Abstract
Introduction
The optimal nadroparin dose in patients undergoing hemodialysis is difficult to determine in clinical practice. Anti-Xa levels ≥ 0.4 IU/mL and < 2.0 IU/mL are suggested to prevent thrombus formation within the extracorporeal circuit whilst minimizing bleeding risk. We aimed to characterize the variability in the association between dose and anti-Xa levels, identify patient and dialysis characteristics that explained this variability, and optimize nadroparin dosing based on the identified characteristics.
Methods
Anti-Xa samples were collected in patients who received intravenous nadroparin as thromboprophylaxis during routine dialysis sessions. A population pharmacodynamic model was developed using non-linear mixed-effects modelling. The percentage of patients ≥ 0.4 IU/mL (efficacy) and < 2.0 IU/mL (safety) was simulated for different doses, patient and dialysis characteristics.
Results
Patients (n = 137) were predominantly receiving standard hemodialysis (84.7% vs. hemodiafiltration 15.3%) and had a mean bodyweight of 76.3 kg (± 16.9). Lean body mass (LBM), mode of dialysis, and dialyzer partially explained between-subject variability in anti-Xa levels. Patients on hemodiafiltration and those receiving hemodialysis with a high LBM (≥ 80 kg) had a low probability (< 29%) of anti-Xa levels ≥ 0.4 IU/mL during the entire dialysis session. All patients, except hemodialysis patients with a low LBM (< 50 kg), had a high probability (> 70%) of peak anti-Xa levels < 2.0 IU/mL.
Conclusion
Mainly patients receiving hemodiafiltration and those receiving hemodialysis with a high LBM can benefit from a higher nadroparin dose than currently used in clinical practice, while having anti-Xa levels < 2.0 IU/mL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our population pharmacodynamic model is able to characterize between-subject variability in the relationship between dose and anti-Xa levels after administration of nadroparin in the clinical hemodialysis setting. |
Lean body mass, mode of dialysis, and dialyzer partially explained between-subject variability in anti-Xa levels during hemodialysis. |
Mainly patients receiving hemodiafiltration and those receiving hemodialysis with a high lean body mass can benefit from a higher nadroparin dose than currently recommended in clinical guidelines to achieve anti-Xa levels ≥ 0.4 IU/mL. |
1 Introduction
Hemodialysis is used to remove toxic metabolites, electrolytes, and excessive fluid from the blood in order to prevent symptoms, such as fatigue, weakness, loss of appetite and nausea, in patients with end-stage kidney disease [1]. Hemodialysis, however, involves contact between a patient’s blood and the extracorporeal circuit, including the membrane of an artificial kidney (i.e., dialyzer), which causes, among others, thrombocyte activation, thrombin generation, and, consequently, an increased risk of thrombus formation [2, 3]. Thrombus formation within the extracorporeal circuit reduces dialyzer clearance and thereby limits the efficiency of dialysis [1, 2, 4, 5].
Nadroparin is a low molecular weight heparin (LMWH) that reduces the risk of thrombus formation by binding to antithrombin, which causes inhibition of factor Xa and, to a lesser extent, factor IIa [2, 6]. Nadroparin can thus be administered to prevent activation of the coagulation pathway in patients during hemodialysis [3, 5]. The nadroparin dose in the first dialysis session is based on a patient’s body weight, but dose adjustments are often required in subsequent sessions and are made on an empirical basis and for a variety of reasons, such as changes in the duration of hemodialysis, visible thrombus formation in the extracorporeal circuit, (a higher risk of) bleeding complications, or concomitant anticoagulant medication [5, 7]. This questions whether the current dosing advice (2850 IU and 3800 IU in patients weighing < 50 kg and ≥ 50 kg, respectively) for thromboprophylaxis with nadroparin is optimal for every patient receiving hemodialysis [5].
The degree of inhibition of factor Xa by nadroparin can be determined using an anti-factor (anti-) Xa assay [8]. Anti-Xa levels ≥ 0.4 IU/mL during the entire dialysis session are suggested to provide adequate anticoagulation [9, 10]. Variability in the anti-Xa levels in patients undergoing hemodialysis may explain the need for nadroparin dose adjustments. Therefore, the aim of this study was to characterize the between-subject variability in the association between nadroparin dose and anti-Xa levels in patients during hemodialysis by developing a population pharmacodynamic model. Furthermore, the influence of several patient and hemodialysis characteristics on the probability of achieving adequate anti-Xa levels during hemodialysis were explored, ultimately to improve and individualize dosing of nadroparin thromboprophylaxis in patients undergoing hemodialysis.
2 Methods
2.1 Patient Population and Study Design
This post hoc analysis was conducted with data obtained from two hospitals in the Netherlands: the University Medical Center Groningen (UMCG) in Groningen and the Haga hospital in The Hague. A waiver (METc 2021/429) was obtained from the medical ethics review committee.
2.1.1 University Medical Center Groningen (UMCG) Dataset
The UMCG dataset consisted of data collected previously in a prospective clinical trial, which was approved by the local medical ethics review committee. All patients provided written informed consent. The patient population, study design, and main results have been published previously [11].
2.1.2 Haga Hospital Dataset
The Haga dataset consisted of data collected during dialysis sessions in routine clinical practice. Data of patients, aged 18 years or older, who were on hemodialysis and received nadroparin as thromboprophylaxis were included. Patients who objected to participating in scientific research were excluded.
2.2 Dialysis Setting
In both datasets, patients receiving hemodialysis (with a session duration of 4 h) used low flux F8HPS or F6HPS dialyzers (Fresenius Medical Care, Bad Homburg, Germany), with an ultrafiltration coefficient (KUF) of 18 and 13 mL/h × mmHg and an effective surface area of 1.8 and 1.3 m2, respectively. Dialysate flow rate for patients receiving hemodialysis (HD) was 500 mL/min. For hemodiafiltration (HDF), the FX800 (Fresenius Medical care) dialyzer with a KUF of 63 mL/h × mmHg and an effective surface area of 1.8 m2 was used. HDF patients were treated with post-dilution hemodiafiltration with a dialysate flow of 500 mL/min. All HDF machines were equipped with AutoSub plus signal analysis software that automatically adapted the substitution fluid flow according to the blood flow, blood viscosity, and dialyzer characteristics. The target convection volume was ≥ 20 L/session.
2.3 Nadroparin Dosing
In both hospitals, nadroparin was administered by an intravenous bolus dose at the start of the dialysis session. Patients included in this study received nadroparin dosages as recommended by the Guideline of the Dutch Federation of Nephrology [5]. According to this Guideline, 2850 IU nadroparin was administered in patients weighing < 50 kg and 3800 IU in patients weighing ≥ 50 kg. Higher dosages are advised in patients with previously observed thrombus formation and lower dosages in patients with an anticipated increased risk of bleeding events [5]. The actual administered dose was recorded at the time of the dialysis session.
2.4 Blood Sampling and Analysis
In patients from the Haga hospital, anti-Xa levels were determined in samples collected at t = 5, t = 30, and t = 240 min after nadroparin administration. In patients from the UMCG, anti-Xa levels were determined in samples collected at t = 0 (immediately before nadroparin administration) and at 60, 180, and 240 min after nadroparin administration. Deviations from these sampling times were recorded.
Samples were collected in 3.2% buffered sodium citrate-containing tubes (0.109 M, BD Vacutainer, Becton Dickinson, UK) and analyzed in the Clinical Chemical Laboratories of the hospitals. Both laboratories are ISO15189 certified. Anti-Xa levels were measured using a two-stage anti-Xa chromogenic assay (Hyphen BioMed, Neuville-sur-Oise, France) and an automated anti-Xa chromogenic assay (Siemens, Marburg, Germany) in the Haga hospital and UMCG, respectively. All assays were subject to internal and external quality assessments.
2.5 Data Collection
All patient and dialysis characteristics were collected from Diamant® (Diasoft, Leusden, the Netherlands), an electronic patient record system specifically developed for dialysis patients. Data on drug use that could potentially influence the required nadroparin dose, such as platelet inhibitors, oral anticoagulants and calcium antagonists, were also extracted from Diamant® [9, 10, 12].
2.6 Development of the Population Pharmacodynamic Model
Pre- and post-processing of data were conducted using R (R version 4.0.3, R Foundation, Vienna, Austria). The population pharmacodynamic model was developed using non-linear mixed-effects modelling (NONMEM) software (version 7.5, ICON Development Solutions, Ellicott City, MD, USA). Model parameters were obtained using the first-order conditional estimation with interaction method.
Before the analysis, the anti-Xa levels were log-transformed to improve model stability. Anti-Xa levels below the limit of quantification (Haga hospital: < 0.01 IU/mL; UMCG < 0.08 IU/mL) were excluded. For the structural model, one- and two-compartmental models with first-order elimination were evaluated. Subsequently, for the stochastic model, between-subject variability was explored on all model parameters by including random effects that assumed a log-normal distribution. Covariance between these random effects was also evaluated. Residual or within-subject variability was modelled using a log additive error. Finally, the influence of covariates on model parameters was explored using a forward selection approach.
The following covariate effects on clearance and volume of distribution parameters were evaluated: age, sex, total body weight (TBW), body mass index (BMI), lean body mass (LBM), residual kidney function (RKF), use of co-medication (i.e., platelet inhibitors, oral anticoagulation, and calcium antagonists), type of dialyzer, mode of dialysis (HD or HDF), total ultrafiltration volume and dialysis center. RKF was defined as diuresis > 200 mL/24 h. LBM was calculated as follows [13]:
LBM and TBW were normalized to 65 kg and 70 kg, respectively, and evaluated using allometric theory with fixed scaling exponents, i.e., 0.75 for clearance parameters and 1.0 for volume of distribution parameters [14]. Categorical covariates were modelled proportionally and continuous covariates were modelled median-normalized using a power model.
Model selection and evaluation were conducted numerically by comparison of the objective function value (OFV) [15]. A significance level of p < 0.05, corresponding to a decrease of 3.84 in OFV, was considered a statistically significant improvement in overall model fit. Furthermore, the relative standard error of the model parameters, shrinkage, and the condition number were evaluated [15, 16]. Visually, model performance was evaluated, using standard goodness-of-fit plots, distribution of the random effects, and individual observed and predicted anti-Xa level versus time curves. Prediction-corrected visual predictive checks were constructed for the final model using 1000 simulations [17].
2.7 Simulations
Model simulations were performed using the RxODE package (version 1.0.9) using R. Per timepoint 10,000 subjects were simulated.
The therapeutic window used in this study was defined as anti-Xa levels of 0.4-2.0 IU/mL during the entire dialysis session. An anti-Xa level ≥ 0.4 IU/mL has been suggested to provide adequate anticoagulation and was therefore selected as the efficacy reference value [9, 10]. The percentage of patients above the efficacy reference value at the end of hemodialysis (i.e., 240 min after nadroparin bolus) was simulated for different doses and patient and dialysis characteristics that significantly influenced anti-Xa levels. Simulations for efficacy were also conducted using anti-Xa levels of 0.2, 0.3, 0.5, 0.7, and 1.0 IU/mL to provide a comprehensive overview.
In patients with subcutaneously administered therapeutically dosed LMWHs, peak anti-Xa levels ≥ 2.0 IU/mL and ≥ 1.0 IU/mL with a once- and twice-daily dosing regimen, respectively, have been associated with a significantly increased bleeding risk [18,19,20]. As nadroparin is administered once before hemodialysis, we considered a peak anti-Xa level (i.e., 5 min after nadroparin bolus) of 2.0 IU/mL as the safety reference value. Simulations for safety were also conducted using peak anti-Xa levels of 1.0, 1.2, 1.4, 1.6, 1.8, and 2.0 IU/mL.
3 Results
3.1 Demographics
A total of 137 dialysis patients were included in this analysis (Table 1). The mean (± standard deviation (SD)]) age was 67.5 years (± 14.2) and the majority of patients were male (65.0%). Most patients were on HD (84.7%) with the F8HPS dialyzer (78.1%). TBW ranged from 48.4 to 126.3 kg and LBM ranged from 45.0 to 89.0 kg.
There were no major differences in patient characteristics between the UMCG (n = 60) and Haga hospital (n = 77). With respect to anticoagulant use, 13.3% of patients from the Haga hospital used oral anticoagulants compared to 29.9% in the UMCG. Moreover, all of the included patients from the UMCG were treated with HD with a F8HPS dialyzer, while this applied to 72.7% of patients in the Haga hospital. Moreover, in both hospitals, the most frequently used nadroparin dose was 2,850 IU (overall dose range: 1430–8550 IU).
3.2 Observed Anti-Xa Levels
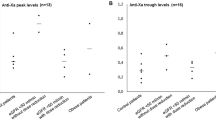
A total of 475 samples were collected from 137 patients, of which 69 samples were excluded due to an anti-Xa level lower than the limit of quantification (n = 60 samples immediately before the administration of nadroparin and n = 9 at later time-points). In total, this resulted in the inclusion of 137 patients with 406 anti-Xa levels ranging from 0.01 to 1.54 IU/mL (Fig. 1). The mean (± SD) observed anti-Xa level was 0.9 (± 0.3), 0.8 (± 0.3), 0.7 (± 0.3), 0.5 (± 0.2) and 0.4 (± 0.2) IU/mL at 5, 30, 60, 180, and 240 min after nadroparin bolus, respectively. The observed anti-Xa level at the end of hemodialysis was ≥ 0.4 IU/mL, the reference value for efficacy, in 38.4% of the patients. The observed peak anti-Xa level was < 2.0 IU/mL, the reference value for safety, in 100.0% of the patients.
3.3 Development of Population Pharmacodynamic Model
For the structural model, a two-compartment model demonstrated a reduction in OFV of 14.7 points (p < 0.05) and was thus deemed superior to a one-compartment model. For the stochastic model, between-subject variability could be identified on the model parameter representing clearance (CL) and volume of distribution (V1). Also, covariance between these parameters was identified.
Allometric scaling of LBM with fixed exponents significantly improved the overall model fit (− 38.1 points in OFV and − 17.3 points compared to TBW). Mode of dialysis on clearance and intercompartmental clearance, mode of dialysis on volume of distribution, and type of dialyzer on clearance also improved the model significantly (− 32.1, − 5.6, − 7.6, and − 10.5 points in OFV). The covariates LBM, mode of dialysis, and type of dialyzer reduced between-subject variability on CL from 105.9 to 87.4%, between-subject variability on V1 from 37.7 to 28.2%, but slightly increased the within-subject variability from 42.0 to 42.3%. The covariates age, sex, TBW, BMI, RKF, use of co-medication (i.e., platelet inhibitors, oral anticoagulation, and calcium antagonists), total ultrafiltration volume, and dialysis center did not significantly improve the model after inclusion of LBM, mode of dialysis, and type of dialyzer.
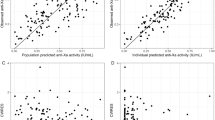
Model parameters were estimated with adequate precision (Table 2). Pharmacokinetic parameters of subgroups by type of dialysis and dialyzer are included in the Online Supplementary Material (OSM), Appendix 1. Goodness-of-fit plots demonstrated good accordance between observed and population predicted and individual predicted anti-Xa levels (OSM, Appendix 2). The conditional weighted residuals (CWRes) over time and the CWRes for population predicted and individual predicted anti-Xa levels did not indicate significant bias (OSM, Appendix 2). No deviation from normality was observed in the distribution of the random effects (OSM, Appendix 3). The results from the prediction-corrected visual predictive check (pcVPC) indicated adequate predictive performance of the population pharmacodynamic model (Fig. 2).
Prediction-corrected visual predictive check (pcVPC). Solid and dashed lines represent the prediction-corrected observed 5th, 50th, and 95th percentiles for all observations. The 95% confidence interval for the 5th, 50th, and 95th percentiles of the model predictions are shown in blue, red, and blue-coloured shaded areas, respectively
3.4 Simulations
The effects of nadroparin dose, LBM, mode of dialysis, and dialyzer were further explored by estimating the probability of reaching the efficacy or safety anti-Xa reference values using model simulations. Table 3 demonstrates the probability of reaching anti-Xa levels ≥ 0.4 IU/mL at the end of dialysis and < 2.0 IU/mL at peak level. A full overview of efficacy and safety simulations with anti-Xa levels 0.2–1.0 IU/mL and 1.0–2.0 IU/mL, respectively, is included in OSM, Appendix 4. Simulation of the anti-Xa levels versus time profile for a typical individual with an LBM of 65 kg, a nadroparin dose of 3800 IU receiving hemodialysis, and hemodiafiltration are shown in Fig. 3.
Simulation of the pharmacodynamic profile. Typical individual used for simulations had a lean body mass of 65 kg, received a dose of 3800 IU/mL and was on standard hemodialysis with a F8HPS dialyzer (left) vs. hemodiafiltration (right). The solid lines represent the median simulated anti-Xa level, the dashed lines represent the 5th and 95th percentiles of the simulated anti-Xa level
3.4.1 Effect of LBM
In patients with an LBM of 50 kg, using a nadroparin dose of 3800 IU and on HD with a F8HPS dialyzer, 66.4% of patients had an anti-Xa level ≥ 0.4 IU/mL during the entire hemodialysis session (efficacy) and 95.3% of patients had a peak anti-Xa level < 2.0 IU/mL (safety). In patients with an LBM of 40 kg and 90 kg with the same dialysis characteristics, these percentages were 77.2% and 15.4% for efficacy, and 82.5% and 100.0% for safety, respectively.
3.4.2 Effect of Mode of Dialysis
In patients with an LBM of 50 kg, using a nadroparin dose of 3800 IU, 0.0% of patients receiving hemodiafiltration with a FX800 dialyzer compared to 66.4% of patients receiving hemodialysis with a F8HPS dialyzer had an anti-Xa level ≥ 0.4 IU/mL. Additionally, 99.9% of patients undergoing hemodiafiltration with a FX800 dialyzer compared to 95.3% of patients receiving hemodialysis with a F8HPS dialyzer had a peak anti-Xa level < 2.0 IU/mL.
3.4.3 Effect of Dialyzer
In HD patients with an LBM of 50 kg, using a nadroparin dose of 3800 IU, 91.7% of patients using an F6HPS dialyzer compared to 66.4% of patients using an F8HPS dialyzer had an anti-Xa level ≥ 0.4 IU/mL. Additionally, 95.2% of patients using an F6HPS dialyzer compared to 95.3% of patients using an F8HPS dialyzer had a peak anti-Xa level < 2.0 IU/mL (Table 3).
4 Discussion
A two-compartment population pharmacodynamic model, with first-order elimination and between-subject variability on nadroparin clearance and volume of distribution, adequately characterized the variability in the association between dose and anti-Xa levels. LBM, mode of dialysis, and dialyzer were incorporated in the model as these characteristics partially explained between-subject variability in nadroparin clearance and volume of distribution. An anti-Xa therapeutic window of 0.4–2.0 IU/mL during the entire hemodialysis session has previously been suggested, which allowed us to estimate the probability of achieving adequate anti-Xa levels for different dosages, patient and dialysis characteristics [9, 10, 18,19,20]. Only 38.4% of patients had observed anti-Xa levels within this therapeutic window during the entire dialysis session. All patients outside the therapeutic window had at least one anti-Xa level < 0.4 IU/mL during dialysis. As demonstrated by the model simulations, mainly patients on HDF and HD patients with an LBM above 80 kg had a low probability of achieving adequate anti-Xa levels.
To our knowledge, only two population pharmacodynamic models of nadroparin, one in obese patients and one in pediatric patients, have been published previously, but none concerned patients receiving hemodialysis nor administration of nadroparin intravenously [21, 22]. Distribution of nadroparin in our population pharmacodynamic model was best described using a two-compartment model, which is similar to the model of Diepstraten et al. in obese patients [21]. In contrast, Laporte et al. used a one-compartment model to describe anti-Xa levels in children, but did not evaluate two-compartment distribution behavior [22]. Large between-subject variability on clearance and volume of distribution was identified in our model, but was also observed in the development of the population pharmacodynamic models in obese and pediatric patients. We could explain part of this variability by introducing several patient and dialysis characteristics into the model.
Introduction of LBM on model parameters for clearance and volume of distribution provided a significantly better description of the observed anti-Xa levels as compared to TBW. This is in contrast to the model of Laporte et al., who used TBW to scale both the apparent clearance and volume of distribution of nadroparin, and also in contrast to the model of Diepstraten et al., who used TBW to scale the apparent clearance of nadroparin [21, 22]. This finding can potentially be explained by the difference in study populations. In the pediatric study population of Laporte et al., introduction of TBW in the model is assumed to represent maturation processes, due to the strong correlation between TBW and maturation in the pharmacokinetics of nadroparin, which are not expected to be present in our adult study population [22]. In the obese study population of Diepstraten et al., introduction of TBW in the model seems to be data driven. This could be explained by differences in the absorption profile of subcutaneously administered nadroparin between obese and non-obese patients (e.g., reduced blood flow in adipose tissue) that can be expected to scale with TBW instead of LBM [21]. Nadroparin is expected to mainly distribute in the blood compartment, which is assumed to scale better with LBM than TBW [23]. We therefore considered implementation of LBM in our population pharmacodynamic model of intravenously administered nadroparin biologically plausible.
Nadroparin is mainly eliminated from the body by renal excretion and, to a lesser extent, by metabolism in the liver [6, 24]. This is confirmed by the reduction in nadroparin clearance in patients with a reduced kidney function [25, 26]. The RKF in the hemodialysis population is most likely too limited to play a significant role in the elimination of nadroparin. Loss of kidney function was expected to result in accumulation of nadroparin in patients receiving hemodialysis, but this and previous studies demonstrated that nadroparin in thromboprophylactic doses does not accumulate in hemodialysis, which could be explained by the remaining hepatic metabolism [3, 8]. This was also confirmed in our study as all patients, where a baseline sample was collected, had anti-Xa levels below the limit of quantification.
In addition to patient characteristics, the type of dialyzer and mode of dialysis also influenced anti-Xa levels. The F6HPS dialyzer decreased nadroparin clearance by 54% as compared to the F8HPS dialyzer, which can be explained by the lower KUF and the smaller effective surface area of the F6HPS compared to the F8HPS dialyzer [27]. HDF with an FX800 dialyzer increased nadroparin clearance and volume of distribution by 142% and 31%, respectively, compared to HD with F8HPS dialyzer. It is, however, unclear if the difference in clearance is due to the mode of dialysis or the dialyzer, since all HDF patients were treated with an FX800 dialyzer. The FX800 dialyzer is a high-flux dialyzer, while the F6HPS and F8HPS dialyzers are low-flux dialyzers. High-flux dialyzers have larger pores and therefore may enhance the clearance of larger molecules such as nadroparin (MW 4.5 kD) [28, 29]. Moreover, HDF as compared to HD removes larger molecules to a greater extent due to use of convection as compared to diffusion, which may also explain the difference in nadroparin clearance between these types of hemodialysis [6, 30, 31]. Some studies reported a significantly lower anti-Xa level in HDF versus high-flux hemodialysis (HF-HD), indicating a higher nadroparin clearance in HDF versus HF-HD [32, 33]. However, Sridharan et al. reported no difference in required LMWH dose to achieve adequate anti-Xa levels between HDF and HF-HD, suggesting no effect of dialysis mode on nadroparin clearance [34]. A possible explanation for the increase in volume of distribution is a greater adsorption of nadroparin to the HDF dialyzer, resulting in a greater loss of nadroparin and therefore an apparent larger volume of distribution.
The therapeutic window used in this study was based on two studies in HD patients who received an intravenous administration of the LMWH dalteparin before the start of HD. The studies showed that dalteparin prevented thrombus formation in patients with anti-Xa levels ≥ 0.4 IU/mL throughout the HD session [9, 10]. It should, however, be acknowledged that both studies had a small sample size, which warrants prospective validation of this reference value. Whether the therapeutic window is different in patients with a high thrombotic or bleeding risk is unknown. It is also unknown whether the anti-Xa level should be ≥ 0.4 IU/mL in patients on HDF as well, as this reference value was not achieved by any of the included patients on HDF in our study throughout the entire dialysis session. From a safety perspective, an anti-Xa level ≥ 2.0 IU/mL was associated with increased bleeding risk in patients with once-daily administered LMWHs [18,19,20]. This safety limit has never been validated for hemodialysis patients and the results should thus be interpreted with caution. Using our population pharmacodynamic model, other reference values can be simulated in case future studies identify more suitable reference values.
Current guidelines recommend dosing nadroparin based on the patient’s body weight [5, 6]. However, it has been stated before that higher nadroparin doses are needed in patients treated with HDF and that the type of dialyzer affects the required nadroparin dose [3, 5, 29, 32,33,34]. This has also been confirmed by our modelling analyses and model simulations, which demonstrated that the majority of patients on HDF had a zero probability of achieving the efficacy reference value throughout the entire HDF session. For patients with an LBM of 40 kg, on HD with an F8HPS dialyzer and a 2850 IU nadroparin dose, the probability of achieving the efficacy reference value throughout HD was estimated to be 56.5%, but this percentage was significantly lower in patients with a higher LBM. Thus, this dose should be considered as relatively low from an efficacy perspective. From a safety perspective, only patients with an LBM < 50 kg and a 5700 IU dose were estimated to have a high probability of anti-Xa levels ≥ 2.0 IU/mL. Based on our model, higher doses should be considered for nadroparin thromboprophylaxis in clinical practice.
Continuous intravenous infusion of nadroparin or a second bolus dose halfway through a dialysis session could result in a longer attainment of the anti-Xa reference value. However, in daily practice this might be less convenient than a single bolus. Another way to improve nadroparin dosing in hemodialysis patients is by implementation of our population pharmacodynamic model in clinical practice. A dosage with adequate anti-Xa levels throughout dialysis can then be predicted. Not all variability in anti-Xa levels could, however, be explained by the factors in our model. To further increase the predictive performance of our model and aid clinical practitioners in dosing decisions, Bayesian forecasting, which individualizes model parameters using observed anti-Xa levels of previous dialysis sessions, could be used. Future research, prospectively validating this approach, is, however, required.
This study has several strengths and limitations. We used a relatively large dataset of 406 anti-Xa levels in 137 patients. Moreover, no strict in- and exclusion criteria were applied in this study, resulting in a diverse patient population with a wide range of TBW and LBM, different dialyzers and concomitant medication, adequately resembling clinical practice. However, due to the study design, the data have been collected retrospectively, which may have introduced some bias. Sampling times were mainly recorded rounded to the nearest planned sampling time, which may have led to errors in the exact time between nadroparin dosing and time of sampling, and thus may have contributed to the observed large variability in anti-Xa levels. Moreover, data on thrombotic or bleeding complications were not monitored, which hampered evaluation of the association between anti-Xa levels and these outcomes. Another limitation is that our anti-Xa level samples date from 2009–2010. Minimal influence can be expected from a bioanalytical perspective, as samples were also analyzed during this period (Haga hospital) or were stored at – 80 °C and analyzed within the long-term stability ranges (UMCG), but changes may have been made in the composition of the dialyzers since then. Furthermore, low-flux dialyzers were used, while an increasing number of HD patients worldwide are currently treated with high-flux instead of low-flux dialyzers. As previously described, high-flux dialyzers can increase nadroparin clearance and thus influence the optimal nadroparin dose. Also, routine clinical care (e.g., differences in co-medication) might have changed, implying that an external validation of the population pharmacodynamic model would be required to further support our conclusions.
5 Conclusion
In this study, we developed a population pharmacodynamic model for nadroparin based on anti-Xa levels that were collected during hemodialysis in a patient population that closely resembles clinical practice. LBM, mode of dialysis (hemodialysis vs. hemodiafiltration), and type of dialyzer (F8HPS vs. F6HPS) affected anti-Xa levels and thus the required nadroparin dose. Mainly patients receiving hemodiafiltration and patients with an LBM ≥ 80 kg had a low probability of achieving anti-Xa levels ≥ 0.4 IU/mL (efficacy reference value). These patients can benefit from a higher nadroparin dose than currently used in clinical practice, while staying below the peak anti-Xa level of 2.0 IU/mL (safety reference value).
References
Thurlow JS, Joshi M, Yan G, Norris KC, Agodoa LY, Yuan CM, et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. 2021;52(2):98–107.
Molnar AO, Bota SE, McArthur E, Lam NN, Garg AX, Wald R, et al. Risk and complications of venous thromboembolism in dialysis patients. Nephrol Dial Transplant. 2017;33(5):874–80.
Coene KLM, Dekker MJE, Kerskes M, Hengst M, Schonck MJM, Konings C, et al. Practical value of anti-Xa activity in the evaluation of extracorporeal circuit anticoagulation during haemodialysis: results of a cross-sectional single-centre study. Nephron. 2017;137(3):205–11.
Sabatino A, Regolisti G, Karupaiah T, Sahathevan S, Sadu Singh BK, Khor BH, et al. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin Nutr. 2017;36(3):663–71.
Nederlandse federatie van Nefrologie (NfN). Richtlijn Antistolling met laagmoleculairgewicht heparines (LMWH) bij nierinsufficiëntie [in Dutch]. Nieuwegein, the Netherlands: NfN; 2012.
Fraxiparine 3.800 IE/0.4 ml oplossing voor injectie (SmPC) 2021. geneesmiddeleninformatiebank.nl.
Kessler M, Moureau F, Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474–89.
Nigten J, de Groot KA, Grootendorst DC, Koolen SL, Herruer MH, Schut NH. Pharmacokinetics of dalteparin during haemodialysis. Nephron Clin Pract. 2013;124(3–4):179–83.
Sagedal S, Hartmann A, Sundstrøm K, Bjørnsen S, Fauchald P, Brosstad F. A single dose of dalteparin effectively prevents clotting during haemodialysis. Nephrol Dial Transplant. 1999;14(8):1943–7.
Grau E, Sigüenza F, Maduell F, Linares M, Olaso MA, Martinez R, et al. Low molecular weight heparin (CY-216) versus unfractionated heparin in chronic hemodialysis. Nephron. 1992;62(1):13–7.
Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, de Jong PE, et al. Hemodialysis-induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin J Am Soc Nephrol. 2012;7(10):1615–23.
Zarbock A, Küllmar M, Kindgen-Milles D, Wempe C, Gerss J, Brandenburger T, et al. Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: a randomized clinical trial. JAMA. 2020;324(16):1629–39.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–65.
Holford NHG, Anderson BJ. Allometric size: the scientific theory and extension to normal fat mass. Eur J Pharm Sci. 2017;109s:S59-s64.
Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometr Syst Pharmacol. 2017;6(2):87–109.
Savic RM, Karlsson MO. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. Aaps J. 2009;11(3):558–69.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. Aaps J. 2011;13(2):143–51.
Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):188s–203s.
Levine MN, Planes A, Hirsh J, Goodyear M, Vochelle N, Gent M. The relationship between anti-factor Xa level and clinical outcome in patients receiving enoxaparine low molecular weight heparin to prevent deep vein thrombosis after hip replacement. Thromb Haemost. 1989;62(3):940–4.
Nieuwenhuis HK, Albada J, Banga JD, Sixma JJ. Identification of risk factors for bleeding during treatment of acute venous thromboembolism with heparin or low molecular weight heparin. Blood. 1991;78(9):2337–43.
Diepstraten J, Janssen EJ, Hackeng CM, van Dongen EP, Wiezer RJ, van Ramshorst B, et al. Population pharmacodynamic model for low molecular weight heparin nadroparin in morbidly obese and non-obese patients using anti-Xa levels as endpoint. Eur J Clin Pharmacol. 2015;71(1):25–34.
Laporte S, Mismetti P, Piquet P, Doubine S, Touchot A, Decousus H. Population pharmacokinetic of nadroparin calcium (Fraxiparine) in children hospitalised for open heart surgery. Eur J Pharm Sci. 1999;8(2):119–25.
Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16(6):773–6.
Johansen KB, Balchen T. Tinzaparin and other low-molecular-weight heparins: what is the evidence for differential dependence on renal clearance? Exp Hematol Oncol. 2013;2(1):21.
Russcher M, Josephus Jitta N, Kraaijenhagen RJ, Fijnheer R, Pasker-de Jong PC, Gaillard CA. Preemptive dosage reduction of nadroparin in patients with renal failure: a retrospective case series. Clin Kidney J. 2013;6(5):473–7.
Goudable C, Saivin S, Houin G, Sie P, Boneu B, Tonthat H, et al. Pharmacokinetics of a low molecular weight heparin (Fraxiparine) in various stages of chronic renal failure. Nephron. 1991;59(4):543–5.
Jager NG, Zandvliet AS, Touw DJ, Penne EL. Optimization of anti-infective dosing regimens during online haemodiafiltration. Clin Kidney J. 2017;10(2):282–90.
McMahon LP, Chester K, Walker RG. Effects of different dialysis membranes on serum concentrations of epoetin alfa, darbepoetin alfa, enoxaparin, and iron sucrose during dialysis. Am J Kidney Dis. 2004;44(3):509–16.
Sombolos KI, Fragia TK, Gionanlis LC, Veneti PE, Bamichas GI, Fragidis SK, et al. The anticoagulant activity of enoxaparin sodium during on-line hemodiafiltration and conventional hemodialysis. Hemodial Int. 2009;13(1):43–7.
Donauer J, Schweiger C, Rumberger B, Krumme B, Böhler J. Reduction of hypotensive side effects during online-haemodiafiltration and low temperature haemodialysis. Nephrol Dial Transplant. 2003;18(8):1616–22.
Shroff R, Smith C, Ranchin B, Bayazit AK, Stefanidis CJ, Askiti V, et al. Effects of hemodiafiltration versus conventional hemodialysis in children with ESKD: the HDF, heart and height study. J Am Soc Nephrol. 2019;30(4):678–91.
Belmouaz M, Goussard G, Joly F, Grand F, Sibille A, Ecotiere L, et al. Comparison of the injection of low-molecular weight heparin in the arterial vs. venous blood line for preventing extracorporeal circuit clotting during hemodialysis. Hemodial Int. 2022;26(3):314-322
Santos A, Macías N, Vega A, Abad S, Linares T, Aragoncillo I, et al. Efficacy of enoxaparin in preventing coagulation during high-flux haemodialysis, expanded haemodialysis and haemodiafiltration. Clin Kidney J. 2021;14(4):1120–5.
Sridharan S, Berdeprado J, Sivalingam M, Farrington K. Dalteparin dosing in high-flux haemodialysis and haemodiafiltration. Nephron Clin Pract. 2012;122(1–2):53–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
Tessa C.C. Jaspers, Charlotte E. Meijer, Louis Jean Vleming, Casper F.M. Franssen, Jerouen Diepstraten, Michael V. Lukens, Patricia M.L.A. van den Bemt, Barbara Maat, Nakisa Khorsand, Daniël J. Touw, and Jeroen V. Koomen declare that they have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Authors’ contributions
TCCJ contributed to study design, development of the population model, simulations with the population model, statistical analysis and writing the initial draft of the manuscript. CEM contributed to the development of the population model and reviewed the manuscript. LV contributed to data collection, interpretation of the results in relation to clinical practice and reviewed the manuscript. CFMF contributed to data collection, interpretation of the results in relation to clinical practice and provided reviews of the manuscript. JD contributed to the development of the population model and reviewed the manuscript. MVL contributed to data analysis and reviewed the manuscript. PMLAvdB contributed to conceptualization of the study, study design and reviewed the manuscript. BM contributed to study design and reviewed the manuscript. NK contributed to study design and reviewed the manuscript. DJT contributed to conceptualization of the study, study design, data collection, interpretation of the results in relation to clinical practice and reviewed the manuscript. JVK contributed to study design, development of the population model, simulations with the population model, statistical analysis and provided vital review of the manuscript.
Ethics approval
A waiver was obtained from the local Medical Ethical Review Committee as this study was not within the scope of the Dutch Human Research Act (METc 2021/429). The UMCG dataset consisted of data collected previously in a prospective clinical trial, which was approved by the local medical ethics review committee (METc 2008/343) and was in accordance with the Declaration of Helsinki.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jaspers, T.C.C., Meijer, C.E., Vleming, L.J. et al. Optimising the Nadroparin Dose for Thromboprophylaxis During Hemodialysis by Developing a Population Pharmacodynamic Model Using Anti-Xa Levels. Clin Pharmacokinet 61, 1559–1569 (2022). https://doi.org/10.1007/s40262-022-01162-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01162-x