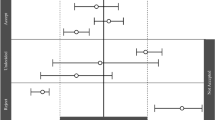
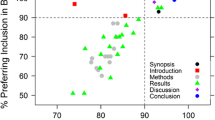
Abstract
Predicting drug exposures using population pharmacokinetic models through Bayesian forecasting software can improve individual pharmacokinetic/pharmacodynamic target attainment. However, selecting the most adapted model to be used is challenging due to the lack of guidance on how to design and interpret external evaluation studies. The confusion around the choice of statistical metrics and acceptability criteria emphasises the need for further research to fill this methodological gap as there is an urgent need for the development of standards and guidelines for external evaluation studies. Herein we discuss the scientific challenges faced by pharmacometric researchers and opportunities for future research with a focus on antibiotics.

Similar content being viewed by others
References
Kantasiripitak W, Van Daele R, Gijsen M, Ferrante M, Spriet I, Dreesen E. Software tools for model-informed precision dosing: how well do they satisfy the needs? Front Pharmacol. 2020;11:620.
Aljutayli A, Thirion DJG, Bonnefois G, Nekka F. Pharmacokinetic equations versus Bayesian guided vancomycin monitoring: Pharmacokinetic model and model-informed precision dosing trial simulations. Clin Transl Sci. 2022;15(4):942–53.
de Velde F, Mouton JW, de Winter BCM, van Gelder T, Koch BCP. Clinical applications of population pharmacokinetic models of antibiotics: challenges and perspectives. Pharmacol Res. 2018;134:280–8.
Frenette C, Sperlea D, German GJ, Afra K, Boswell J, Chang S, et al. The 2017 global point prevalence survey of antimicrobial consumption and resistance in Canadian hospitals. Antimicrob Resist Infect Control. 2020;9(1):104.
Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54(1):109–15.
Peters L, Olson L, Khu DTK, Linnros S, Le NK, Hanberger H, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: A cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS ONE. 2019;14(5): e0215666.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55. https://doi.org/10.1016/S0140-6736(21)02724-0. Erratum in: Lancet. 2022;400(10358):1102.
Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86(2):156–67.
Hughes DM, Goswami S, Keizer RJ, Hughes MA, Faldasz JD. Bayesian clinical decision support-guided versus clinician-guided vancomycin dosing in attainment of targeted pharmacokinetic parameters in a paediatric population. J Antimicrob Chemother. 2020;75(2):434–7.
Frymoyer A, Schwenk HT, Zorn Y, Bio L, Moss JD, Chasmawala B, et al. Model-informed precision dosing of vancomycin in hospitalized children: implementation and adoption at an Academic Children’s Hospital. Front Pharmacol. 2020;11:551.
Brooks JT, Keizer RJ, Long-Boyle JR, Kharbanda S, Dvorak CC, Friend BD. Population pharmacokinetic model development of tacrolimus in pediatric and young adult patients undergoing hematopoietic cell transplantation. Front Pharmacol. 2021;12: 750672.
Darwich AS, Ogungbenro K, Vinks AA, Powell JR, Reny JL, Marsousi N, et al. Why has model-informed precision dosing not yet become common clinical reality? Lessons from the past and a roadmap for the future. Clin Pharmacol Ther. 2017;101(5):646–56.
Heus A, Uster DW, Grootaert V, Vermeulen N, Somers A, In't Veld DH, Wicha SG, De Cock PA. Model-informed precision dosing of vancomycin via continuous infusion: a clinical fit-for-purpose evaluation of published PK models. Int J Antimicrob Agents. 2022;59(5):106579. https://doi.org/10.1016/j.ijantimicag.2022.106579.
Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51(1):1–13.
Aljutayli A, Marsot A, Nekka F. An update on population pharmacokinetic analyses of vancomycin, part I. In adults. Clin Pharmacokinet. 2020;59(6):671–98.
Aljutayli A, El-Haffaf I, Marsot A, Nekka F. An update on population pharmacokinetic analyses of vancomycin, part II: in pediatric patients. Clin Pharmacokinet. 2022;61(1):47–70.
Chung E, Sen J, Patel P, Seto W. Population pharmacokinetic models of vancomycin in paediatric patients: a systematic review. Clin Pharmacokinet. 2021;60(8):985–1001.
Duffull SB, Wright DF. What do we learn from repeated population analyses? Br J Clin Pharmacol. 2015;79(1):40–7. https://doi.org/10.1111/bcp.12233.
Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn. 2001;28(2):171–92.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–12.
Nguyen THT, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacomet Syst Pharmacol. 2017;6(2):87–109.
US FDA. Population pharmacokinetics: guidance for industry. 2022. https://www.fda.gov/media/128793/download. Accessed 03 Feb 2023.
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, et al. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007;46(3):221–34.
Cheng Y, Wang C-y, Li Z-r, Pan Y, Liu M-b, Jiao Z. Can population pharmacokinetics of antibiotics be extrapolated? Implications of external evaluations. Clin Pharmacokinet. 2021;60(1):53–68.
Mentre F, Ebelen ME. Validation of population pharmacokinetic–pharmacodynamic analyses: review of proposed approaches. In: Balant LP, Aarons L, editors. The population approach: measuring and managing variability in response concentration and dose (COST B1). Brussels: Office for Official Publications of the European Communities; 1997. p. 146–60.
Short TG, Willemsen G. Total intravenous anesthesia and target controlled infusions: a comprehensive global anthology. Anesth Analg. 2018;126(2):718.
Kanji S, Hayes M, Ling A, Shamseer L, Chant C, Edwards DJ, et al. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK statement. Clin Pharmacokinet. 2015;54(7):783–95.
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, et al. Are population pharmacokinetic and/ or pharmacodynamic models adequately evaluated? Clin Pharmacokinet. 2007;46(3):221–34.
Short TG, Aun CS, Tan P, Wong J, Tam YH, Oh TE. A prospective evaluation of pharmacokinetic model controlled infusion of propofol in paediatric patients. Br J Anaesth. 1994;72(3):302–6.
Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991;67(1):41–8.
El Hassani M, Simard C, Pilote S, Cloutier I, Soufsaf S, Marsot A. Consideration of height-based tobramycin dosing regimens for the treatment of adult cystic fibrosis pulmonary exacerbations. Br J Clin Pharmacol. 2021;88(5):2246–55.
Zhao CY, Jiao Z, Mao JJ, Qiu XY. External evaluation of published population pharmacokinetic models of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol. 2016;81(5):891–907.
Sheiner LB, Beal SL, Dunne A. Analysis of nonrandomly censored ordered categorical longitudinal data from analgesic trials. J Am Stat Assoc. 1997;92(440):1235–44.
Zhao W, Kaguelidou F, Biran V, Zhang D, Allegaert K, Capparelli EV, et al. External Evaluation of Population Pharmacokinetic Models of Vancomycin in Neonates: the transferability of published models to different clinical settings. Br J Clin Pharmacol. 2013;75(4):1068–80.
Lv C, Lu J, Jing L, Liu TT, Chen M, Zhang R, et al. Systematic external evaluation of reported population pharmacokinetic models of vancomycin in Chinese children and adolescents. J Clin Pharm Ther. 2021;46(3):820–31.
Marsot A, Michel F, Chasseloup E, Paut O, Guilhaumou R, Blin O. Phenobarbital in intensive care unit pediatric population: predictive performances of population pharmacokinetic model. Fundam Clin Pharmacol. 2017;31(5):558–66.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Mentré F, Escolano S. Prediction discrepancies for the evaluation of nonlinear mixed-effects models. J Pharmacokinet Pharmacodyn. 2006;33(3):345–67.
Brendel K, Comets E, Laffont C, Laveille C, Mentré F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006;23(9):2036–49.
Zhang R, Chen M, Liu T-t, Lu J-J, Lv C-l. Comparison of the predictive performance between cystatin c and serum creatinine by vancomycin via a population pharmacokinetic models: a prospective study in a Chinese population. Eur J Drug Metab Pharmacokinet. 2020;45(1):135–49.
Hu C, Yin W-j, Li D-y, Ding J-j, Zhou L-y, Wang J-l, et al. Evaluating tacrolimus pharmacokinetic models in adult renal transplant recipients with different CYP3A5 genotypes. Eur J Clin Pharmacol. 2018;74(11):1437–47.
Mao J-J, Jiao Z, Yun H-Y, Zhao C-Y, Chen H-C, Qiu X-Y, et al. External evaluation of population pharmacokinetic models for ciclosporin in adult renal transplant recipients. Br J Clin Pharmacol. 2018;84(1):153–71.
Varvel JR, Donoho DL, Shafer SL. Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm. 1992;20(1):63–94.
Miyabe-Nishiwaki T, Masui K, Kaneko A, Nishiwaki K, Nishio T, Kanazawa H. Evaluation of the predictive performance of a pharmacokinetic model for propofol in Japanese macaques (Macaca fuscata fuscata). J Vet Pharmacol Ther. 2013;36(2):169–73.
Glass PS, Shafer S, Reves JG. Intravenous drug delivery systems. In: Miller RD, editor. Miller’s Anesthesia. Churchill Livingstone: Elsevier; 2004. p. 439–80.
Sepúlveda P, Cortínez LI, Sáez C, Penna A, Solari S, Guerra I, et al. Performance evaluation of paediatric propofol pharmacokinetic models in healthy young children. Br J Anaesth. 2011;107(4):593–600.
Masui K, Upton RN, Doufas AG, Coetzee JF, Kazama T, Mortier EP, et al. The performance of compartmental and physiologically based recirculatory pharmacokinetic models for propofol: a comparison using bolus, continuous, and target-controlled infusion data. Anesth Analg. 2010;111(2):368–79.
Coppens M, Van Limmen JG, Schnider T, Wyler B, Bonte S, Dewaele F, et al. Study of the time course of the clinical effect of propofol compared with the time course of the predicted effect-site concentration: Performance of three pharmacokinetic-dynamic models. Br J Anaesth. 2010;104(4):452–8.
Yang N, Wang J, Xie Y, Ding J, Wu C, Liu J, et al. External evaluation of population pharmacokinetic models to inform precision dosing of meropenem in critically ill patients. Front Pharmacol. 2022;13: 838205.
Thibault C, Zuppa AF. Dexmedetomidine in children on extracorporeal membrane oxygenation: pharmacokinetic data exploration using previously published models. Front Pediatr. 2022;10:924829. https://doi.org/10.3389/fped.2022.924829.
Guo T, van Hest RM, Roggeveen LF, Fleuren LM, Thoral PJ, Bosman RJ, van der Voort PHJ, Girbes ARJ, Mathot RAA, Elbers PWG. External evaluation of population pharmacokinetic models of vancomycin in large cohorts of intensive care unit patients. Antimicrob Agents Chemother. 2019;63(5):e02543–18. https://doi.org/10.1128/AAC.02543-18
Alghanem S, Paterson I, Touw DJ, Thomson AH. Influence of multiple courses of therapy on aminoglycoside clearance in adult patients with cystic fibrosis. J Antimicrob Chemother. 2013;68(6):1338–47.
Wicha SG, Märtson A-G, Nielsen EI, Koch BCP, Friberg LE, Alffenaar J-W, et al. From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin Pharmacol Ther. 2021;109(4):928–41.
Zhou Y, Long E, Shi T, Wang Z, Zhao J, Liu H, et al. External validation of vancomycin population pharmacokinetic models in ten cohorts of infected Chinese patients. J Glob Antimicrob Resist. 2022;30:163–72.
Keizer RJ, Ter Heine R, Frymoyer A, Lesko LJ, Mangat R, Goswami S. Model-informed precision dosing at the bedside: scientific challenges and opportunities. CPT Pharmacometrics Syst Pharmacol. 2018;7(12):785–7. https://doi.org/10.1002/psp4.12353.
Graves DA, Locke CS Jr, Muir KT, Miller RP. The influence of assay variability on pharmacokinetic parameter estimation. J Pharmacokinet Biopharm. 1989;17(5):571–92. https://doi.org/10.1007/BF01071350.
El Hassani M, Marsot A. Impact of sampling times on the predictive performance of tobramycin population pharmacokinetic models [abstract no 24]. Population Approach Group in Europe 2021.
Wang YL, Guilhaumou R, Blin O, Velly L, Marsot A. External evaluation of population pharmacokinetic models for continuous administration of meropenem in critically ill adult patients. Eur J Clin Pharmacol. 2020;76(9):1281–9.
Verrier D, Sivapregassam S, Solente AC. Dose-finding studies, MCP-Mod, model selection, and model averaging: Two applications in the real world. Clin Trials. 2014;11(4):476–84.
Aoki Y, Hooker AC. Model averaging and selection methods for model structure and parameter uncertainty quantification. In: PAGE. Abstracts of the Annual Meeting of the Population Approach Group in Europe. ISSN 1871-6032; 2016.
Aoki Y, Röshammar D, Hamrén B, Hooker AC. Model selection and averaging of nonlinear mixed-effect models for robust phase III dose selection. J Pharmacokinet Pharmacodyn. 2017;44(6):581–97. https://doi.org/10.1007/s10928-017-9550-0.
Kantasiripitak W, Outtier A, Wicha SG, Kensert A, Wang Z, Sabino J, et al. Multi-model averaging improves the performance of model-guided infliximab dosing in patients with inflammatory bowel diseases. CPT Pharmacometrics Syst Pharmacol. 2022;11(8):1045–59.
Heus A, Uster DW, Grootaert V, Vermeulen N, Somers A, Huis in’t Veld D, et al. Model-informed precision dosing of vancomycin via continuous infusion: a clinical fit-for-purpose evaluation of published PK models. Int J Antimicrobi Agents. 2022;59(5):106579. https://doi.org/10.1016/j.ijantimicag.2022.106579.
Uster DW, Stocker SL, Carland JE, Brett J, Marriott DJE, Day RO, et al. A model averaging/selection approach improves the predictive performance of model-informed precision dosing: vancomycin as a case study. Clin Pharmacol Ther. 2021;109(1):175–83.
Sarker IH. AI-based modeling: techniques, applications and research issues towards automation, intelligent and smart systems. SN Comput Sci. 2022;3(2):158.
Brier ME, Zurada JM, Aronoff GR. Neural network predicted peak and trough gentamicin concentrations. Pharm Res. 1995;12(3):406–12.
Huang X, Yu Z, Bu S, Lin Z, Hao X, He W, et al. An ensemble model for prediction of vancomycin trough concentrations in pediatric patients. Drug Des Devel Ther. 2021;15:1549–59.
Hughes JH, Keizer RJ. A hybrid machine learning/pharmacokinetic approach outperforms maximum a posteriori Bayesian estimation by selectively flattening model priors. CPT Pharmacometrics Syst Pharmacol. 2021;10(10):1150–60.
Woillard J-B, Labriffe M, Debord J, Marquet P. Tacrolimus exposure prediction using machine learning. Clin Pharmacol Ther. 2021;110(2):361–9.
Lee S, Song M, Han J, Lee D, Kim BH. Application of machine learning classification to improve the performance of vancomycin therapeutic drug monitoring. Pharmaceutics. 2022;14(5):1023. https://doi.org/10.3390/pharmaceutics14051023
Kim YK, Lee JH, Jang HJ, Zang DY, Lee DH. Predicting antibiotic effect of vancomycin using pharmacokinetic/pharmacodynamic modeling and simulation: dense sampling versus sparse sampling. antibiotics (Basel). 2022;11(6):743. https://doi.org/10.3390/antibiotics11060743.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Mehdi El Hassani received a scholarship from Université de Montréal, and Amélie Marsot acknowledges support from the Fonds de Recherche du Québec-Santé (FRQS) Research Scholars – Junior 1 (Young Researcher Establishment) Career Scholarship.
Conflicts of interest
Mehdi El Hassani and Amélie Marsot declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Author contributions
ME and AM conceptualised the manuscript. ME performed the literature search and wrote the manuscript. AM revised the manuscript.
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analysed.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Hassani, M., Marsot, A. External Evaluation of Population Pharmacokinetic Models for Precision Dosing: Current State and Knowledge Gaps. Clin Pharmacokinet 62, 533–540 (2023). https://doi.org/10.1007/s40262-023-01233-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01233-7