Abstract
Purpose
Beta-lactams (BL), the most commonly prescribed class of antibiotics, are recommended as the first-line therapy for multiple indications in infectious disease guidelines. Meropenem (MERO) is frequently used in intensive care units (ICU) to treat bacterial infections with or without sepsis. The pharmacokinetics of MERO display a large variability in patients admitted to ICUs due to altered pathophysiology. The aim of this study was to perform an external evaluation of published population pharmacokinetic models of MERO in order to test their predictive performance in a cohort of ICU adult patients.
Methods
A literature search in PubMed/Medline database was made following the PRISMA statement. External evaluation was performed using NONMEM software, and the bias and inaccuracy values were calculated.
Results
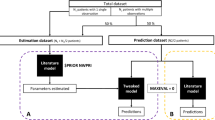
An external validation dataset from the Timone Hospital in Marseille, France, included 84 concentration samples from 27 patients. Four models of MERO were identified according to the inclusion criteria of the study. None of the models presented acceptable values of bias and inaccuracy.
Conclusion
While performing external evaluations on some populations may confirm a model’s suitability to diverse groups of patients, there is still some variability that cannot be explained nor solved by the procedure. This brings to light the difficulty to develop only one model for ICU patients and the need to develop one specific model to each population of critically ill patients.



Similar content being viewed by others
References
Wiseman LR, Wagstaff AJ, Brogden RN, Bryson HM (1995) Meropenem. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs. 50(1):73–101
Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL (2005) Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother 55(5):601–607
Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P et al (2019) Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit Care (London, England) 23(1):104
Chow KM, Hui AC, Szeto CC (2005) Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis 24(10):649–653
Mould DR, Upton RN (2013) Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2(4):e38-e
Zhao W, Kaguelidou F, Biran V, Zhang D, Allegaert K, Capparelli EV, Holford N, Kimura T, Lo YL, Peris JE, Thomson A, van den Anker JN, Fakhoury M, Jacqz-Aigrain E (2013) External evaluation of population pharmacokinetic models of vancomycin in neonates: the transferability of published models to different clinical settings. Br J Clin Pharmacol 75(4):1068–1080
Lee JY, Garnett CE, Gobburu JV, Bhattaram VA, Brar S, Earp JC et al (2011) Impact of pharmacometric analyses on new drug approval and labelling decisions: a review of 198 submissions between 2000 and 2008. Clin Pharmacokinet 50(10):627–635
Varghese JM, Roberts JA, Lipman J (2010) Pharmacokinetics and pharmacodynamics in critically ill patients. Curr Opin Anaesthesiol 23(4):472–478
Verdier MC, Tribut O, Tattevin P, Le Tulzo Y, Michelet C, Bentue-Ferrer D (2011) Simultaneous determination of 12 beta-lactam antibiotics in human plasma by high-performance liquid chromatography with UV detection: application to therapeutic drug monitoring. Antimicrob Agents Chemother 55(10):4873–4879
EMA. guideline-bioanalytical-method-validation_en.pdf [Internet]. Available from: https://wwwemaeuropaeu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_enpdf. [cited 2019 Apr 24].
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Guang W, Baraldo M, Furlanut M (1995) Calculating percentage prediction error: a user’s note. Pharmacol Res 32(4):241–248
Varvel JR, Donoho DL, Shafer SL (1992) Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm 20(1):63–94
Hara M, Masui K, Eleveld DJ, Struys M, Uchida O (2017) Predictive performance of eleven pharmacokinetic models for propofol infusion in children for long-duration anaesthesia. Br J Anaesth 118(3):415–423
Brendel K, Comets E, Laffont C, Laveille C, Mentre F (2006) Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23(9):2036–2049
Broeker A, Nardecchia M, Klinker KP, Derendorf H, Day RO, Marriott DJ et al (2019) Towards precision dosing of vancomycin: a systematic evaluation of pharmacometsric models for Bayesian forecasting. Clin Microbiol Infect 25(10):1286.e1–1286.e7
Delattre IK, Musuamba FT, Jacqmin P, Taccone FS, Laterre P-F, Verbeeck RK, Jacobs F, Wallemacq P (2012) Population pharmacokinetics of four β-lactams in critically ill septic patients comedicated with amikacin. Clin Biochem 45(10):780–786
Li C, Kuti JL, Nightingale CH, Nicolau DP (2006) Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol 46(10):1171–1178
Mattioli F, Fucile C, Del Bono V, Marini V, Parisini A, Molin A et al (2016) Population pharmacokinetics and probability of target attainment of meropenem in critically ill patients. Eur J Clin Pharmacol 72(7):839–848
Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J (2009) Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 64(1):142–150
Delattre IK, Musuamba FT, Nyberg J, Taccone FS, Laterre P-F, Verbeeck RK, Jacobs F, Wallemacq PE (2010) Population pharmacokinetic modeling and optimal sampling strategy for Bayesian estimation of amikacin exposure in critically ill septic patients. Ther Drug Monit 32(6):749–756
Jaruratanasirikul S, Thengyai S, Wongpoowarak W, Wattanavijitkul T, Tangkitwanitjaroen K, Sukarnjanaset W, Jullangkoon M, Samaeng M (2015) Population pharmacokinetics and Monte Carlo dosing simulations of meropenem during the early phase of severe sepsis and septic shock in critically ill patients in intensive care units. Antimicrob Agents Chemother 59(6):2995–3001
Kees MG, Minichmayr IK, Moritz S, Beck S, Wicha SG, Kees F, Kloft C, Steinke T (2016) Population pharmacokinetics of meropenem during continuous infusion in surgical ICU patients. J Clin Pharmacol 56(3):307–315
Minichmayr IK, Roberts JA, Frey OR, Roehr AC, Kloft C, Brinkmann A (2018) Development of a dosing nomogram for continuous-infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J Antimicrob Chemother 73(5):1330–1339
Lodise TP, Nau R, Kinzig M, Drusano GL, Jones RN, Sorgel F (2007) Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J Antimicrob Chemother 60(5):1038–1044
Crandon JL, Ariano RE, Zelenitsky SA, Nicasio AM, Kuti JL, Nicolau DP (2010) Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med 37(4):632–638
Lodise TP, Sorgel F, Melnick D, Mason B, Kinzig M, Drusano GL (2011) Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 55(4):1606–1610
Mathew SK, Mathew BS, Neely MN, Naik GS, Prabha R, Jacob GG, K S, Fleming DH (2016) A nonparametric pharmacokinetic approach to determine the optimal dosing regimen for 30-minute and 3-hour meropenem infusions in critically ill patients. Ther Drug Monit 38(5):593–599
Cojutti P, Sartor A, Righi E, Scarparo C, Bassetti M, Pea F (2017) Population pharmacokinetics of high-dose continuous-infusion meropenem and considerations for use in the treatment of infections due to KPC-producing <span class=“named-content genus-species” id=“named-content-1”>Klebsiella pneumoniae</span>. Antimicrob Agents Chemother 61(10):e00794–e00717
Sjovall F, Alobaid AS, Wallis SC, Perner A, Lipman J, Roberts JA (2018) Maximally effective dosing regimens of meropenem in patients with septic shock. J Antimicrob Chemother 73(1):191–198
Dhaese SAM, Farkas A, Colin P, Lipman J, Stove V, Verstraete AG, Roberts JA, de Waele JJ (2019) Population pharmacokinetics and evaluation of the predictive performance of pharmacokinetic models in critically ill patients receiving continuous infusion meropenem: a comparison of eight pharmacokinetic models. J Antimicrob Chemother 74(2):432–441
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B et al (2007) Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet 46(3):221–234
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL, International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases (2014) Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14(6):498–509
Sunder S, Jayaraman R, Mahapatra HS, Sathi S, Ramanan V, Kanchi P, Gupta A, Daksh S, Ram P (2014) Estimation of renal function in the intensive care unit: the covert concepts brought to light. J Intensive Care 2(1):31
Marsot A, Guilhaumou R, Riff C, Blin O (2017) Amikacin in critically ill patients: a review of population pharmacokinetic studies. Clin Pharmacokinet 56(2):127–138
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R (2004) A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350(22):2247–2256
Roberts JA, Pea F, Lipman J (2013) The clinical relevance of plasma protein binding changes. Clin Pharmacokinet 52(1):1–8
Duffull S, Waterhouse T, Eccleston J (2005) Some considerations on the design of population pharmacokinetic studies. J Pharmacokinet Pharmacodyn 32(3-4):441–457
Alihodzic D, Broeker A, Baehr M, Kluge S, Langebrake C, Wicha SG (2020) Impact of inaccurate documentation of sampling and infusion time in model-informed precision dosing. Front Pharmacol 11:172. https://doi.org/10.3389/fphar.2020.00172
Grahl JJ, Stollings JL, Rakhit S, Person AK, Wang L, Thompson JL, Pandharipande PP, Ely EW, Patel MB (2018) Antimicrobial exposure and the risk of delirium in critically ill patients. Crit Care 22(1):337
Bhattacharyya S, Darby RR, Raibagkar P, Gonzalez Castro LN, Berkowitz AL (2016) Antibiotic-associated encephalopathy. Neurology. 86(10):963–971
Funding
Internal funding supported this study.
Author information
Authors and Affiliations
Contributions
YW and AM analyzed the data. AM performed population pharmacokinetic analysis. LV included patient. YW and AM wrote the manuscript. OB validated the manuscript. AM and RG conceived and designed the study. AM supervised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 67.1 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Guilhaumou, R., Blin, O. et al. External evaluation of population pharmacokinetic models for continuous administration of meropenem in critically ill adult patients. Eur J Clin Pharmacol 76, 1281–1289 (2020). https://doi.org/10.1007/s00228-020-02922-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02922-z