Abstract
Background and Objective
Low-molecular-weight heparins are routinely administered to patients in the intensive care unit to prevent venous thromboembolisms. There is considerable evidence that low-molecular-weight heparin doses should be personalised based on anti-Xa levels, but pharmacokinetic data in intensive care unit patients are lacking. This study aimed to characterise the pharmacokinetics and associated variability of the low-molecular-weight heparin nadroparin in critically ill patients.
Methods
Critically ill adult patients who were admitted to the intensive care unit and received nadroparin for prophylaxis of venous thromboembolism were included in a study. Population pharmacokinetic analysis was performed by means of parametric non-linear mixed-effects modelling (NONMEM).
Results
A total of 30 patients were enrolled with 12 patients undergoing continuous veno-venous hemodialysis and 18 patients not undergoing continuous veno-venous hemodialysis. Very high variability in pharmacokinetics was observed with an inter-individual variability in the volume of distribution of 63.7% (95% confidence interval 46.5–90.6), clearance of 166% (95% confidence interval 84.7–280) and relative bioavailability of 40.2% (95% confidence interval 29.5–52.6). We found that standard doses of 2850 IE and 5700 IE of nadroparin resulted in sub-prophylactic exposure in critically ill patients.
Conclusions
Low exposure and highly variable pharmacokinetics of nadroparin were observed in intensive care unit patients treated with a prophylactic dose. It can be debated whether nadroparin is currently dosed optimally in intensive care unit patients and our findings encourage the investigation of higher and tailored dosing of nadroparin in the critically ill.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is considerable evidence that low-molecular-weight heparin doses should be personalised based on anti-Xa levels, but little is known on how this should be performed in intensive care unit patients. |
In this prospective pharmacokinetic clinical study, anti-Xa levels of 30 critically ill adult patients with or without continuous veno-venous hemodialysis receiving low-molecular-weight heparin nadroparin prophylaxis were analysed using non-linear mixed-effects modelling (NONMEM). |
Highly variable and low exposure was observed in our population and simulations showed that higher doses might be required. |
It can be debated whether nadroparin is currently dosed optimally in intensive care unit patients and our findings encourage the investigation of higher and tailored dosing of nadroparin in the critically ill. |
1 Introduction
Critically ill patients show alterations in various physiological functions that can influence the pharmacokinetics (PK) of drugs and, thus, also influence the efficacy of drugs [1]. Because of the highly variable PK, intensive care unit (ICU) patients are at risk for inadequate or toxic drug exposure. Subcutaneously administered low-molecular-weight heparins (LMWHs) are widely used for the prevention of venous thromboembolism in critically ill patients [2,3,4,5]. However, the appropriateness of the subcutaneous route and the recommended prophylactic dose of LMWH in these patients are under debate [6,7,8].
The PK of LMWHs is measured indirectly by quantification of anti-Xa activity, as LMWH concentrations cannot be directly measured [9]. An anti-Xa target range for LMWH prophylaxis of 0.2–0.5 IU/mL has been suggested [10]. However, large variability and low levels of anti-Xa in critically ill patients treated with LMWH enoxaparin have been observed [11, 12] and individualised dosing seems indicated, which is not reflected in current dosing protocols that, more or less, advise fixed dosing [13].
A limited number of studies has been performed in ICU patients studying prophylactic dosing individualisation of LMWH, but these focused specifically on estimating effects on the PK of continuous veno-venous hemodialysis (CVVHD) [14], or were performed in a selected cohort of patients with normal renal function [15]. A detailed understanding of the PK of LMWH is a prerequisite to enable physicians to ensure that individual patients receive the optimal dose [16]. Therefore, we aimed to characterise the PK and associated variability of the LMWH nadroparin in critically ill patients being treated in the ICU as a step towards evidence-based, individualised, prophylactic LMWH nadroparin dosing in these patients.
2 Materials and Methods
2.1 Patients
Adult ICU patients were included in this prospective clinical study if they received a standard, subcutaneously administered prophylactic dose of 2850 IE (= 0.3 mL) of nadroparin once daily subcutaneously or 5700 IE (= 0.6 mL) for body weights of 100 kg or higher following hospital protocol. Exclusion criteria included a therapeutic LMWH dose, morbid obesity (body mass index [BMI] ≥ 40 kg/m2) and admission to the ICU for routine post-operative intensive care monitoring. Patients included those undergoing continuous veno-venous hemodialysis (CVVHD) using a high-flux filter Ultraflux® AV1000S. The CVVHD settings are based on bodyweight on the day of admission with a dialysate flow of 2000 mL/h and a blood flow of 100 mL/h. Regional citrate anticoagulation was used with an initial dosing of 4 mmol/L blood. The study was carried out in The Netherlands in accordance with the ethical standards and the Helsinki Declaration of 1975. The Institutional Review Board (Commissie Mensgebonden Onderzoek, Arnhem-Nijmegen) waived the need for informed consent because of the observational nature and negligible burden associated with this study (registration number 2019-5226).
2.2 Blood Sampling Scheme Justification
Blood samples for the determination of anti-Xa levels were collected at t = 0, 0.5, 1, 3, 6, 12 and 24 h (just before the second dose) after nadroparin dosing and at 0.5, 1, 3, 6, 12 and 24 h after the second nadroparin dose, based on the D-optimal design theory [17] and previous pharmacokinetic studies of other LMWHs [18, 19].
2.3 Blood Collection and Anti-Xa Determination
Blood samples were collected in 3.2% buffered sodium citrate-containing tubes and were immediately stored on ice until centrifugation. All samples were double centrifuged at 20 °C within 1 h after collection to obtain plasma samples and stored at − 80 °C until analysis within 2 weeks after collection. Plasma levels of anti-Xa activity were measured with a CS2100 (Siemens Healthcare Diagnostic Products, Marburg, Germany) using an anti-Xa clotting assay (STA®-liquid ANTI-Xa; Diagnostica Stago, Asnières, France). The rate of chromophobe appearance at 405 nm was measured. Calibration occurred with five concentrations of nadroparin. The calibration curve was found to be linear between 0.1 and 2.00 IU/mL.
2.4 Pharmacokinetic Analysis
The obtained pharmacokinetic data were analysed by means of parametric non-linear mixed-effects modelling using the software package NONMEM. In short, a linear pharmacokinetic model was fitted to the data, describing the absorption of nadroparin from the subcutaneous compartment to the central compartment. After development of the base model, the following covariates for the pharmacokinetics of nadroparin were investigated based on their physiological plausibility: the influence of estimated glomerular filtration (estimated glomerular filtration rate) and CVVHD on clearance, the influence of vasopressor use on bioavailability and clearance, and the fluid impact of fluid balance on the volume of distribution. Anti-Xa observations that were below the limit of quantitation, yet above the limit of detection were included in the pharmacokinetic analysis using the “all data” approach, as previously proposed by Keizer et al., as this is known to result in superior estimation of pharmacokinetic parameters compared with censoring [20]. The pharmacokinetic analysis is described in detail in the Electronic Supplementary Material (ESM).
2.5 Dose Evaluations
Using the developed pharmacokinetic model, we simulated concentration–time profiles for typical individuals of 70 kg to illustrate the impact of covariates and different dosing regimes on the PK of nadroparin on a population level. Furthermore, we performed a Monte Carlo simulation exploring the effect of different dosing regimes on steady-state peak anti-Xa levels (4 h after administration) for nadroparin in critically ill patients. We simulated dosing regimes of 2850 IE (0.3 mL), 5700 IE (0.6 mL) and 9500 IE (1 mL) of anti-Xa nadroparin once daily. For each regime, we simulated 1000 virtual individuals, with a typical weight of 70 kg, drawn from a log-normal distribution with an inter-individual variability of 20% and a typical glomerular filtration rate of 90 mL/min, drawn from a log-normal distribution with an inter-individual variability of 30%.
3 Results
3.1 Patients and Data
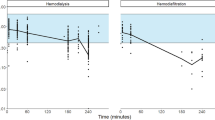
Demographic characteristics are listed in Table 1. A total of 30 patients were enrolled in this study with 12 CVVHD patients and 18 non-CVVHD patients. Figure 1 shows the total number of anti-Xa level samples included versus time (192), showing most of the concentrations were below the lower bound of the prophylactic range (0.2 IU/mL) at the peak concentrations 4 h after the dose. From visual inspection, no accumulation of nadroparin was observed during the study period. Although a BMI > 40 was an exclusion criterion, one person with a BMI of 41 was included because of a rounding error in the initial calculation of the BMI. The majority (89%) of the doses administered were 2850 IU anti-Xa. The average dose administered was 38 IU per kilogram bodyweight with a relative standard deviation of 24%.
3.2 Pharmacokinetic Analysis
The proposed linear pharmacokinetic model, describing first-order absorption from a subcutaneous compartment and first-order elimination from the central compartment, described the data well using allometric scaling. Renal function and the use of CVVHD were covariates for clearance of nadroparin (p < 0.05). Furthermore, the volume of distribution of nadroparin increased with an increasing fluid balance (p < 0.05). The parameter estimates for the final model are shown in Table 2. The results of the pharmacokinetic analysis are described in detail in the ESM, together with the model evaluation through goodness-of-fit plots. Notably, in the final model including covariates, a very high variability in PK was observed with an inter-individual variability in the volume of distribution of 63.7% (95% confidence interval 46.5–90.6), inter-individual variability in clearance of 166% (95% confidence interval 84.7–280) and an intra-individual variability in relative bioavailability of 40.2% (95% confidence interval 29.5–52.6).
3.3 Dose Evaluations
The results of the dose evaluation simulations are presented in Figs. 2 and 3. Figure 2A shows the impact of different dosing regimens of nadroparin (2850 IE, 5700 IE and 9500 IE of nadroparin once daily) on population anti-Xa levels. This figure shows that standard doses of 2850 IE and 5700 IE of nadroparin result in sub-prophylactic exposure in a typical individual of 70 kg in the ICU. Only a dose of 9500 IE of nadroparin results in anti-Xa levels within the prophylactic range. Figure 2B shows the impact of renal function and the use of CVVHD on anti-Xa levels after a nadroparin dose of 2850 IE. It can be observed that anti-Xa levels are higher in ICU patients with a low renal function and the use of CVVHD. Last, Fig. 2C shows the impact of fluid balance (4000 mL vs 0 mL) on anti-Xa levels after a nadroparin dose given at a dose of 2850 IE once daily. From this figure, it can be observed that the population-predicted changes in peak levels of anti-Xa are small. Figure 3 shows the predicted anti-Xa peak levels (4 h after administration) when dosing nadroparin to 1000 virtual critically ill patients with different dosing regimes of 2850 IE, 5700 IE and 9500 IE. As observed, predicted exposure is highly variable and does not result in anti-Xa peak levels within the prophylactic range at standard doses of 2850 IE and 5700 IE.
A Impact of different nadroparin doses (2850 IE, 5700 IE and 9500 IE once daily) on anti-Xa levels over time in a typical individual of 70 kg in the intensive care unit with normal renal function (estimated glomerular filtration rate [eGFR] of 90 mL/min) and a fluid balance of 0 mL. The horizontal lines depict the reference range for peak concentrations. B The impact of fluid balance (4000 mL vs 0 mL) on anti-Xa levels over time in a typical individual of 70 kg in the intensive care unit with a normal renal function (eGFR of 90 mL/min) after a daily dose of nadroparin of 2850 IE. Note that the lower prophylactic bound of nadroparin is 0.2 IE/mL. C Impact of renal function and continuous veno-venous hemodialysis (CVVHD) on anti-Xa levels over time in a typical individual of 70 kg in the intensive care unit after a once-daily dose of nadroparin of 2850 IE. Note that the lower prophylactic bound of nadroparin is 0.2 IE/mL
Predicted peak anti-Xa levels at 4 h with different dosing regimens of nadroparin (2850 IE, 5700 IE and 9500 IE) to 1000 virtual intensive care unit patients. The boxplots show the minimum, first quartile, median, third quartile and maximum predicted peak anti-Xa level. The dotted lines represent the prophylactic range of anti-Xa (lower and upper bound)
4 Discussion and Conclusions
To our knowledge, this is the first study that describes the PK of nadroparin in ICU patients including patients undergoing CVVHD. We found that the majority of anti-Xa levels measured were sub-prophylactic with the standard recommended dose of 2850 IE and 5700 IE. Consequently, higher doses of approximately 9500 IE per dose are needed in order to achieve anti-Xa target peak levels in the LMWH prophylactic range of 0.2–0.5 IU/mL [10]. Furthermore, large inter-individual variability in pharmacokinetic parameters was observed in bioavailability, volume of distribution and clearance, which could not fully be explained by the covariates in the final model.
We found that renal function and the use of CVVHD were significant covariates for the clearance of nadroparin, which also has been described in the literature for other LMWHs [9, 14, 21,22,23]. Furthermore, we found that fluid balance was a covariate for the volume of distribution of nadroparin. This effect can probably be explained by the hydrophilic properties of LMWHs and the large range of fluid balances observed in our study and this effect has not been described before. In our study, both apparent volume of distribution and apparent clearance were lower than previously found for nadroparin, indicating that the fraction of nadroparin reaching the systemic circulation (bioavailability) is decreased [24]. This decreased bioavailability may also explain the exceptionally low anti-Xa activities observed in our population. Reduced bioavailability of LMWHs has been described before in ICU patients probably owing to the use of vasopressors [7]. Cihlar et al. suggested to use intravenous LMWH in ICU patients in order to achieve higher and more predictable anti-Xa levels [8]. In addition, in ICU patients with coronavirus disease 2019, higher doses of LMWH were suggested in order to achieve prophylactic anti-Xa levels [25, 26].
Although there was a wide variability in anti-Xa levels between these patients, the increased dose of LMWH resulted mostly in anti-Xa levels within the prophylactic range [25, 26]. The proposed prophylactic range for anti-Xa peak levels of 0.2–0.5 IU/mL is based on retrospective data in non-ICU patients receiving a prophylactic dose of LMWH [10]. Furthermore, for this pharmacokinetic analysis, the surrogate endpoint anti-Xa level was used instead of the relationship between deep vein thrombosis (DVT) and anti-Xa. However, nadroparin at the usual prophylactic dose has been found to reduce the incidence of DVT in ICU patients [27], it remains unknown whether this dose is optimal. Research also suggests that in addition to peak anti-Xa levels, the cumulative exposure of anti-Xa during a dosing interval (area under the concentration–time curve) may be a better surrogate efficacy endpoint, especially in ICU patients, to predict the clinical effect [8].
Our cross-sectional pharmacokinetic study of nadroparin reflected a real-world ICU population, which can be considered as a strong point of our study, but may partly also explain the high variability in the observed PK. The high diversity in underlying disease and comorbidities of our critically ill population obscures the findings of our pharmacokinetic study by a high level of variability. Although one may argue that the current study in 30 patients was in a relatively limited population. Nonetheless, this was the largest study thus far studying the PK of nadroparin in critically ill patients. Moreover, the prospective character of our study and the sampling on two separate dosing occasions further strengthened our analysis. Extrapolation of our conclusions to other LMWHs should be done with caution as PK may differ [28]. More accurate measurements would have increased our understanding of renal involvement and body compensation of nadroparin PK, but would not have changed our conclusions on the low and highly variable exposure of nadroparin in ICU patients.
Furthermore, we did not collect data on peripheral oedema and did not test oedema as a covariate for the bioavailability of nadroparin. This may be considered a limitation of our study, yet as nadroparin was administered in the abdomen per hospital protocol to limit the effect of oedema on subcutaneous drug absorption, this may only play a minor role. Additionally, it can be discussed that the used algorithm to estimate renal function based on serum creatinine is optimal in an unstable and critically ill population. For estimation of renal function in this population, exogenous markers such as iohexol or chromium-51-labeled EDTA may be the best choices [29]. However, our objective was not to investigate the best renal function marker to predict nadroparin clearance, but to investigate potential covariates that are available in route clinical practice. Last, it is known that in rare cases (e.g. rare genetic variants or liver failure) antithrombin III may impact anti-Xa measurements [30]. This may partially explain some variability in our analysis, but we did not have these data available.
Notably, in our study we did not find an effect of vasopressor use on the bioavailability of nadroparin. Although our study was not designed to detect this effect, our findings contrast with early study results [31], but in line with recent results in patients with coronavirus disease 2019 [26]. Whether this is owing to the fact that the landscape of vasopressor use in the ICU has changed [32] (e.g. a shift towards the use of vasopressin instead of norepinephrine since 2002,) or the fact that perhaps the effect is not as profound as thought earlier, remains speculative.
Based on our findings, it can be debated whether nadroparin using a fixed dose of 285O IE or 5700 IE is optimal as exposure is low and highly variable. We postulate that higher doses, leading to peak levels in the prophylactic range, might be of benefit for critically ill patients and could prevent more DVTs. Furthermore, considering the high pharmacokinetic variability of nadroparin in ICU patients, it may be argued that nadroparin dose individualisation based on anti-Xa monitoring may further aid the prevention of DVT or bleedings as a result of extremely low or high nadroparin exposure at an increased prophylactic dose. A well-designed randomised study is warranted to investigate the superiority in efficacy of high-dose individualised nadroparin versus standard of care, with DVTs and bleedings as primary clinical endpoints. Furthermore, such a study may serve as validation of the prophylactic range of anti-Xa levels in the critically ill population.
References
Power BM, Forbes AM, van Heerden PV, Ilett KF. Pharmacokinetics of drugs used in critically ill adults. Clin Pharmacokinet. 1998;34(1):25–56.
Bergmann JF, Mouly S. Thromboprophylaxis in medical patients: focus on France. Semin Thromb Hemost. 2002;28(Suppl. 3):51–5.
Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337(10):688–98.
Wong GC, Giugliano RP, Antman EM. Use of low-molecular-weight heparins in the management of acute coronary artery syndromes and percutaneous coronary intervention. JAMA. 2003;289(3):331–42.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–143.
Priglinger U, Delle Karth G, Geppert A, Joukhadar C, Graf S, Berger R, et al. Prophylactic anticoagulation with enoxaparin: Is the subcutaneous route appropriate in the critically ill? Crit Care Med. 2003;31(5):1405–9.
Dorffler-Melly J, de Jonge E, Pont AC, Meijers J, Vroom MB, Buller HR, et al. Bioavailability of subcutaneous low-molecular-weight heparin to patients on vasopressors. Lancet. 2002;359(9309):849–50.
Cihlar R, Sramek V, Papiez A, Penka M, Suk P. Pharmacokinetic comparison of subcutaneous and intravenous nadroparin administration for thromboprophylaxis in critically ill patients on vasopressors. Pharmacology. 2020;105(1–2):73–8.
Feng Y, Green B, Duffull SB, Kane-Gill SL, Bobek MB, Bies RR. Development of a dosage strategy in patients receiving enoxaparin by continuous intravenous infusion using modelling and simulation. Br J Clin Pharmacol. 2006;62(2):165–76.
Wei MY, Ward SM. The anti-factor Xa range for low molecular weight heparin thromboprophylaxis. Hematol Rep. 2015;7(4):5844.
Haas CE, Nelsen JL, Raghavendran K, Mihalko W, Beres J, Ma Q, et al. Pharmacokinetics and pharmacodynamics of enoxaparin in multiple trauma patients. J Trauma. 2005;59(6):1336–43 (discussion 43–4).
Mayr AJ, Dunser M, Jochberger S, Fries D, Klingler A, Joannidis M, et al. Antifactor Xa activity in intensive care patients receiving thromboembolic prophylaxis with standard doses of enoxaparin. Thromb Res. 2002;105(3):201–4.
Guyatt GH, Akl EA, Crowther M, Schunemann HJ, Gutterman DD, Lewis SZ. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl.):48S-52S.
van der Voort PH, Postma SR, Kingma WP, Boerma EC, de Heide LJ, Bakker AJ. An observational study on the effects of nadroparin-based and citrate-based continuous venovenous hemofiltration on calcium metabolism. Blood Purif. 2007;25(3):267–73.
Gouya G, Palkovits S, Kapiotis S, Madl C, Locker G, Stella A, et al. Bioactivity of enoxaparin in critically ill patients with normal renal function. Br J Clin Pharmacol. 2012;74(5):806–14.
Roberts JA, Taccone FS, Lipman J. Understanding PK/PD. Intensive Care Med. 2016;42(11):1797–800.
Mentre F, Mallet A, Baccar D. Optimal design in random-effects regression. Biometrika. 1997;84(2):429–42.
van Hasselt JG, Green B, Morrish GA. Leveraging physiological data from literature into a pharmacokinetic model to support informative clinical study design in pregnant women. Pharm Res. 2012;29(6):1609–17.
Green B, Duffull SB. Prospective evaluation of a D-optimal designed population pharmacokinetic study. J Pharmacokinet Pharmacodyn. 2003;30(2):145–61.
Keizer RJ, Jansen RS, Rosing H, Thijssen B, Beijnen JH, Schellens JH, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect. 2015;3(2): e00131.
Mismetti P, Laporte-Simitsidis S, Navarro C, Sie P, d’Azemar P, Necciari J, et al. Aging and venous thromboembolism influence the pharmacodynamics of the anti-factor Xa and anti-thrombin activities of a low molecular weight heparin (nadroparin). Thromb Haemost. 1998;79(6):1162–5.
Green B, Greenwood M, Saltissi D, Westhuyzen J, Kluver L, Rowell J, et al. Dosing strategy for enoxaparin in patients with renal impairment presenting with acute coronary syndromes. Br J Clin Pharmacol. 2005;59(3):281–90.
Bazinet A, Almanric K, Brunet C, Turcotte I, Martineau J, Caron S, et al. Dosage of enoxaparin among obese and renal impairment patients. Thromb Res. 2005;116(1):41–50.
Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26(Suppl. 2):24–38.
Vlot EA, Van den Dool EJ, Hackeng CM, Sohne M, Noordzij PG, Van Dongen EPA. Anti Xa activity after high dose LMWH thrombosis prophylaxis in covid 19 patients at the intensive care unit. Thromb Res. 2020;196:1–3.
van der Heijden C, Ter Heine R, Kooistra EJ, Bruggemann RJ, Walburgh Schmidt JWJ, de Grouw E, et al. Effects of dalteparin on anti-Xa activities cannot be predicted in critically ill COVID-19 patients. Br J Clin Pharmacol. 2022;88(6):2982–7.
McLeod AG, Geerts W. Venous thromboembolism prophylaxis in critically ill patients. Crit Care Clin. 2011;27(4):765–80.
Samama MM, Gerotziafas GT. Comparative pharmacokinetics of LMWHs. Semin Thromb Hemostasis. 2000;26(Suppl. 1):31–8.
Soveri I, Berg UB, Björk J, Elinder C-G, Grubb A, Mejare I, et al. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64(3):411–24.
Croles FN, Lukens MV, Mulder R, De Maat MP, Mulder AB, Meijer K. Monitoring of heparins in antithrombin-deficient patients. Thromb Res. 2019;175:8–12.
Dörffler-Melly J, de Jonge E, de Pont A-C, Meijers J, Vroom MB, Büller HR, et al. Bioavailability of subcutaneous low-molecular-weight heparin to patients on vasopressors. Lancet. 2002;359(9309):849–50.
Russell JA, Gordon AC, Williams MD, Boyd JH, Walley KR, Kissoon N, editors. Vasopressor therapy in the intensive care unit. Semin Respir Crit Care Med. 2021;42(1):59–77
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Marieke J. H. A. Kruip received an unrestricted research grant from Sobi, payment to her institute and speakers fees from SOBI, Roche, and Bristol Myers Squibb. Jeroen Diepstraten, Anne van Rongen, Marianne P. Zijlstra, Pim L. J van der Heiden and Rob ter Heine have no potential conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study was carried out in The Netherlands in accordance with the ethical standards and the Helsinki Declaration of 1975.
Consent to participate
The Institutional Review Board (Commissie Mensgebonden Onderzoek, Arnhem-Nijmegen) waived the need for informed consent because of the observational nature and negligible burden associated with this study (registration number 2019-5226).
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
JD: performed the research, designed the study, contributed essential reagents or tools, analysed the data and wrote the paper. AvR: performed the research, designed the study, contributed essential reagents or tools, analysed the data and wrote the paper. MZ: contributed essential reagents or tools, analysed the data and wrote the paper. MK: analysed the data and wrote the paper. RtH: designed the study, analysed the data and wrote the paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diepstraten, J., van Rongen, A., Zijlstra, M.P. et al. Low and Highly Variable Exposure to Prophylactic LMWH Nadroparin in Critically Ill Patients: Back to the Drawing Board for Prophylactic Dosing?. Clin Pharmacokinet 62, 297–305 (2023). https://doi.org/10.1007/s40262-022-01202-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01202-6