Abstract
Background and Objective
Tucatinib, a highly selective tyrosine kinase inhibitor of the human epidermal growth factor receptor 2 (HER2) approved for HER2-positive metastatic breast cancer, is cleared by hepatic metabolism and subsequent biliary excretion. Liver disease can alter drug disposition and pharmacokinetics (PK). The objective of this study is to characterize PK and safety of tucatinib in volunteers with hepatic impairment.
Methods
This Phase 1 study compared the PK and safety of a single 300-mg oral dose of tucatinib in volunteers with mild, moderate, and severe hepatic impairment (Child-Pugh A/B/C) to healthy volunteers matched for sex, age, and body mass index. Pharmacokinetic parameters were determined for tucatinib and its predominant metabolite ONT-993.
Results
Compared with healthy volunteers, tucatinib exposure was similar in volunteers with mild impairment and increased in those with moderate or severe impairment without reaching statistical significance. Respective fold increases in geometric mean ratios for AUC0-t and AUC0-∞ were 1.13 and 1.15 in moderate impairment, and 1.43 and 1.61 in severe impairment compared with healthy volunteers. Three treatment-emergent adverse events (nausea, dermatitis, and increased transaminases) were reported in three volunteers and showed no obvious association with hepatic impairment status.
Conclusion
The 1.61-fold geometric mean ratio AUC0-∞ increase in volunteers with severe hepatic impairment supports the recommendation in the tucatinib prescribing information to reduce the dose from 300 mg twice daily to 200 mg twice daily in patients with severe impairment; no dose adjustment is recommended for patients with mild or moderate hepatic impairment.
This trial (NCT03722823) was registered on October 29, 2018.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The increased exposure to tucatinib was greater in volunteers with severe hepatic impairment than in those with moderate impairment (1.61- and 1.15-fold geometric mean ratio AUC0-∞ increases, respectively). The plasma exposures of tucatinib were similar between volunteers with normal hepatic function and mild hepatic impairment. |
These results support the prescribing information recommendation to reduce the tucatinib dose from 300 mg twice-daily to 200 mg twice-daily in patients with severe hepatic impairment. |
Tucatinib was well tolerated in volunteers with hepatic impairment, with treatment-emergent adverse events showing no obvious association with hepatic impairment status. |
1 Introduction
Human epidermal growth factor receptor 2 (HER2) gene amplification or overexpression is an oncogenic driver in various solid tumors including breast, gastric, lung, and colon cancers [1]. HER2 has become a well-established therapeutic target in breast and gastric cancers [1]. As of 2022, eight HER2-targeted agents have received US Food and Drug Administration (FDA) approval in HER2-positive breast cancer [2, 3] and two have been approved in gastric cancer [4, 5].
Tucatinib is a highly selective tyrosine kinase inhibitor (TKI) of the HER2 receptor [6]. Tucatinib, in combination with trastuzumab and capecitabine, is approved in several countries for adult patients with advanced, unresectable or metastatic HER2-positive breast cancer who have received prior anti-HER2 therapy [7, 8]. This approval is based on results from the pivotal HER2CLIMB trial, in which tucatinib (300 mg twice daily) significantly prolonged progression-free survival (including in patients with brain metastases) and overall survival in patients who had previously received trastuzumab, pertuzumab, and trastuzumab emtansine [9, 10]. Given the high selectivity of tucatinib for the HER2 kinase domain without significant inhibition of the epidermal growth factor receptor (EGFR) [11], patients receiving tucatinib may have decreased potential for EGFR-related toxicities, which have been observed with the dual HER2/EGFR TKI lapatinib and the pan-HER TKI neratinib [12,13,14,15].
In vitro metabolism studies in human liver microsomes suggest tucatinib is predominantly cleared by the drug metabolizing enzyme cytochrome P450 (CYP) 2C8, to a lesser extent by CYP3A4 and CYP3A5, and subsequent biliary excretion [16]. The predominant metabolite of tucatinib, ONT-993, is formed via hydroxylation by CYP2C8 [16]. The cytotoxic potency of ONT-993 is two- to three-fold less than that of tucatinib and the potency adjusted exposure of ONT-993 is < 10% of the total pharmacological activity [17]. Therefore, ONT-993 is not expected to meaningfully contribute to the efficacy or safety of tucatinib. Liver disease can result in reduced hepatic blood flow and drug-metabolizing enzyme activity, potentially causing alterations in drug distribution and decreased hepatic drug clearance [18]. In addition, liver failure can affect the binding of drugs to plasma proteins, altering the free fraction in plasma [18]. As tucatinib is eliminated via hepatic metabolism and biliary routes [16], this study was conducted to characterize the pharmacokinetics (PK) and safety of tucatinib in volunteers with hepatic impairment.
2 Methods
2.1 Study Conduct
Before the start of this study, the study protocol and informed consent form were approved by a central institutional review board (Advarra d/b/a Schulman Associates IRB, Cincinnati, OH). All amendments to the protocol were also approved by the institutional review board. The study was conducted in accordance with the requirements of Title 21 of the FDA Code of Federal Regulations, Good Clinical Practice, and Declaration of Helsinki and Internal Conference on Harmonisation Guidelines. All volunteers provided written informed consent for participation and publication prior to study enrollment.
This Phase 1, open-label, single-dose, parallel-group study evaluated the safety, tolerability, and PK of a single oral dose of tucatinib 300 mg in volunteers with hepatic impairment compared with matched-control healthy volunteers with normal hepatic function (NCT03722823). Twenty-four volunteers with hepatic impairment, and a minimum of six volunteers with normal hepatic function were planned for recruitment. The study was conducted at four sites in the USA (Orlando Clinical Research Center, Orlando, FL; New Orleans Center for Clinical Research, Knoxville, TN; Orange County Research Center, Tustin, CA; and American Research Corporation at the Texas Liver Institute, San Antonio, TX).
2.2 Study Volunteers
Male and female volunteers aged 18–75 years, with a body mass index (BMI) of 18.0 to 37.0 kg/m2 were eligible for inclusion. Volunteers were enrolled after they were confirmed to be in good general health, except for hepatic impairment, based on their medical history, physical examination, hematology, blood chemistry, negative human immunodeficiency viruses and hepatitis B and C serology, and urinalysis. Female volunteers were required to be of nonchildbearing potential and male volunteers were also required to be of nonchildbearing potential or agreed to use contraception if sexually active.
To be classified as having hepatic impairment, volunteers were required to have a Child Pugh (CP) score of 5–6 (mild; CP-A), 7–9 (moderate; CP-B), or 10–14 (severe; CP-C), with a known medical history of liver disease (with or without a known history of alcohol abuse). Hepatic impairment must have been clinically stable (no acute episodes of illness due to deterioration in hepatic function) for at least 1 month prior to screening. Volunteers were required to be on a stable medication regimen at the time of screening (defined as not starting any new drug(s) or significantly changing drug dosage(s) within 30 days prior to administration of study drugs). Healthy volunteers were matched to volunteers with hepatic impairment by sex, age (±10 years), and BMI (±20%); each matched-control healthy volunteer may have been matched with up to one volunteer within each hepatic impairment group.
Volunteers were excluded if they had received drugs or substances known to inhibit or induce CYP3A4 or CYP2C8 within 30 days prior to study initiation. Use of tobacco or other nicotine-containing products within 3 months prior to screening and throughout the study for healthy volunteers, and within 2 h pre-dosing and 4 h post-dosing for volunteers with hepatic impairment was prohibited. Volunteers were also excluded if they consumed foods or beverages containing poppy seeds, grapefruit, grapefruit juice, or Seville oranges within 7 days prior to study initiation and throughout the study. Consumption of alcohol-, citric acid-, caffeine-, or xanthine-containing foods or beverages within 48 h prior to study initiation and throughout the study was not permitted unless deemed acceptable by the Investigator.
2.3 Study Design
After the screening period (up to 28 days), eligible volunteers were assigned to groups according to hepatic function, matched-control healthy volunteers with normal hepatic function, and mild, moderate, or severe hepatic impairment.
Tucatinib was administered orally as a single 300-mg dose with 240 mL of water after ≥ 2 h fasting on the morning of Day 1. No food was allowed for up to 1 h after the tucatinib dose. Volunteers were admitted to the clinical research center on the day before initiating treatment, discharged on Day 3, and received a follow-up phone call on Day 7 (± 2 days).
2.4 PK Assessments
The key objectives of the study were to evaluate the PK profiles of tucatinib and ONT-993 in volunteers with impaired hepatic function compared with healthy controls. Blood samples were collected for PK analysis pre-dose, and 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 24, 36, and 48 h post-dose.
2.4.1 Plasma Concentrations of Tucatinib and ONT-993
Plasma concentrations of tucatinib and ONT-993 were determined using validated bioanalytical procedures, performed by Covance (Madison, WI) using high-performance liquid chromatography (LC) with tandem mass spectrometry (MS), as described previously [19].
2.4.2 Fraction Unbound of Tucatinib
The fraction unbound (fu) of tucatinib was determined using an equilibrium dialysis procedure with a sample of pre-dose plasma from each volunteer. A high-throughput dialysis apparatus (model HTD96b, HTDialysis LLC, Gales Ferry, CT) was assembled with hydrated dialysis membrane strips (molecular weight cut-off of 6–8 kDa) according to manufacturer’s recommendations. The pre-dose plasma samples were fortified with tucatinib (636 ng/mL), and the dialysis time was determined from the time-to-equilibrium experiment. Fortified plasma was added to the donor side and plasma ultrafiltrate was added to the receiver side of the equilibrium dialysis plate. Samples were incubated at 37 °C for 5 h, under 5% CO2 in saturated humidity. All protein binding determinations were in quadruplicate. At equilibrium, the donor side plasma samples were diluted with Dulbecco’s phosphate buffered saline (DPBS) and the receiver side samples were diluted with control plasma to provide a common analytical matrix (90:10 DPBS:plasma) before LC-MS/MS analysis. Calibration and quality control standards were prepared in a matrix identical to the study samples. The fu of tucatinib was calculated applying the following equation:
where Cp and Cd were concentrations of tucatinib in donor side plasma and receiver side dialysate at equilibrium, respectively.
The performance of the test system was verified using the positive control warfarin (3000 ng/mL) in control human plasma for the calculated percent bound for warfarin of at least 95%, which was included on each day of dialysis of clinical plasma samples.
2.4.3 PK Parameters
Pharmacokinetic parameters were determined from plasma concentrations of tucatinib and ONT-993 using noncompartmental methods, performed using Phoenix WinNonlin Version 8.1 (Certara L.P., Princeton, NJ). The PK parameters evaluated were area under the plasma concentration-time curve (AUC) from time 0 to the time of last quantifiable concentration (AUC0-t), AUC from time 0 to infinity (AUC0-∞), maximum observed plasma concentration (Cmax), time to Cmax (Tmax), apparent plasma terminal elimination half-life (t½), apparent total plasma clearance (CL/F; tucatinib only), metabolic ratio based on AUC0-∞ (MRAUC; ONT-993 only), and metabolic ratio based on Cmax (MRCmax; ONT-993 only). The fu was used to calculate AUC0-t,u, AUC0-∞,u, Cmax,u, CL/Fu and the unbound tucatinib for each individual volunteer.
2.5 Safety
Safety was assessed based on the frequency and severity of treatment-emergent adverse events (TEAEs), clinical laboratory parameters, vital signs, 12-lead electrocardiogram (ECG), and physical examination. TEAEs were assessed and graded applying the Common Terminology Criteria for Adverse Events version 5.0 and coded according to the Medical Dictionary for Regulatory Activities (MedDRA; version 21.1). TEAEs were summarized by preferred terms and system organ class.
2.6 Statistical Analysis
Descriptive statistics were applied to summarize demographic, safety, and PK parameters.
The primary analysis planned was to evaluate the PK of tucatinib in volunteers with hepatic impairment (test) compared with volunteers with normal hepatic function (reference). If an individual healthy volunteer was matched to one volunteer from any or all hepatic impairment groups, the primary PK parameters, Cmax, AUC0-t, and AUC0-∞, were log-transformed for tucatinib and ONT-993 and analyzed using a paired t-test to estimate the mean difference between the hepatic impairment groups and the normal hepatic function group for each parameter. The mean difference and its 90% confidence interval (CI) were back-transformed to give a geometric mean ratio, together with the corresponding 90% CI.
Determination of the sample size was based on historical studies of similar nature, with no formal sample size calculation. At least six volunteers each per hepatic function group were planned to complete the study; this was considered sufficient to evaluate the PK of tucatinib and ONT-993 in hepatic impairment.
The safety population consisted of all volunteers who received tucatinib and had at least one post-dose safety assessment. The PK population consisted of all volunteers who received tucatinib and had evaluable PK data; volunteers may have been excluded from the PK summary statistics and statistical analysis if they vomited at or within approximately two times median Tmax.
3 Results
3.1 Study Volunteers
Overall, 37 volunteers were enrolled in the study: 15 volunteers with normal hepatic function, eight with mild hepatic impairment, eight with moderate hepatic impairment and six with severe hepatic impairment (Table 1). All volunteers received a single dose of tucatinib per protocol, completed the study, and were evaluable for PK and safety analyses. Overall, most volunteers were white (83.8%), male (73.0%), mean age was 54 years, and mean BMI was 29.2 kg/m2 (Table 1).
3.2 PK of Tucatinib
3.2.1 Total Plasma Tucatinib PK
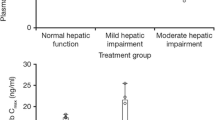
Plasma concentrations of tucatinib over time are shown in Fig. 1. Following administration of a single 300-mg oral dose, tucatinib was rapidly absorbed in all volunteers irrespective of hepatic impairment, with median Tmax ranging from 1.0 to 2.0 h (Table 2). The geometric mean and individual values for AUC0-∞ and Cmax for tucatinib in healthy volunteers and the hepatic impairment groups are shown in Table 2 and Fig. 2, respectively. The plasma exposures and other PK parameters (t1/2 and CL/F) of tucatinib were similar between volunteers with normal hepatic function and mild hepatic impairment (Table 2).
AUC0-∞ (a) and Cmax (b) for tucatinib in volunteers with hepatic impairment and healthy volunteers. Solid lines inside the box plot represent the median. The boxes represent the 25th and 75th percentile and the whiskers represent the minimum and maximum values. AUC0–∞ area under the concentration-time curve from time 0 to infinity, Cmax maximum observed concentration. aAUC0–∞ data are only shown for five subjects with severe hepatic impairment
In general, plasma exposures of tucatinib were increased in volunteers with moderate and severe hepatic impairment compared with healthy controls (Table 2 and Fig. 2). In volunteers with moderate impairment, geometric mean ratios increased 1.15- and 1.13-fold for AUC0-∞ and AUC0-t, respectively, compared with healthy controls, whereas the geometric mean ratio for Cmax was 0.89 (Fig. 3 and Supplementary Table 1). Geometric mean ratios for the severe impairment versus normal hepatic function groups were 1.61, 1.43, and 1.17 for AUC0-∞, AUC0-t, and Cmax, respectively. The trend of increased plasma exposure for total tucatinib did not reach statistical significance in volunteers with moderate or severe impairment compared with those with normal hepatic function, likely due to observed high variability in plasma concentrations in the volunteers with hepatic impairment (Fig. 1). Compared with healthy volunteers, geometric mean CL/F decreased by 12.39% and 42.29% in volunteers with moderate and severe hepatic impairment, respectively. While the arithmetic mean t1/2 was similar between volunteers with normal hepatic function and moderate impairment, t1/2 increased by 1.43-fold in those with severe impairment.
Statistical analysis comparing plasma tucatinib primary PK parameters for volunteers with hepatic impairment vs volunteers with normal hepatic function. Data are GMR and 90% CI for each comparison. AUC0-∞ AUC from time 0 to infinity, CI confidence interval, Cmax maximum observed plasma concentration, GMR geometric mean ratio, PK pharmacokinetics. aGMR for natural log transformed parameter, natural log transformed back to the linear scale. b90% CI for GMR of natural log transformed parameter, natural log transformed back to the linear scale. cN = 5 (one subject was not included in regression-based parameter calculations due to the terminal phase not being well defined for tucatinib [R2 adjusted < 0.8])
3.2.2 Unbound Tucatinib PK
The geometric mean fu of tucatinib was similar in all groups irrespective of hepatic function and ranged from 0.027 to 0.040 (Table 2). The PK parameters for unbound tucatinib were similar between healthy controls and volunteers with mild hepatic impairment (Supplementary Table 2). Similar to the results for total tucatinib, there was a trend for increased plasma exposure (based on geometric mean AUC0-∞) for unbound tucatinib in volunteers with moderate and severe hepatic impairment versus healthy controls. As for total tucatinib, the geometric mean ratios for AUC0-t, u, and AUC0-∞, u did not reach statistical significance for volunteers with moderate or severe impairment versus normal hepatic function based on 90% CI values (Supplementary Table 3 and Supplementary Fig. 1).
3.3 PK of ONT-993
The plasma concentrations of ONT-993 over time and the PK of ONT-993 are shown in Fig. 1 and Supplementary Table 4, respectively. The plasma concentrations of ONT-993 were lower in volunteers with hepatic impairment than in healthy controls. This is reflected in lower MRCmax as the level of hepatic impairment increased; the geometric mean MRCmax was 0.125 in healthy volunteers and decreased to 0.0806, 0.0698, and 0.0275 in volunteers with mild, moderate, and severe impairment, respectively (Table 2). There was a lesser effect of hepatic impairment level on the geometric mean MRAUC, which was 0.159 in healthy volunteers and decreased to 0.146 and 0.129 in volunteers with mild and moderate impairment, respectively. There were insufficient data to calculate MRAUC for volunteers with severe impairment.
The plasma exposure of ONT-993 based on Cmax incrementally decreased as the level of hepatic impairment increased, with geometric mean ratios reaching statistical significance in volunteers with moderate or severe impairment versus normal hepatic function based on 90% CI values (Supplementary Table 5 and Supplementary Fig. 3). In contrast, the ONT-993 AUC0-t and AUC0-∞ geometric mean ratios did not reach statistical significance in any group based on 90% CI values.
3.4 Safety
Overall, three of 37 (8.1%) volunteers experienced a total of three TEAEs following a single oral dose of tucatinib 300 mg, two of which were considered related to study drug; all were resolved on or before Day 10. Grade 1 nausea, considered study-drug related, was reported in one volunteer with normal hepatic function. The other two TEAEs were reported in volunteers in the mild hepatic impairment group and comprised Grade 1 dermatitis considered unrelated to tucatinib, and Grade 2 increased transaminases considered study-drug related. Except for the one case of increased transaminases, there were no clinically significant findings in clinical laboratory evaluations, vital signs, ECGs, or physical examinations. There were no serious TEAEs and no TEAEs required concomitant medication.
4 Discussion
Liver disease is relatively common in patients with cancer and may be caused by multiple factors including the use of anti-cancer therapies and the presence of hepatic metastases, which are common in several tumor types including breast, gastric, pancreatic, colorectal, and lung [20, 21]. Liver disease can lead to increased drug exposure due to reduced drug-metabolizing enzyme activity and subsequent hepatic clearance [18]. Drugs that cause hepatotoxicity may do so at lower doses in patients with hepatic impairment than those with normal hepatic function; also idiosyncratic drug reactions typically occur more frequently in patients with liver disease [22]. Therefore, as tucatinib is predominantly cleared by the liver [16], this study was conducted to investigate the impact of hepatic impairment on tucatinib PK and safety. A single-dose, parallel design was used as it is the standard design of studies investigating the PK of drugs in volunteers with hepatic impairment. Evaluating the PK and safety profile of tucatinib in healthy volunteers rather than patients ensured these parameters were unaffected by cancer [23]. As the PK of tucatinib are linear, this approach was considered appropriate to evaluate the impact of hepatic impairment on tucatinib PK.
In comparison with demographically matched volunteers with normal hepatic function, the plasma exposures of tucatinib and unbound tucatinib based on AUC0-t, and AUC0-∞ were similar in volunteers with mild hepatic impairment. Although the exposures were generally increased in volunteers with moderate or severe impairment compared to volunteers with normal hepatic function, the geometric mean ratios showed a less than two-fold increase in AUC0-t and AUC0-∞ for both groups. Moreover, none of these changes reached statistical significance in volunteers with hepatic impairment versus healthy controls due to the high variability in tucatinib plasma concentrations observed in the moderate and severe impairment groups.
The increases in tucatinib and unbound tucatinib exposures in the moderate and severe impairment groups appeared to be driven by lower CL/F compared with volunteers with normal hepatic function. This lower clearance resulted in a longer t1/2 in the severe impairment group.
These results, which are indicative of reduced hepatic metabolism of tucatinib in the moderate and severe impairment groups, are further supported by the PK data for the predominant metabolite, ONT-993. As the severity of hepatic impairment increased, the metabolite to parent ratio decreased. This decrease was most pronounced for MRCmax, with values ranging from 0.125 in healthy volunteers, to 0.0806 in volunteers with mild impairment, and 0.0275 in those with severe impairment.
In this study, three volunteers (one in the normal hepatic function group and two in the mild impairment group) experienced a single TEAE, each of mild or moderate severity, all of which resolved by the end of the study. The TEAEs of nausea and increased transaminases were considered related to tucatinib, whereas a case of Grade 1 dermatitis was considered unrelated. In a Phase 1 dose-escalation and dose-expansion study of 50 patients with HER2-positive advanced solid tumors who were treated with twice-daily oral tucatinib until disease progression or intolerance to study treatment, nausea was the most frequently reported TEAE related to tucatinib occurring in 34% of patients [11]. Tucatinib-related elevated alanine aminotransferase and aspartate aminotransferase TEAEs were each reported in 12% of patients and, in the main, occurred within 1 week of tucatinib initiation. These reports of elevated liver enzymes did not lead to tucatinib discontinuation and tucatinib was reinitiated with no safety concerns in almost all patients who experienced dose modifications due to elevated transaminases [11]. In addition, elevated liver enzymes detected by laboratory evaluation were predominantly Grade 1 and were reversible upon interruption or dose reduction of tucatinib [11].
Following a single dose of tucatinib there was no obvious pattern of association between the incidence of TEAEs and hepatic impairment status. However, this was a small single-dose study. Furthermore, there was high variability in the plasma exposure of tucatinib in individual volunteers from the moderate and severe hepatic impairment groups. The study was also conducted in volunteers of generally good health except for their hepatic impairment, rather than patients with cancer. In addition, when interpreting these results, the likeliness that the hepatic impairment of volunteers was due to different causes should be taken into consideration.
In conclusion, these results demonstrate that the increased exposure to tucatinib was greater in volunteers with severe impairment than in those with moderate impairment. While the tucatinib geometric mean AUC0-∞ and Cmax increases in volunteers with severe hepatic impairment were approximately 1.73- and 1.08-fold, respectively (Table 2), the maximum observed increases were up to 3.7- and 3.8-fold in AUC0-∞ and Cmax in one subject with severe hepatic impairment. In addition, PK data in volunteers with severe hepatic impairment were limited (only six subjects were evaluable for Cmax and five for AUC0-∞). Therefore, these results support the dosing recommendations outlined in the tucatinib prescribing information; the tucatinib 300-mg twice-daily dose should be reduced to a 200-mg twice-daily dose for patients with severe hepatic impairment, while no dose modification is needed for patients with mild or moderate impairment [7, 8].
References
Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz H, McCall S, Penault-Llorca F, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29(5):1108–19.
Choong GM, Cullen GD, O’Sullivan CC. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J Clin. 2020;70(5):355–74.
Lau KH, Tan AM, Shi Y. New and emerging targeted therapies for advanced breast cancer. Int J Mol Sci. 2022;23:4.
Daiichi Sankyo. Enhertu (trastuzumab deruxtecan) US prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761139s011lbl.pdf. Accessed November 2022.
Genentech Inc. Herceptin (trastuzumab) US prescribing information. 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf. Accessed November 2022.
Kulukian A, Lee P, Taylor J, Rosler R, de Vries P, Watson D, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–87.
Seagen Inc. Tukysa (tucatinib) US prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213411s000lbl.pdf. Accessed November 2022.
Seagen Inc. Tukysa (tucatinib) European summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/tukysa-epar-product-information_en.pdf. Accessed November 2022.
Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609.
Curigliano G, Mueller RP, Borges V, Hamilton E, Hurvitz S, Loi S, et al. Updated results of tucatinib vs placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB). J Clin Oncol. 2021;39(Suppl 15):1043.
Moulder SL, Borges VF, Baetz T, McSpadden T, Fernetich G, Murthy RK, et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2(+)-advanced solid tumors, with an expansion cohort in HER2(+) metastatic breast cancer (MBC). Clin Cancer Res. 2017;23(14):3529–36.
Sonnenblick A, de Azambuja E, Agbor-Tarh D, Bradbury I, Campbell C, Huang Y, et al. Lapatinib-related rash and breast cancer outcome in the ALTTO phase III randomized trial. J Natl Cancer Inst. 2016;108:8.
Capri G, Chang J, Chen SC, Conte P, Cwiertka K, Jerusalem G, et al. An open-label expanded access study of lapatinib and capecitabine in patients with HER2-overexpressing locally advanced or metastatic breast cancer. Ann Oncol. 2010;21(3):474–80.
Saura C, Oliveira M, Feng Y-H, Dai M-S, Hurvitz SA, Kim S-B, et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. J Clin Oncol. 2019;37(15):1002.
Mortimer J, Di Palma J, Schmid K, Ye Y, Jahanzeb M. Patterns of occurrence and implications of neratinib-associated diarrhea in patients with HER2-positive breast cancer: analyses from the randomized phase III ExteNET trial. Breast Cancer Res. 2019;21(1):32.
Sun H, Cardinal K, Wienkers L, Chin A, Kumar V, Neace C, et al. Elimination of tucatinib, a small molecule kinase inhibitor of HER2, is primarily governed by CYP2C8 enantioselective oxidation of gem dimethyl. Cancer Chemother Pharmacol. 2022;2:2.
Food and Drug Administration. Multi-disciplinary review and evaluation - NDA 213411 (tucatinib). 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213411Orig1s000MultidisciplineR.pdf. Accessed November 2022.
Rodighiero V. Effects of liver disease on pharmacokinetics An update. Clin Pharmacokinet. 1999;37(5):399–431.
Topletz-Erickson A, Lee A, Rustia EL, Sun H, Mayor JG, Abdulrasool LI, et al. Evaluation of safety and clinically relevant drug-drug interactions with tucatinib in healthy volunteers. Clin Pharmacokinet. 2022;10:1417–26.
Diamond JR, Finlayson CA, Borges VF. Hepatic complications of breast cancer. Lancet Oncol. 2009;10(6):615–21.
Wang S, Feng Y, Swinnen J, Oyen R, Li Y, Ni Y. Incidence and prognosis of liver metastasis at diagnosis: a pan-cancer population-based study. Am J Cancer Res. 2020;10(5):1477–517.
National Institure for Health and Care Excellence. Prescribing in hepatic impairment. 2022. https://bnf.nice.org.uk/guidance/prescribing-in-hepatic-impairment.html. Accessed November 2022.
Karakunnel JJ, Bui N, Palaniappan L, Schmidt KT, Mahaffey KW, Morrison B, et al. Reviewing the role of healthy volunteer studies in drug development. J Transl Med. 2018;16(1):336.
Acknowledgments
The authors would like to thank the study participants involved in the study. The authors would also like to thank the investigators Eric Lawitz, Thomas Marbury, Joel Neutel, and William Smith. Medical writing support was provided by Charlotte Simpson, PhD, and editorial support, including formatting, proofreading, and submission, was provided by George Chappell, MSc, both of Scion, London, UK, supported by Seagen according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288). The sponsor was involved in the study design, and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors. Some of these results were presented at the 2021 American Association for Cancer Research (AACR) annual meeting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this publication was provided by Seagen Inc. (Bothell, Washington, USA).
Conflict of Interest
This study was funded by Seagen Inc. The authors wrote the article with the assistance of a medical writer funded by the sponsor. All the authors had full access to the relevant data, vouch for the completeness and accuracy of the data and for adherence of the trial to the protocol, assume final responsibility for the content of the article, and for the decision to submit the article for publication. A.R. Topletz-Erickson, A.J. Lee, J.G. Mayor, H. Sun, L.I. Abdulrasool, and C.J. Endres are employees of Seagen Inc., and hold stocks and shares in Seagen Inc. E.L. Rustia is an employee of Gilead Sciences. L.N. Walker is an employee of Harpoon Therapeutics.
Ethics Approval
Advarra d/b/a Schulman Associates IRB, a central institutional review board, reviewed and approved all study materials prior to initiating recruitment and data collection.
Consent to Participate
Informed consent was obtained from all participants prior to commencing enrollment.
Consent for Publication
All authors provided this consent.
Availability of Data and Material
Qualified researchers may request access to certain data and related study documents consistent with the Principles for Responsible Clinical Trial Data Sharing. Interested researchers can use http://www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care.
Code Availability
Not applicable.
Authors’ Contributions
All authors contributed to the study design, and analysis and interpretation of data. All authors contributed to critical revisions of the article and provided their final approval for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Topletz-Erickson, A.R., Lee, A.J., Mayor, J.G. et al. The Pharmacokinetics and Safety of Tucatinib in Volunteers with Hepatic Impairment. Clin Pharmacokinet 61, 1761–1770 (2022). https://doi.org/10.1007/s40262-022-01183-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01183-6