Abstract
Background and Objectives
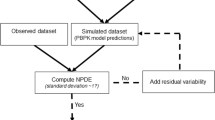
Precision dosing requires selecting the appropriate population pharmacokinetic model, which can be assessed through external evaluations (EEs). The lack of understanding of how different study design factors influence EE study outcomes makes it challenging to select the most suitable model for clinical use. This study aimed to evaluate the impact of sample size, sampling strategy, and handling of concentrations below the lower limit of quantification (BLQ) on the outcomes of EE for four population pharmacokinetic models using vancomycin and tobramycin as examples.
Methods
Three virtual patient populations undergoing vancomycin or tobramycin therapy were simulated with varying sample size and sampling scenarios. The three approaches used to handle BLQ data were to (1) discard them, (2) impute them as LLOQ/2, or (3) use a likelihood-based approach. EEs were performed with NONMEM and R.
Results
Sample size did not have an important impact on the EE results for a given scenario. Increasing the number of samples per patient did not improve predictive performance for two out of the three evaluated models. Evaluating a model developed with rich sampling did not result in better performance than those developed with regular therapeutic drug monitoring. A likelihood-based method to handle BLQ samples impacted the outcomes of the EE with lower bias for predicted troughs.
Conclusions
This study suggests that a large sample size may not be necessary for an EE study, and models selected based on TDM may be more generalizable. The study highlights the need for guidelines for EE of population pharmacokinetic models for clinical use.










Similar content being viewed by others

References
Marsot A. Pharmacokinetic variability in pediatrics and intensive care: toward a personalized dosing approach. J Pharm Pharm Sci. 2018;21(1):354–62.
Powell JR, Cook J, Wang Y, Peck R, Weiner D. Drug dosing recommendations for all patients: a roadmap for change. Clin Pharmacol Ther. 2021;109(1):65–72.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacomet Syst Pharmacol. 2013;2(4): e38.
Kantasiripitak W, Van Daele R, Gijsen M, Ferrante M, Spriet I, Dreesen E. Software tools for model-informed precision dosing: how well do they satisfy the needs? Front Pharmacol. 2020;11:620.
Aljutayli A, Thirion DJG, Bonnefois G, Nekka F. Pharmacokinetic equations versus Bayesian guided vancomycin monitoring: pharmacokinetic model and model-informed precision dosing trial simulations. Clin Transl Sci. 2022;15(4):942–53.
Gu JQ, Guo YP, Jiao Z, Ding JJ, Li GF. How to handle delayed or missed doses: a population pharmacokinetic perspective. Eur J Drug Metab Pharmacokinet. 2020;45(2):163–72.
Patanwala AE, Spremo D, Jeon M, Thoma Y, Alffenaa JWC, Stocker S. Discrepancies between Bayesian vancomycin models can affect clinical decisions in the critically ill. Crit Care Res Pract. 2022;2022:7011376.
El Hassani M, Marsot A. External evaluation of population pharmacokinetic models for precision dosing: current state and knowledge gaps. Clin Pharmacokinet. 2023;62(4):533–40.
Cheng Y, Wang C-Y, Li Z-R, Pan Y, Liu M-B, Jiao Z. Can population pharmacokinetics of antibiotics be extrapolated? Implications of external evaluations. Clin Pharmacokinet. 2021;60(1):53–68.
Kim YK, Lee JH, Jang HJ, Zang DY, Lee DH. Predicting antibiotic effect of vancomycin using pharmacokinetic/pharmacodynamic modeling and simulation: dense sampling versus sparse sampling. Antibiotics (Basel). 2022;11(6):743.
Aarons L, Ogungbenro K. Optimal design of pharmacokinetic studies. Basic Clin Pharmacol Toxicol. 2010;106(3):250–5.
Beal SL. Sample size determination for confidence intervals on the population mean and on the difference between two population means. Biometrics. 1989;45(3):969–77.
Grieve AP. Confidence intervals and sample sizes. Biometrics. 1991;47(4):1597–602.
Ogungbenro K, Aarons L. How many subjects are necessary for population pharmacokinetic experiments? Confidence interval approach. Eur J Clin Pharmacol. 2008;64(7):705–13.
Chan Kwong AHXP, Calvier EAM, Fabre D, Gattacceca F, Khier S. Prior information for population pharmacokinetic and pharmacokinetic/pharmacodynamic analysis: overview and guidance with a focus on the NONMEM PRIOR subroutine. J Pharmacokinet Pharmacodyn. 2020;47(5):431–46.
Keizer RJ, Jansen RS, Rosing H, Thijssen B, Beijnen JH, Schellens JH, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect. 2015;3(2): e00131.
Huang S, Ding Q, Yang N, Sun Z, Cheng Q, Liu W, et al. External evaluation of published population pharmacokinetic models of posaconazole. Front Pharmacol. 2022;13:1005348.
Konecki C, Feliu C, Cazaubon Y, Giusti D, Tonye-Libyh M, Brixi H, et al. External evaluation of population pharmacokinetic models and bayes-based dosing of infliximab. Pharmaceutics. 2021;13(8):1191.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Santacana E, Rodríguez-Alonso L, Padullés A, Guardiola J, Rodríguez-Moranta F, Serra K, et al. External evaluation of population pharmacokinetic models of infliximab in patients with inflammatory bowel disease. Ther Drug Monit. 2018;40(1):120–9.
Hanafin PO, Nation RL, Scheetz MH, Zavascki AP, Sandri AM, Kwa AL, et al. Assessing the predictive performance of population pharmacokinetic models for intravenous polymyxin B in critically ill patients. CPT Pharmacomet Syst Pharmacol. 2021;10(12):1525–37.
Yang N, Wang J, Xie Y, Ding J, Wu C, Liu J, et al. External evaluation of population pharmacokinetic models to inform precision dosing of meropenem in critically ill patients. Front Pharmacol. 2022;13: 838205.
Nguyen TH, Comets E, Mentré F. Extension of NPDE for evaluation of nonlinear mixed effect models in presence of data below the quantification limit with applications to HIV dynamic model. J Pharmacokinet Pharmacodyn. 2012;39(5):499–518.
Irby DJ, Ibrahim ME, Dauki AM, Badawi MA, Illamola SM, Chen M, et al. Approaches to handling missing or “problematic” pharmacology data: pharmacokinetics. CPT Pharmacomet Syst Pharmacol. 2021;10(4):291–308.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35(4):401–21.
Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11(2):371–80.
Mehrotra N, Tang L, Phelps SJ, Meibohm B. Evaluation of vancomycin dosing regimens in preterm and term neonates using Monte Carlo simulations. Pharmacotherapy. 2012;32(5):408–19.
Dong M, Rodriguez AV, Blankenship CA, McPhail G, Vinks AA, Hunter LL. Pharmacokinetic modelling to predict risk of ototoxicity with intravenous tobramycin treatment in cystic fibrosis. J Antimicrob Chemother. 2021;76(11):2923–31.
El Hassani M, Simard C, Pilote S, Cloutier I, Soufsaf S, Marsot A. Consideration of height-based tobramycin dosing regimens for the treatment of adult cystic fibrosis pulmonary exacerbations. Br J Clin Pharmacol. 2021;88(5):2246–55.
Smit C, Wasmann RE, Wiezer MJ, van Dongen HPA, Mouton JW, Brüggemann RJM, et al. Tobramycin clearance is best described by renal function estimates in obese and non-obese individuals: results of a prospective rich sampling pharmacokinetic study. Pharm Res. 2019;36(8):112.
Koloskoff K, Thirion DJG, Matouk E, Marsot A. New recommendations of a height-based dosing regimen of tobramycin for cystic fibrosis in adults: a population pharmacokinetic analysis. Ther Drug Monit. 2022. https://doi.org/10.1097/FTD.0000000000001021.
Alghanem S, Paterson I, Touw DJ, Thomson AH. Influence of multiple courses of therapy on aminoglycoside clearance in adult patients with cystic fibrosis. J Antimicrob Chemother. 2013;68(6):1338–47.
Teutonico D, Musuamba F, Maas HJ, Facius A, Yang S, Danhof M, et al. Generating virtual patients by multivariate and discrete re-sampling techniques. Pharm Res. 2015;32(10):3228–37.
Owen JS, Fiedler-Kelly J (2014) Introduction to population pharmacokinetic/pharmacodynamic analysis with nonlinear mixed effects models. John Wiley & Sons, Hoboken, NJ
Germovsek E, Osborne L, Gunaratnam F, Lounis SA, Busquets FB, Standing JF, et al. Development and external evaluation of a population pharmacokinetic model for continuous and intermittent administration of vancomycin in neonates and infants using prospectively collected data. J Antimicrob Chemother. 2019;74(4):1003–11.
Vancocin (vancomycin hydrochloride) [package insert]. Baudette, MN: ANI Pharmaceuticals; 2017
Aljutayli A, El-Haffaf I, Marsot A, Nekka F. An update on population pharmacokinetic analyses of vancomycin, part II: in pediatric patients. Clin Pharmacokinet. 2022;61(1):47–70.
Smit C, Wasmann RE, Goulooze SC, Hazebroek EJ, Van Dongen EPA, Burgers DMT, et al. A prospective clinical study characterizing the influence of morbid obesity on the pharmacokinetics of gentamicin: towards individualized dosing in obese patients. Clin Pharmacokinet. 2019;58(10):1333–43.
Crass RL, Pai MP. Optimizing estimated glomerular filtration rate to support adult to pediatric pharmacokinetic bridging studies in patients with cystic fibrosis. Clin Pharmacokinet. 2019;58(10):1323–32.
Frymoyer A, Hersh AL, El-Komy MH, Gaskari S, Su F, Drover DR, et al. Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob Agents Chemother. 2014;58(11):6454–61.
Bauer RJ. NONMEM tutorial part II: estimation methods and advanced examples. CPT Pharmacomet Syst Pharmacol. 2019;8(8):538–56.
Maitre PO, Ausems ME, Vozeh S, Stanski DR. Evaluating the accuracy of using population pharmacokinetic data to predict plasma concentrations of alfentanil. Anesthesiology. 1988;68(1):59–67.
Hara M, Masui K, Eleveld DJ, Struys MMRF, Uchida O. Predictive performance of eleven pharmacokinetic models for propofol infusion in children for long-duration anaesthesia. Br J Anaesth. 2017;118(3):415–23.
Miyabe-Nishiwaki T, Masui K, Kaneko A, Nishiwaki K, Nishio T, Kanazawa H. Evaluation of the predictive performance of a pharmacokinetic model for propofol in Japanese macaques (Macaca fuscata fuscata). J Vet Pharmacol Ther. 2013;36(2):169–73.
Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90(2):154–66.
Hughes JH, Tong DMH, Faldasz JD, Frymoyer A, Keizer RJ. Evaluation of neonatal and paediatric vancomycin pharmacokinetic models and the impact of maturation and serum creatinine covariates in a large multicentre data set. Clin Pharmacokinet. 2023;62(1):67–76.
Heus A, Uster DW, Grootaert V, Vermeulen N, Somers A, In’t Veld DH, et al. Model-informed precision dosing of vancomycin via continuous infusion: a clinical fit-for-purpose evaluation of published PK models. Int J Antimicrob Agents. 2022;59(5): 106579.
Colin PJ, Allegaert K, Thomson AH, Touw DJ, Dolton M, de Hoog M, et al. Vancomycin pharmacokinetics throughout life: results from a pooled population analysis and evaluation of current dosing recommendations. Clin Pharmacokinet. 2019;58(6):767–80.
Cunio CB, Uster DW, Carland JE, Buscher H, Liu Z, Brett J et al. Towards precision dosing of vancomycin in critically ill patients: an evaluation of the predictive performance of pharmacometric models in ICU patients. Clin Microbiol Infect. 2021;27(5):783.e7–783.e14
Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacomet Syst Pharmacol. 2017;6(2):87–109.
Bensken WP, Pieracci FM, Ho VP. Basic introduction to statistics in medicine, part 1: describing data. Surg Infect (Larchmt). 2021;22(6):590–6.
Hsu LF. A survey of population pharmacokinetic reports submitted to the USFDA: an analysis of common issues in NDA and BLA from 2012 to 2021. Clin Pharmacokinet. 2022;61(12):1697–703.
Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11(3):558–69.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
ME and AM designed the research. ME performed research and analyzed data. ME wrote the manuscript. UL and AM reviewed the manuscript.
Funding
This work was supported by the Fonds de Recherche du Québec-Santé (FRQS); the Réseau Québécois de Recherche sur les Médicaments and Canada Foundation for Innovation.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Code Availability
Available on request from the corresponding author.
Conflict of interest
Mehdi El Hassani, Uwe Liebchen and Amélie Marsot declare no conflicts of interest.
Ethical Approval
No ethics approval was necessary given that no clinical trial data were used for the simulations. We relied on published data and models to perform simulations.
Consent to Participate
Not applicable as this was a simulation study.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Hassani, M., Liebchen, U. & Marsot, A. Does Sample Size, Sampling Strategy, or Handling of Concentrations Below the Lower Limit of Quantification Matter When Externally Evaluating Population Pharmacokinetic Models?. Eur J Drug Metab Pharmacokinet (2024). https://doi.org/10.1007/s13318-024-00897-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s13318-024-00897-1