerable difference in weight loss from baseline (RYGB: 30 ± 7%, diet: 3 ± 6%). At baseline, the area under the concentration–time curve from zero to infinity was −5.5 µg h/L [95% CI −8.5, −2.5] (−26%) lower in patients with obesity (RYGB plus diet) than in normal weight individuals scheduled for a cholecystectomy. Further, patients undergoing an RYGB had a 0.05 fmol/µg [95% CI 0.00, 0.10] (29%) higher hepatic P-gp level than the normal weight individuals.
Conclusions
Changes in digoxin pharmacokinetics following weight loss induced by a pre-operative low-energy diet and an RYGB or a strict diet (a low-energy diet plus a very-low-energy diet) were minor and unlikely to be clinically relevant. The lower systemic exposure of digoxin in patients with obesity suggests that these patients may have increased biliary excretion of digoxin possibly owing to a higher expression of P-gp in the liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The systemic exposure of digoxin, a P-glycoprotein probe drug, is lower in patients with obesity than in normal weight individuals, which may be due to an increased biliary excretion of digoxin caused by higher hepatic P-gp expression in patients with obesity. |
Only minor, not clinically relevant, changes in digoxin pharmacokinetics were observed following weight loss induced by a pre-operative low-energy diet and Roux-en-Y gastric bypass or a strict diet. |
Dose adjustment of digoxin following bariatric surgery and/or weight loss does not seem necessary. |
1 Introduction
Bariatric surgery induces a substantial long-term weight loss with significant improvements in obesity-related comorbidities such as non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus [1,2,3]. As such, it has become an appropriate treatment option for patients with severe obesity and a high risk of comorbidity and mortality. A remaining challenge in patients undergoing bariatric surgery is to predict pharmacokinetic changes for a specific drug [4]. Aside from the subsequent weight loss, the anatomical and physiological alterations in the gastrointestinal tract following bariatric surgery may influence drug disposition [5]. This is particularly relevant for a Roux-en-Y gastric bypass (RYGB), in which the stomach is reduced to a small gastric pouch and a significant length (75–150 cm) of the proximal intestine is bypassed. Several studies have investigated how an RYGB influences the pharmacokinetics of drugs primarily metabolized by the cytochrome P450 (CYP) superfamily [6,7,8,9,10,11], but data regarding the effect of an RYGB on drug transporters are still limited [10, 12].
P-glycoprotein (P-gp), also known as ATP-binding cassette sub-family B member 1 (ABCB1), is an efflux transporter with broad substrate specificity found in various tissues such as the intestine, blood–brain barrier, kidneys, and liver [13]. In the intestine, P-gp acts as a barrier to drug absorption, thus restricting the oral bioavailability of substrate drugs [14]. P-glycoprotein expression has been shown to increase along the small intestine with the lowest expression in the proximal parts [15, 16]. Because of the gastrointestinal rearrangement following an RYGB, orally administered drugs are promptly delivered to the more distal parts of the intestine with a higher expression of P-gp, which may decrease oral bioavailability of substrate drugs. Digoxin is a P-gp substrate and is recommended by the US Food and Drug Administration to use as a probe drug to assess P-gp activity in drug–drug interaction studies [17]. Digoxin is well absorbed with a high oral bioavailability (70–80%) [18], is widely distributed, and has a long elimination half-life that varies between 26 and 45 hours in healthy individuals [19]. Although the primary route of digoxin elimination is through glomerular filtration and P-gp-mediated tubular secretion of the unchanged drug, up to 30% of a digoxin dose may be eliminated by hepatic P-gp into the bile [19]. Following an RYGB, the subsequent weight loss with improvement in comorbidities may lead to a tissue-specific alteration of P-gp activity and therefore also influence digoxin pharmacokinetics. In fact, hepatic P-gp has been shown to be upregulated in patients with NAFLD [20], suggesting that P-gp activity in the liver may decrease following weight loss because of an improvement in NAFLD status.
Only one small study, using digoxin as a probe drug, has previously investigated the effect of an RYGB on P-gp activity [10]. In this study, the authors found an increased absorption rate of digoxin 3 and 12 months post-surgery, but no change in the systemic exposure. There is still a lack of knowledge on how body weight, weight loss, and an RYGB influences P-gp activity in vivo. We therefore investigated short (6 weeks) and long-term (2 years) changes in digoxin pharmacokinetics as a measure of P-gp activity in a clinical study including patients with obesity undergoing an RYGB or non-surgical calorie restriction. The study also included a cross-sectional comparison of digoxin pharmacokinetics and hepatic P-gp expression between patients with obesity and mainly normal weight individuals scheduled for a cholecystectomy.
2 Methods
2.1 Study Population
The present analyses are part of the extensive COCKTAIL study (NCT02386917), an open, non-randomized, three-armed, controlled study performed at Vestfold Hospital Trust in Norway, investigating the effect of an RYGB and non-surgical calorie restriction on the pharmacokinetics of a cocktail of probe drugs (caffeine [CYP1A2], losartan [CYP2C9], omeprazole [CYP2C19], midazolam [CYP3A], rosuvastatin [organic anion transporting polypeptide 1B1], and digoxin [P-gp]) [21]. Data on CYP1A2, CYP2C9, CYP2C19, CYP3A, and organic anion transporting polypeptide 1B1 activities have been published previously [6, 7, 12]. Patients with severe obesity scheduled for weight loss treatment with an RYGB or non-surgical calorie restriction based on clinical indications were included and followed for 2 years. A cross-sectional group of mainly normal weight individuals scheduled for a cholecystectomy was also included. As described in detail previously, patients aged 18 years and older with a body mass index ≥18.5 kg/m2 and a stable body weight over the last 3 months were eligible for inclusion [21]. Key exclusion criteria included previous bariatric or upper gastrointestinal surgery, estimated glomerular filtration rate (eGFR) ≤30 mL/min/1.73 m2, or treatment with substances that may influence the pharmacokinetics of the probe drugs in close approximation to the investigations.
2.2 Study Procedures
The present analyses included pharmacokinetic data from all three groups at baseline (week 0), and from the weight loss groups at the follow-up visits at week 3, week 9, and year 2. Proteomics data from liver and jejunal biopsies (only RYGB) obtained at the time of surgery in the patients undergoing an RYGB (week 3) or cholecystectomy (week 0) were also included in the analyses [22]. The two weight loss groups started a 3-week low-energy diet (LED; <1200 kcal/day) immediately after the pharmacokinetic investigation at baseline. The RYGB was performed at week 3, after the pre-operative LED. Between weeks 3 and 9, both groups followed a 6-week strict caloric regimen consisting of <800 kcal/day induced by an RYGB or a very-low-energy diet (VLED). The patients followed local treatment guidelines between the follow-up visits at week 9 and year 2.
The pharmacokinetic investigational days have been described in detail previously [21]. In short, patients abstained from food and drugs from 10:00 p.m. the evening before the investigations. On the study day, patients first met for baseline blood sampling (07:30 a.m.) followed by administration of the cocktail of probe drugs. Oral digoxin (0.5 mg) was administered at 09:00 a.m. Blood samples for the analysis of digoxin were collected from a peripheral venous catheter at 0.25, 0.5, 1, 1.5, 2, 3, 4, 4.25, 4.5, 5, 5.5, 6, 8, 10, 12, 23, and 24 hours. Blood samples were drawn in K2-EDTA vacutainer tubes and centrifuged for 10 minutes at 4 °C (1800 g). Plasma was then separated into Cryovials and frozen within 1 hour at −70 °C until analysis.
2.3 Bioanalytical Assay and Clinical Chemistry Analyses
Plasma concentrations of digoxin were determined by Covance Laboratories (Madison, WI, USA) using validated liquid chromatography followed by tandem mass spectrometry methods. The standard curve ranged from 0.05 to 10.0 ng/mL. The inter-run precisions, assessed as coefficients of variation, were 5.8%, 3.6%, and 10.1% at 0.150 ng/mL, 3.00 ng/mL, and 7.50 ng/mL, respectively (n = 120). The inter-run accuracies ranged between 99.3 and 97.6%.
Standard clinical chemistry analyses were performed in fresh blood samples at the Department of Laboratory Medicine, Vestfold Hospital Trust, Tønsberg, Norway. Plasma levels of high-sensitivity C-reactive protein were measured using immunoturbidimetry (Advia Chemistry XPT Systems; Siemens, Erlangen, Germany) at Fürst Medical Laboratory, Oslo, Norway. Body weight (kg) and body composition were measured with the Inbody 720 Body Composition Analyzer (Biospace, Seoul, South Korea).
2.4 Quantification of ABCB1 (P-gp) in Hepatic and Jejunal Biopsies
Proteins were extracted from liver and jejunal biopsies in a SDS-containing (2% w/v) lysis buffer and quantified as previously described [22]. In short, samples were digested using multi-enzyme digestion in a filter-aided sample preparation protocol with LysC and trypsin. A proteomics analysis was performed with Q Exactive HF or Q Exactive HF-X. MS data were processed with MaxQuant using the human UniProtKB, and spectral raw intensities were normalized with variance stabilization. The protein levels were calculated using the total protein approach [23].
2.5 Population Pharmacokinetic Modeling
A population pharmacokinetic model was developed to estimate individual digoxin pharmacokinetics at the different study visits. In short, a non-parametric adaptive grid approach implemented in Pmetrics 1.9.4 for R 4.2.3 was used [24, 25]. A total of 3410 digoxin concentrations across 308 pharmacokinetic profiles from 98 patients were included, of which 197 and 111 were 8-point and 18-point profiles, respectively. The rich data from all patients allowed the model to make accurate individual predictions without the need for covariates, making it fit for purpose in combination with the linear mixed models (see below). The model was internally validated on 25% of the dataset, which comprised solely 8-point pharmacokinetic profiles. A full description of the model development and metrics is available in the Electronic Supplementary Material (ESM).
2.6 Pharmacokinetic Calculations
Posterior individual parameter values, as well as posterior individually predicted concentrations obtained from the final population pharmacokinetic model run with the complete dataset, were used for all pharmacokinetic calculations. Predictions were made in 1-min intervals, including each sample point. Area under the concentration–time curve from zero to infinity (AUC0–∞) was calculated with the trapezoidal approximation from individual posterior-predicted concentrations implemented in the ‘makeAUC’ function in the Pmetrics [24] package for R [25]. Maximum plasma concentration (Cmax) and time to reach Cmax (Tmax) were obtained directly from the individual predictions.
2.7 Data and Statistical Analyses
Linear mixed models were used in the longitudinal analysis to estimate within-group changes and between-group differences (RYGB vs diet). The pharmacokinetic parameter was treated as a dependent variable, whereas time (visit), group (RYGB and diet), and their interaction (time × group) were treated as fixed effects. The unique patient identifier was used as a random effect (individual intercepts). Contrast analyses were performed for parameters of interest. In the cross-sectional analyses, the Welch two-sample t-test was used to compare patients with obesity (RYGB plus diet) and the mainly normal weight individuals (cholecystectomy). To describe the relationship between variables of interest, linear regression analyses were performed. Estimated glomerular filtration rate was calculated using the CKD-EPI formula [26]. The NAFLD liver fat score was calculated as suggested by Kotronen et al. and values greater than −0.640 were indicative of NAFLD [27]. Data are presented as mean ± standard deviation or mean [95% confidence interval (CI)] unless otherwise stated. Confidence intervals from linear mixed models were adjusted with Tukey’s method. With the preetermined α = 0.05, 95% CIs not including zero and p-values <0.05 were considered statistically significant. All statistical analyses were performed using R 4.2.3 [25].
3 Results
3.1 Patients
A total of 108 patients were included in the COCKTAIL study. The patient flow is described in the Fig. S4 of the ESM. Briefly, eight patients withdrew or were excluded before study start. Further, two patients were excluded from the pharmacokinetic analyses because of severe liver cirrhosis and technical difficulties with the venous catheter. Thus, 98 patients (40, 40, and 18 in the RYGB group, diet group, and cholecystectomy group, respectively) supplied at least one 24-hour pharmacokinetic profile during the study period and were included in the present analyses.
Patient characteristics at baseline are given in Table 1. On average, age (46 ± 11 years) and kidney function (eGFR 108 ± 12 mL/min/1.73 m2) were similar in the three groups. In line with the inclusion criteria, patients with obesity (RYGB plus diet) had a higher mean body weight than the patients scheduled for cholecystectomy (mean difference 57 kg [95% CI 49, 64]). Most patients with obesity also had NAFLD (86%), compared with 11% in the cholecystectomy group.
3.2 Changes in Body Weight and Kidney Function
For patients included in the pharmacokinetic analyses, mean weight loss in the two intervention groups was similar after the initial 3-week LED (RYGB: 4.8 ± 1.2%, diet: 4.5 ± 1.9%) and at week 9 after additional 6 weeks of calorie restriction induced by an RYGB (13 ± 2.3%) or VLED (11 ± 3.6%). Thereafter, the RYGB group continued to lose weight with a mean weight loss of 19 ± 9% from week 9 to year 2. In contrast, the diet group regained weight in the same period (10 ± 8%). No change in eGFR was observed in any of the groups in the first 9 weeks of the study. Between week 9 and year 2 a minor, but statistically significant decline in eGFR was observed in the diet group (−5 mL/min/1.73 m2 [95% CI −9, −1]), while no change was observed in the RYGB group.
3.3 Baseline Digoxin Pharmacokinetics
As shown in Fig. 1a, mean AUC0–∞ of digoxin was −5.5 µg h/L [95% CI −8.5, −2.5] (26%) lower in patients with obesity compared with normal weight individuals scheduled for a cholecystectomy. The patients with obesity also had lower Cmax and longer Tmax than the cholecystectomy group: −0.59 µg/L [95% CI −1.1, −0.08] (−23%) and 0.22 hours [95% CI 0.10, 0.34] (28%), respectively (shown in Fig. 1b and c). Both body weight and eGFR were inversely associated with the systemic exposure of digoxin: ß = −0.11, R2adj = 0.42, p < 0.001 and ß = −0.12, R2adj = 0.07, p =0.004, respectively (shown in Fig. 2a and b).
Comparison of (a) the systemic exposure (area under the concentration–time curve from zero to infinity [AUC0–Inf]) of digoxin, (b) maximum plasma concentration (Cmax), (c) time to Cmax (Tmax) at baseline between mainly normal weight individuals scheduled for cholecystectomy (CHOL) and patients with severe obesity scheduled for treatment with Roux-en-Y gastric bypass (RYGB) or a very-low-energy diet (DIET), and (d) hepatic P-gp (ATP-binding cassette sub-family B member 1 [ABCB1]) level at the time of surgery between CHOL and RYGB. Hepatic biopsies were only available in 56 patients (CHOL, n = 18 and RYGB, n = 38)
Association between (a) body weight, (b) estimated glomerular filtration rate (eGFR), and digoxin systemic exposure (area under the concentration–time curve from zero to infinity [AUC0–Inf]) in the Roux-en-Y gastric bypass (RYGB) group, diet group, and cholecystectomy (CHOL) group at baseline. Association between (c) hepatic ATP-binding cassette sub-family B member 1 (ABCB1) levela, (d) jejunal ABCB1 concentrationb, and digoxin systemic exposure (AUC0–∞) in patients undergoing surgery (week 3). R is the Pearson correlation coefficient. aOnly available in patients undergoing surgery (RYGB = 38, CHOL = 18). bOnly available in patients undergoing an RYGB (n = 36)
3.4 Protein Levels of P-gp in the Liver and Jejunal Biopsies
The hepatic ABCB1 level was 0.05 fmol/µg [95% CI 0.00, 0.10] (29%) higher in the RYGB group than in the cholecystectomy group (shown in Fig. 1d). When comparing patients with (n = 37) and without NAFLD (n = 18), mean hepatic ABCB1 level was 0.07 fmol/µg [95% CI 0.03, 0.11] (42%) higher in patients with NAFLD. The individual hepatic levels of ABCB1 were associated with digoxin AUC0–∞ (ß = −26, R2adj = 0.12, p = 0.006, shown in Fig. 2c). Jejunal ABCB1 levels, only available in the patients undergoing an RYGB, were not associated with digoxin AUC0–∞ (p = 0.38, shown in Fig. 2d). Body weight was positively associated with hepatic (ß = 0.001, R2adj = 0.13, p =0.004), but not jejunal (p = 0.59) levels of ABCB1.
3.5 Longitudinal Changes in Digoxin Pharmacokinetics
Observed data for digoxin AUC0–∞, Cmax, and Tmax in the two intervention groups are presented in Table 2. Results from the mixed-model analysis of longitudinal changes in digoxin pharmacokinetics are given in Table 3. At week 9, AUC0–∞ was similarly increased in both weight loss groups compared with baseline (RYGB: 16% [95% CI 4.5, 26], diet: 16% [95% CI 3.6, 26]) (Fig. 3a). No statistically significant changes in AUC0–∞ was observed long term (year 2) in any of the groups (Table 3, Fig. 3a). Furthermore, no between-group differences were observed (Fig. 3d). Longitudinal changes in the concentration–time profiles (0–6 hours) of digoxin in the RYGB group and diet group are shown in Fig. 4.
Changes in digoxin (a) systemic exposure (area under the concentration–time curve from zero to infinity [AUC0–Inf]), (b) maximum plasma concentration (Cmax), and (c) time to Cmax (Tmax) in the Roux-en-Y gastric bypass (RYGB) and DIET groupsa. A linear mixed model was used to estimate mean change over time [95% confidence interval]. Statistically significant differences (p < 0.05) within groups (RYGB or DIET) compared with baseline are symbolized by *. Between-group differences in within-group changes for the RYGB and DIET groups for digoxin (d) AUC0–∞, (e) Cmax, and (f) Tmax. Difference between groups was calculated with diet as the reference group and presented as the marginal mean with 95% CI. aEstimated marginal means from the linear mixed model
A faster absorption of digoxin, reflected by a 25% [95% CI 4.3, 52] shorter mean Tmax, was observed 6 weeks after an RYGB and was still present at the study visit at year 2 (Table 3, Fig. 3c). The patients undergoing an RYGB also had a lower Cmax early after surgery that remained decreased long term (Table 3, Fig. 3b). In contrast, neither digoxin Cmax nor Tmax demonstrated any change in the diet group during the study period (Table 3, Fig. 3b and c). As such, there was a statistically significant difference in the change between the RYGB group and the diet group for these two parameters (Fig. 3e and f).
4 Discussion
The available literature on how body weight, weight loss, and an RYGB influence drug transporters such as P-gp is sparse. In this study, changes in digoxin pharmacokinetics were investigated to provide an insight into P-gp activity before and after weight loss induced by an RYGB or a strict diet. We observed a similar increase in the systemic exposure of digoxin in the two intervention groups in the early phase after a pre-operative LED and an RYGB or a strict diet (LED plus VLED) [weeks 0–9]. The different effect on systemic exposure by the common LED intervention during the first 3 weeks in the RYGB group and the diet group was minor and not considered to be of clinical importance. Although the baseline investigation showed that patients with obesity had a lower systemic exposure of digoxin than normal weight individuals scheduled for a cholecystectomy, we did not observe any changes in systemic exposure following the substantial weight loss of ~30% in the RYGB group at year 2. This indicates that substantial weight loss does not lead to the reversal of obesity-induced alteration in digoxin pharmacokinetics mediated by P-gp or other factors.
Given that kidney function was comparable between patients with obesity and normal weight individuals, the difference in digoxin AUC0–∞ is likely to be explained by differences in non-renal clearance. The proteomics data indicated that this is at least partly due to a higher hepatic expression of P-gp in patients with obesity. The weak, but significant, association that was observed between hepatic P-gp expression and AUC0–∞ in the present study suggests that biliary elimination is of some importance for the systemic exposure of digoxin. We can only speculate on the mechanism for the upregulation of hepatic P-gp in patients with obesity, but our data suggested that NAFLD may play a role. Patients with NAFLD had a higher hepatic P-gp expression than patients without NAFLD. In line with this finding, Hardwick et al. found an increased expression of P-gp in human liver samples with the progression of NAFLD [28]. Other studies have also demonstrated a higher hepatic P-gp expression, as well as lower AUC following intravenous and oral dosing of digoxin in rats with NAFLD [29, 30]. Nevertheless, in the study by Abernethy et al., there was no difference in the clearance of digoxin between patients with obesity and normal weight controls following intravenous administration [31]. The presence of NAFLD was not reported in this study and may not have been present in the same extent as in our study population. We did not observe any association between jejunal P-gp expression and digoxin AUC0–∞ in our study, but jejunal biopsies were only obtained in the patients undergoing an RYGB and not the normal weight individuals scheduled for a cholecystectomy. However, the lack of association may be explained by the fact that most of the orally administered digoxin is absorbed in the proximal part of the intestine [19]. In a previous study with healthy volunteers, the systemic exposure of oral digoxin decreased significantly during treatment with the P-gp inducer rifampin because of an increase in duodenal P-gp expression [32]. It is challenging to say if the patients with obesity in our study also had an increased expression of duodenal P-gp contributing to the lower AUC0–∞ in these patients compared with the normal weight individuals.
The RYGB led to a faster absorption of digoxin 6 weeks and 2 years after surgery. This is in line with what Chan et al. found for digoxin Tmax 3 and 12 months after an RYGB [10]. A faster absorption following an RYGB has also been shown for other drugs [8, 11, 12] and may be explained by an accelerated gastric emptying time due to the anatomical alterations in the gastrointestinal tract [4]. Interestingly, we observed a lower Cmax of digoxin after surgery, while Chan et al. found no changes in their study [10]. Normally, most of the digoxin is absorbed in the proximal part of the small intestine [19]. However, because an RYGB places orally administered drugs directly in the more distal parts with higher P-gp expression [15, 16], there may be an increased efflux of digoxin back into the lumen, which limits the absorption. The reduced absorption surface in the small intestine may also be of importance [5]. In this work, the altered absorption of digoxin following an RYGB was not followed by any significant changes in AUC0–∞. The initial weight loss induced by the pre-operative LED and an RYGB led to a temporary, minor increase in digoxin AUC0–∞ that returned to baseline values at year 2 despite most patients having lost a substantial proportion of their body weight. Interestingly, AUC0–∞ changed similarly in the diet group throughout the study period, although their body weight was regained at year 2. This suggests that weight loss induced by LED followed by an RYGB or VLED does not influence digoxin AUC0–∞, and therefore P-gp activity, to any significant degree. In line with our results, Chan et al. found no difference in the AUC0–∞ of digoxin following an RYGB in their study [10]. Additionally, Ewy et al. found no difference in blood concentrations of digoxin following a single intravenous dose before and after weight loss induced by LED [33].
The major strength of the present study is the large sample size and long follow-up time compared with previous studies with similar objectives. The dietary control group with matched short-term weight loss enabled us to differentiate the effect of an RYGB and weight loss on digoxin pharmacokinetics. Additionally, individual digoxin pharmacokinetic variables and parameters were calculated using a population pharmacokinetic model based on rich data. The study has some limitations. Although digoxin remains the commonly used probe drug for P-gp activity, its sensitivity and specificity have been questioned [17]. However, to date, an ideal probe drug for P-gp activity does not exist. Further, no covariates were included in the population pharmacokinetic model. Because only individual predictions of pharmacokinetic parameters were of interest in this analysis, the statistical analyses were based on a linear mixed model with an individual random intercept instead of estimating all relevant interactions within the population pharmacokinetic model. In addition, the ABCB1 genotype was not included in the analyses. Conflicting evidence exists with regard to the impact of the ABCB1 polymorphism on digoxin pharmacokinetics [34,35,36]. The kidney function was not measured but estimated using the CKD-EPI formula, which may overestimate GFR 6 months following an RYGB [37]. As all patients in the present study had normal kidney function throughout the study, we do not believe that changes in renal clearance have led to the minor observed changes in digoxin AUC0–∞. Furthermore, as NAFLD was predicted using a score based on metabolic factors, it was not possible to confirm the NAFLD status or to determine the severity of NAFLD.
5 Conclusions
Patients with obesity demonstrate a lower systemic exposure of digoxin than normal weight individuals scheduled for a cholecystectomy. This may be explained by a higher hepatic expression of P-gp in patients with obesity leading to increased biliary excretion of digoxin. Our findings may be relevant for dosing of other P-gp substrates, but differences in factors such as substrate specificity, metabolic pathways, and physicochemical properties make it challenging to extrapolate the findings to other drugs transported by P-gp. Even though patients with obesity lose a substantial amount of weight as observed in the RYGB group, P-gp activity does not seem to return to that of normal weight individuals. Roux-en-Y gastric bypass leads to an earlier onset of absorption following oral dosing of digoxin, but neither weight loss induced by LED following an RYGB nor VLED changes the systemic exposure of digoxin to any clinically relevant degree. Dose adjustment of digoxin following an RYGB and/or weight loss does therefore not seem necessary in patients with satisfactory serum concentrations of digoxin prior to the intervention. However, the relatively narrow therapeutic range of digoxin should be kept in mind.
References
Elsaid MI, Li Y, Bridges JFP, Brock G, Minacapelli CD, Rustgi VK. Association of bariatric surgery with cardiovascular outcomes in adults with severe obesity and nonalcoholic fatty liver disease. JAMA Netw Open. 2022;5(10): e2235003. https://doi.org/10.1001/jamanetworkopen.2022.35003.
Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149(3):275–87. https://doi.org/10.1001/jamasurg.2013.3654.
Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397(10287):1830–41. https://doi.org/10.1016/s0140-6736(21)00591-2.
Angeles PC, Robertsen I, Seeberg LT, Krogstad V, Skattebu J, Sandbu R, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299–311. https://doi.org/10.1111/obr.12869.
Kingma JS, Burgers DMT, Monpellier VM, Wiezer MJ, Blussé van Oud-Alblas HJ, Vaughns JD, et al. Oral drug dosing following bariatric surgery: general concepts and specific dosing advice. Br J Clin Pharmacol. 2021;87(12):4560–76. https://doi.org/10.1111/bcp.14913.
Kvitne KE, Krogstad V, Wegler C, Johnson LK, Kringen MK, Hovd MH, et al. Short- and long-term effects of body weight, calorie restriction and gastric bypass on CYP1A2, CYP2C19 and CYP2C9 activity. Br J Clin Pharmacol. 2022;88(9):4121–33. https://doi.org/10.1111/bcp.15349.
Kvitne KE, Robertsen I, Skovlund E, Christensen H, Krogstad V, Wegler C, et al. Short- and long-term effects of body weight loss following calorie restriction and gastric bypass on CYP3A-activity: a non-randomized three-armed controlled trial. Clin Transl Sci. 2022;15(1):221–33. https://doi.org/10.1111/cts.13142.
Brill MJ, van Rongen A, van Dongen EP, van Ramshorst B, Hazebroek EJ, Darwich AS, et al. The pharmacokinetics of the CYP3A substrate midazolam in morbidly obese patients before and one year after bariatric surgery. Pharm Res. 2015;32(12):3927–36. https://doi.org/10.1007/s11095-015-1752-9.
Puris E, Pasanen M, Ranta VP, Gynther M, Petsalo A, Käkelä P, et al. Laparoscopic Roux-en-Y gastric bypass surgery influenced pharmacokinetics of several drugs given as a cocktail with the highest impact observed for CYP1A2, CYP2C8 and CYP2E1 substrates. Basic Clin Pharmacol Toxicol. 2019;125(2):123–32. https://doi.org/10.1111/bcpt.13234.
Chan LN, Lin YS, Tay-Sontheimer JC, Trawick D, Oelschlager BK, Flum DR, et al. Proximal Roux-en-Y gastric bypass alters drug absorption pattern but not systemic exposure of CYP3A4 and P-glycoprotein substrates. Pharmacotherapy. 2015;35(4):361–9. https://doi.org/10.1002/phar.1560.
Goday Arno A, Farré M, Rodríguez-Morató J, Ramon JM, Pérez-Mañá C, Papaseit E, et al. Pharmacokinetics in morbid obesity: influence of two bariatric surgery techniques on paracetamol and caffeine metabolism. Obes Surg. 2017;27(12):3194–201. https://doi.org/10.1007/s11695-017-2745-z.
Hovd M, Robertsen I, Johnson LK, Krogstad V, Wegler C, Kvitne KE, et al. Neither gastric bypass surgery nor diet-induced weight-loss affect OATP1B1 activity as measured by rosuvastatin oral clearance. Clin Pharmacokinet. 2023. https://doi.org/10.1007/s40262-023-01235-5.
Staud F, Ceckova M, Micuda S, Pavek P. Expression and function of p-glycoprotein in normal tissues: effect on pharmacokinetics. Methods Mol Biol. 2010;596:199–222. https://doi.org/10.1007/978-1-60761-416-6_10.
Takano M, Yumoto R, Murakami T. Expression and function of efflux drug transporters in the intestine. Pharmacol Ther. 2006;109(1–2):137–61. https://doi.org/10.1016/j.pharmthera.2005.06.005.
Mai Y, Dou L, Yao Z, Madla CM, Gavins FKH, Taherali F, et al. Quantification of P-glycoprotein in the gastrointestinal tract of humans and rodents: methodology, gut region, sex, and species matter. Mol Pharm. 2021;18(5):1895–904. https://doi.org/10.1021/acs.molpharmaceut.0c00574.
Mouly S, Paine MF. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm Res. 2003;20(10):1595–9. https://doi.org/10.1023/a:1026183200740.
Nader AM, Foster DR. Suitability of digoxin as a P-glycoprotein probe: implications of other transporters on sensitivity and specificity. J Clin Pharmacol. 2014;54(1):3–13. https://doi.org/10.1002/jcph.200.
Beveridge T, Nüesch E, Ohnhaus EE. Absolute bioavailability of digoxin tablets. Arzneimittelforschung. 1978;28(4):701–3.
Iisalo E. Clinical pharmacokinetics of digoxin. Clin Pharmacokinet. 1977;2(1):1–16. https://doi.org/10.2165/00003088-197702010-00001.
Omidkhoda N, Zare S, Mahdiani S, Samadi S, Akhlaghi F, Mohammadpour AH. Hepatic transporters alternations associated with non-alcoholic fatty liver disease (NAFLD): a systematic review. Eur J Drug Metab Pharmacokinet. 2023;48(1):1–10. https://doi.org/10.1007/s13318-022-00802-8.
Hjelmesæth J, Åsberg A, Andersson S, Sandbu R, Robertsen I, Johnson LK, et al. Impact of body weight, low energy diet and gastric bypass on drug bioavailability, cardiovascular risk factors and metabolic biomarkers: protocol for an open, non-randomised, three-armed single centre study (COCKTAIL). BMJ Open. 2018;8(5): e021878. https://doi.org/10.1136/bmjopen-2018-021878.
Wegler C, Wiśniewski JR, Robertsen I, Christensen H, Kristoffer Hertel J, Hjelmesaeth J, et al. Drug disposition protein quantification in matched human jejunum and liver from donors with obesity. Clin Pharmacol Ther. 2022;111(5):1142–54. https://doi.org/10.1002/cpt.2558.
Wiśniewski JR, Rakus D. Multi-enzyme digestion FASP and the 'total protein approach’-based absolute quantification of the Escherichia coli proteome. J Proteomics. 2014;109:322–31. https://doi.org/10.1016/j.jprot.2014.07.012.
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34(4):467–76. https://doi.org/10.1097/FTD.0b013e31825c4ba6.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2023. Available from: https://www.R-project.org/. (Accessed 22 Oct 2023).
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–72. https://doi.org/10.1053/j.gastro.2009.06.005.
Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2011;39(12):2395–402. https://doi.org/10.1124/dmd.111.041012.
Jeong HJ, Lee SH, Kang HE. Changes in digoxin pharmacokinetics associated with hepatic P-glycoprotein upregulation in rats with non-alcoholic fatty liver disease. Fundam Clin Pharmacol. 2021;35(6):1100–8. https://doi.org/10.1111/fcp.12687.
Li Z, Zhang J, Zhang Y, Zhou L, Zhao J, Lyu Y, et al. Intestinal absorption and hepatic elimination of drugs in high-fat high-cholesterol diet-induced non-alcoholic steatohepatitis rats: exemplified by simvastatin. Br J Pharmacol. 2021;178(3):582–9. https://doi.org/10.1111/bph.15298.
Abernethy DR, Greenblatt DJ, Smith TW. Digoxin disposition in obesity: clinical pharmacokinetic investigation. Am Heart J. 1981;102(4):740–4. https://doi.org/10.1016/0002-8703(81)90100-9.
Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104(2):147–53. https://doi.org/10.1172/jci6663.
Ewy GA, Groves BM, Ball MF, Nimmo L, Jackson B, Marcus F. Digoxin metabolism in obesity. Circulation. 1971;44(5):810–4. https://doi.org/10.1161/01.cir.44.5.810.
Talameh JA, Lanfear DE. Pharmacogenetics in chronic heart failure: new developments and current challenges. Curr Heart Fail Rep. 2012;9(1):23–32. https://doi.org/10.1007/s11897-011-0076-2.
Oni-Orisan A, Lanfear DE. Pharmacogenomics in heart failure: where are we now and how can we reach clinical application? Cardiol Rev. 2014;22(5):193–8. https://doi.org/10.1097/crd.0000000000000028.
Hsin CH, Stoffel MS, Gazzaz M, Schaeffeler E, Schwab M, Fuhr U, et al. Combinations of common SNPs of the transporter gene ABCB1 influence apparent bioavailability, but not renal elimination of oral digoxin. Sci Rep. 2020;10(1):12457. https://doi.org/10.1038/s41598-020-69326-y.
von Scholten BJ, Persson F, Svane MS, Hansen TW, Madsbad S, Rossing P. Effect of large weight reductions on measured and estimated kidney function. BMC Nephrol. 2017;18(1):52. https://doi.org/10.1186/s12882-017-0474-0.
Acknowledgments
The authors thank the study participants, the surgical staff, and the study personnel involved in the COCKTAIL study at Vestfold Hospital Trust. The authors also thank the Swedish Research Council, approval numbers 5715 and 01951 (CW and PA) for supporting the proteomics analyses. This article is based on data from the COCKTAIL study, which also is part of Kine Eide Kvitne’s PhD at the University of Oslo. A copy of the thesis is available from the University of Oslo website.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital). Vestfold Hospital Trust, Norway, Department of Pharmacy, University of Oslo, Norway, and AstraZeneca, Sweden funded the preparation of this article.
Conflicts of Interest/Competing Interests
Cecilia Karlsson, Shalini Andersson, and Rasmus Jansson-Löfmark are AstraZeneca employees and own shares in AstraZeneca, while Christine Wegler is a former AstraZeneca employee. Kine Eide Kvitne, Markus Hovd, Line Kristin Johnson, Per Artursson, Rune Sandbu, Jøran Hjelmesæth, Eva Skovlund, Hege Christensen, Anders Åsberg, and Ida Robertsen have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The COCKTAIL study was approved by the Regional Committee for Medical and Health Research Ethics (2013/2379/REK) and performed according to the Declaration of Helsinki.
Consent to Participate
All patients gave written informed consent as part of the COCKTAIL study.
Consent for Publication
Not applicable.
Availability of Data and Material
Access to data collected from this study, including anonymized individual-participant data, may potentially be made available following publication upon request to the corresponding author. After approval of a proposal, data will be shared with investigators whose proposed use of the data has been approved by the COCKTAIL Steering Committee, according to the consent given by the participants and Norwegian laws and legislations.
Code Availability
Not applicable.
Authors’ Contributions
KEK drafted the manuscript. MH developed the population pharmacokinetic model. KEK, MH, and IR analyzed and interpreted the data. JH, AÅ, SA, CK, HC, ES, and RS designed the research. IR, LKJ, PA, CW, and RJ performed the research. All authors reviewed and approved the manuscript in its final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kvitne, K.E., Hovd, M., Johnson, L.K. et al. Digoxin Pharmacokinetics in Patients with Obesity Before and After a Gastric Bypass or a Strict Diet Compared with Normal Weight Individuals. Clin Pharmacokinet 63, 109–120 (2024). https://doi.org/10.1007/s40262-023-01320-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01320-9