Abstract
Background
Multiple clinical, demographic, and genetic factors affect the pharmacokinetics of tacrolimus in children, yet in daily practice, a uniform body-weight based starting dose is used. It can take weeks to reach the target tacrolimus pre-dose concentration.
Objectives
The objectives of this study were to determine the pharmacokinetics of tacrolimus immediately after kidney transplantation and to find relevant parameters for dose individualization using a population pharmacokinetic analysis.
Methods
A total of 722 blood samples were collected from 46 children treated with tacrolimus over the first 6 weeks after renal transplantation. Non-linear mixed-effects modeling (NONMEM®) was used to develop a population pharmacokinetic model and perform a covariate analysis. Simulations were performed to determine the optimal starting dose and to develop dosing guidelines.
Results
The data were accurately described by a two-compartment model with allometric scaling for bodyweight. Mean tacrolimus apparent clearance was 50.5 L/h, with an inter-patient variability of 25%. Higher bodyweight, lower estimated glomerular filtration rate, and higher hematocrit levels resulted in lower total tacrolimus clearance. Cytochrome P450 3A5 expressers and recipients who received a kidney from a deceased donor had a significantly higher tacrolimus clearance. The model was successfully externally validated. In total, these covariates explained 41% of the variability in clearance. From the significant covariates, the cytochrome P450 3A5 genotype, bodyweight, and donor type were useful to adjust the starting dose to reach the target pre-dose concentration. Dosing guidelines range from 0.27 to 1.33 mg/kg/day.
Conclusion
During the first 6 weeks after transplantation, the tacrolimus weight-normalized starting dose should be higher in pediatric kidney transplant recipients with a lower bodyweight, those who express the cytochrome P450 3A5 genotype, and those who receive a kidney from a deceased donor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the first 6 weeks post-transplantation bodyweight, the cytochrome P450 3A5 genotype, donor type (deceased vs. living), estimated glomerular filtration rate, and hematocrit significantly influence the clearance of tacrolimus in children receiving a kidney transplant |
The tacrolimus weight-normalized starting dose in pediatric kidney transplant recipients should be higher in children with a lower bodyweight, those who express the cytochrome P450 3A5 genotype, and in patients who receive a kidney from a deceased donor |
1 Introduction
The use of tacrolimus-based immunosuppressive therapy following pediatric renal transplantation has drastically improved patient and graft survival. There still is, however, a long way to go regarding the reduction in tacrolimus treatment-related co-morbidities [1, 2]. Adverse events associated with the use of tacrolimus include nephrotoxicity, neurotoxicity, alopecia, gastrointestinal disturbances, hypertension, and post-transplantation diabetes mellitus [3,4,5]. These side effects contribute to patient non-adherence, limited long-term kidney allograft survival, and high cardiovascular morbidity and mortality of transplant recipients [6, 7]. High tacrolimus concentrations are associated with toxicity and lower concentrations seem to be related to an increased risk of acute rejection episodes [8, 9]. Glucocorticoid-sparing protocols are becoming more common, making it even more crucial to reach the tacrolimus target concentration as soon as possible to reduce the risk of acute rejection [8].
Tacrolimus is a critical dose drug with a narrow therapeutic index for which therapeutic drug monitoring (TDM) is routinely performed. Multiple factors, including bodyweight [10, 11], age [12, 13], drug interactions [14], hematocrit [10, 15], ethnicity [16], treatment with glucocorticoids [17], and cytochrome P450 (CYP) 3A genotype [10] affect the pharmacokinetics of tacrolimus. This has been extensively investigated in adults; however, data in children are limited. Children aged younger than 5 years appear to need higher weight-normalized doses of tacrolimus than older children to reach the target range [12]. The reason for this age-related increased clearance (CL) is unknown. It has been demonstrated that CYP3A5 expressers (those with the CYP3A5*1/1 or CYP3A5*1/*3 genotype) require at least a 1.5-fold higher tacrolimus dose compared with CYP3A5 non-expressers (CYP3A5*3/*3 genotype) [10, 12, 18,19,20,21]. CYP3A4*22 is associated with lower tacrolimus dose requirements post-renal transplantation [22,23,24]. The CYP3A4*1G allele is associated with a higher tacrolimus dose requirement; however, its contribution to tacrolimus exposure is less than half of that of the CYP3A5 genotype [25]. This effect is thought to be independent of CYP3A5 status [16]. In routine clinical practice, these factors are not taken into consideration. The starting dose of tacrolimus is usually based on bodyweight and then adjusted by means of TDM. Therapeutic drug monitoring limits the time a patient is exposed to sub- and supra-therapeutic tacrolimus concentrations, but it can take up to 2 weeks to reach the target exposure range [26].
The use of a population pharmacokinetic model may help in predicting an individual’s response to tacrolimus and can be applied before the start of therapy. To date, four models have been developed for pediatric renal transplant recipients [20, 27,28,29,30]. Of these models, only one was developed using transplant recipients in the immediate post-transplant phase and could therefore be used to determine the starting tacrolimus dose [10]. However, this model was developed with children treated in nine hospitals with different immunosuppressive regimens, had sparse tacrolimus sampling, important covariates such as ethnicity were not included, and the model was not externally validated.
The aim of the current study was to describe the population pharmacokinetics of twice-daily immediate-release tacrolimus in the first weeks after pediatric renal transplantation and to develop a dosing guideline for the starting dose. In contrast to previous studies, all children were treated with the same immunosuppressive regimen, had an abbreviated time profile measured with additional extensive tacrolimus sampling, important covariates such as ethnicity and CYP3A5 genotype were included, and the model was externally validated.
2 Materials and Methods
2.1 Study Design
A retrospective analysis of pediatric transplant recipients who received a donor kidney between November 2009 and April 2016 was performed. Clinical and demographic data were retrieved from the medical records for the first 6 weeks after pediatric renal transplantation. Data were collected in the pediatric nephrology department at the Erasmus Medical Center, Sophia Children’s Hospital, Rotterdam, the Netherlands. Eligible for enrolment were patients aged younger than 18 years, who received a kidney from an ABO compatible living or a deceased donor, and were treated with tacrolimus as part of their initial immune suppressive regimen. All clinical values were collected from 24 h before transplantation until 6 weeks post-transplantation.
External validation of the pharmacokinetic model was performed on an independent dataset consisting of 23 children who underwent transplantation between March 2012 and July 2015 in the Radboud University Medical Center, Amalia Children’s Hospital, Nijmegen, The Netherlands. These children were not included in the initial model building dataset, and were selected using the same inclusion criteria.
The study was designed in accordance with the Declaration of Helsinki of 1975. For the model building dataset, all laboratory analyses were performed for routine clinical practice. The Ethics Review Board of the Erasmus Medical Center decided that the rules laid down in the Medical Research Involving Human Subjects Act do not apply to this study (Medical Ethical Review Board No. 2017-092). The extra genotyping was approved by the Ethics Review Board of the Erasmus Medical Center (No. 2010-219). For the validation dataset, the Ethics Review Board of the Radboud University Medical Center approved the genotyping for CYP3A5 in leftover material (No. 2014-1282). The parents or legal caregivers of all participants signed an informed consent prior to DNA collection.
2.2 Immunosuppression
All patients were treated according to the TWIST protocol with basiliximab, tacrolimus, mycophenolic acid, and a 5-day course of glucocorticoids [31]. Both the twice-daily formulations Prograft® (Astellas Pharma, Leiden, The Netherlands) and Modigraf® (Astellas Pharma, Leiden, The Netherlands) granules for suspension were used. An extemporaneously prepared suspension from the Prograft® capsules in an oral suspending vehicle from our Good Manufacturing Practice-certified pharmacy was used by some children. All children received an initial tacrolimus dose of 0.3 mg/kg/day divided into two doses every 12 h. The subsequent doses were adjusted using TDM.
In our hospital, it is common to measure the tacrolimus pre-dose concentration (C 0) for the first time after four to five dosages of tacrolimus, i.e., approximately 3 days after transplantation. As this is a study with data obtained in routine clinical practice, not all patients had their first tacrolimus C0 measurement on day 3. This depended on multiple factors, including clinical factors (e.g., signs of tacrolimus toxicity or rejection), as well as logistic factors (tacrolimus concentrations are not routinely measured during the weekend in our hospital). In the first 3 weeks post-transplantation, the target C 0 was 10–15 ng/mL, from then onwards the target C 0 was 7–12 ng/mL. Approximately 2 weeks after transplantation, an abbreviated 4-h tacrolimus concentration vs. time profile was obtained. For these profiles, blood samples were taken before tacrolimus administration, and 10, 30, 90, 120, and 240 min post-ingestion. The dose of immunosuppression and other co-medication was recorded in the electronic prescribing system during the entire study period. Tacrolimus sample collection times and time of latest ingestion were also recorded.
The following clinical data were collected retrospectively from medical records: weight, height, time post-transplant, sex, age, ethnicity, hematocrit, creatinine, aspartate aminotransferase, albumin, C-reactive protein, total protein, CYP3A4 genotype, CYP3A5 genotype, co-medication, glucocorticoid dose, primary diagnosis, previous transplantations, renal replacement therapy prior to transplantation (pre-emptive, hemodialysis or peritoneal dialysis), donor (living or post-mortal), human leukocyte antigen mismatches. The estimated glomerular filtration rate (eGFR) was calculated using the adapted Schwartz formula (K * height (cm)/serum creatinine (µmol/L) with a K value of 36.5 and the body surface area using the formula according to Mosteller [32, 33].
2.3 Laboratory Analysis
Genotyping for CYP3A5 and CYP3A4 was performed as described previously [34]. For CYP3A4, the *1G and *22 polymorphisms were tested, and for CYP3A5 we tested for the *3 and *7 alleles. Deviations from the Hardy–Weinberg equilibrium were tested using the Chi squared goodness-of-fit test. Most tacrolimus concentrations were analyzed in whole-blood samples using a validated liquid chromatography-tandem mass spectrometry (LC–MS/MS) method, with a lower limit of quantification of 1.0 ng/mL. The remaining tacrolimus concentrations of the model building dataset (9%) were measured before the introduction of the LC–MS/MS method using the immunoassay with a lower limit of quantification of 1.5 ng/mL. The accuracy of the quality-control samples was between 85 and 115% and the intra- and inter-assay imprecision was less than 15% during the study period. As it is known that there is a difference between tacrolimus concentrations measured using an LC–MS/MS and an immunoassay, this was built into the residual error model.
2.4 Population Pharmacokinetic Modeling
Pharmacokinetic analysis was conducted by non-linear mixed-effects modeling using NONMEM® Version 7.2 (FOCE+I; ICON Development Solutions, Ellicott City, MD, USA) and PsN® Version 4.6.0. Pirana® software was used as an interface between NONMEM®, R (version 3.2.2) and Xpose (version 4).
2.4.1 Base Model Development
Based on visual inspection of the data and a review of the literature, one- and two-compartment models were considered to describe the concentration–time data. Typical values for lag-time, absorption rate constant (ka), volume of distribution, CL, and inter-compartmental clearance (Q) were estimated. Bioavailability could not be quantified; therefore, certain parameters were estimated as ratios: apparent oral clearance (CL/F), Q/F, and apparent volume of distribution. Inter-individual variability (IIV) and inter-occasion variability (IOV) were modeled for each pharmacokinetic parameter using an exponential model, and residual variability was incorporated as an additive and proportional error for each analytical method. Allometric scaling was used to account for variability in pharmacokinetic parameters owing to differences in bodyweight. Shrinkage was calculated for all model parameters for which IIV was estimated. A shrinkage value below 20% was considered acceptable [35]. Model selection was based on minimum objective function values (OFVs), parameter precision, error estimates, shrinkage values, and visual inspection of the goodness-of-fit plots.
2.4.2 Covariate Model Development
Covariates were selected on the basis of their known or theoretical relationships with tacrolimus pharmacokinetics. Demographic, clinical, and genetic characteristics were evaluated as potential model covariates, all selected covariates are shown in Table 1. Co-medications known to interact with the tacrolimus concentration were included. The differences between the capsules and suspension (Modigraf® and the extemporaneously prepared suspension) were also tested as a covariate.
The relationship between covariates and IIV was first investigated graphically and covariates with a visually apparent relationship were singly added to the model. A univariate analysis was performed to determine if they improved the model. The forward inclusion-backward elimination method was used [36]. Covariates that significantly improved the model (p < 0.05) were added to the full model. A backward elimination process was then performed with a stricter statistical significance of p < 0.001 (OFV > 10.83). To be accepted in the model, the covariate also needed to reduce the IIV of the pharmacokinetic parameter involved. A shark plot was generated for each covariate for case-deletion diagnostics.
2.4.3 Model Evaluation
Multiple procedures were used to validate the final model. First of all, a bootstrap resampling method was applied [37]. Five hundred bootstrap datasets were generated by sampling randomly from the original dataset with replacement. The validity of the model was evaluated by comparing the median values and their corresponding 95% confidence intervals of the bootstrap replicates with the estimates of the original dataset. The model was further validated using the visual predictive check (VPC) by simulating 500 datasets [38], and a normalized prediction distribution errors (NPDE) analysis [39]. To investigate how the OFV differences between the covariate and base model is distributed across individuals, a shark plot was generated. Finally, an independent dataset containing 23 children treated with the same immunosuppressive regimen was used for external validation using a VPC. The VPCs were prediction corrected and stratified for the covariates included in the final model.
To evaluate the effect of the significant covariates, simulations were performed using the final population pharmacokinetic model with varying parameters for the covariate. For each covariate, concentration–time profiles were simulated for 1000 patients. All other parameters were fixed to the median. To develop the dosing guidelines for the starting dose of tacrolimus, simulations were also performed using a simulation model, which only included the covariates that significantly influence the starting dose. For each combination of these covariates, concentration–time profiles were simulated for 1000 patients, and a new starting dose was calculated to reach a target level of 12.5 ng/mL.
Statistical analyses other than those mentioned above, were performed using SPSS® Version 23 (SPSS Inc., Chicago, IL, USA). Data on patients’ baseline characteristics were presented as median and range for continuous variables.
3 Results
A total of 46 children were included in the model building group. Patient characteristics are presented in Table 1. From these patients, 722 blood samples were collected and analyzed for tacrolimus concentrations (range 2–109 ng/mL). Each patient had at least one pharmacokinetic profile over 4 h approximately 2 weeks (range 8–42 days) post-transplantation. The median number of tacrolimus concentrations per patient was 16 (range 9–23). None of the samples were below the lower limit of quantification. There was no deviation from the Hardy–Weinberg equilibrium.
3.1 Base Model
The data were best described by a two-compartment model. The residual error was described with a combined additive and proportional error model. Allometric scaling with fixed exponents [0.75 (CL/F and Q/F) and 1 (V 1/F and V 2/F)] significantly improved the model (p < 0.001). Estimation of the exponents did not improve the model and resulted in values not significantly different than the fixed values. Including IIV on CL/F, V 1/F, V 2/F, and k a significantly improved the model fit. The OFV decreased further after introduction of IOV on CL/F and V 2/F. Parameter estimates of the base model, final model, and simulation model are presented in Table 2.
3.2 Covariate Analysis
The base two-compartment model with allometric scaling was used as a reference for the covariate analysis. After graphical analysis, the univariate analysis resulted in ten significant covariates, as shown in Table 3. After backward elimination, only CYP3A5 genotype, donor, hematocrit, and eGFR were found to correlate significantly with CL/F, and remained in the final model. The following equation described the final model for estimation of tacrolimus CL/F (L/h) in the first 6 weeks post-transplant:
3.3 Evaluation of the Final Model
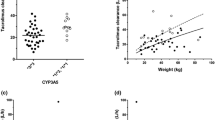
All estimates were within the limits, given the criteria as defined under Sect. 2, except for shrinkage on V 2, which was 27%. Goodness-of-fit plots of the final model showed the population predictions and individual predictions were evenly distributed around the line of unity, and the conditional weighted residuals were normally distributed (Fig. 1).
Goodness-of-fit plots of the final model. a Observed concentration (DV) plotted against predicted concentration (PRED). b DV plotted against individual predicted concentration (IPRED). c The correlation of conditional weighted residuals (CWRES) with the time after the tacrolimus dose. d The correlation of CWRES with PRED. The line represents the line of identity. OBS observed concentration
A bootstrap analysis with 500 bootstrap replicates was performed to obtain 95% confidence intervals for all pharmacokinetic parameters. Owing to minimization and boundary errors, the bootstrap results were recomputed without filtering these samples. Results of the bootstrap are shown in Table 2. Visual predictive checks showed that the median and the variability fell within the corresponding simulations (Fig. 2a). This demonstrates the good predictive performance of the final model in the internal validation. Evaluation of the predictive performance by NPDE analysis showed adequate predictive ability, with distribution of the NPDEs not significantly deviating from a normal distribution and the majority of the values were between −2 and 2. Evaluation of the individual’s influence on a change in OFV by the shark plot showed 70% of the included children had a decrease in OFV with the final model vs. the base model. In the external validation, the median and variability were adequately described, confirming the validation of the model (Fig. 2b). There was an insufficient number of CYP3A5 expressers in this validation cohort to validate the algorithm for this subgroup.
Prediction-corrected visual predictive check (VPC) showing how well the average trend of the observations (solid line) and how well the variability of the observed data (two dashed lines) fall within the model simulated average trend (red shaded area) and the model-simulated variability (blue shaded areas) represented as a 95% confidence interval (CI). The average and the variability of the observed data both fall within the corresponding simulations. a Internal VPC of the final model. b External VPC of the final model
3.4 Simulations
Based on the final model, CYP3A5 expressers had a two times higher CL/F. An increase in eGFR from 30 to 90 mL/min resulted in 19% higher CL/F, whereas a decrease in hematocrit levels from 0.3 to 0.25 L/L corresponded with a 20% higher tacrolimus CL/F. Deceased donor was associated with a 35% higher tacrolimus CL/F than living donor. The effects of CYP3A5 genotype, hematocrit, eGFR, donor, and bodyweight on CL/F are shown in Fig. 3. In total, these covariates explained 41% of the variability in CL/F.
Simulated plasma profiles of tacrolimus at first steady state after transplantation. a Simulated plasma profiles of tacrolimus for cytochrome P450 (CYP) 3A5 non-expressers (CYP3A5*3/*3) and CYP3A5 expressers (CYP3A5*1/*1 or CYP3A5*1/*3). b Simulated plasma profiles of tacrolimus for patients receiving a kidney from a living or deceased donor. c Simulated plasma profiles of tacrolimus for patients with a bodyweight of 15, 30, 55, or 80 kg. d Simulated plasma profiles of tacrolimus for patients with plasma hematocrit (Ht) levels of 0.2, 0.25, and 0.3 L/L. e Simulated plasma profiles of tacrolimus for patients with an estimated glomerular filtration rate (eGFR) of 10, 50, or 90 mL/min. conc concentration
3.5 Dosing Guidelines
The model was used to design a dosing algorithm for the starting dose of tacrolimus after pediatric kidney transplantation. The last measured eGFR and hematocrit before transplantation did not significantly influence the CL/F and because these parameters change tremendously post-transplantation, were not incorporated in the algorithm for the starting dose. The following equation described the estimation of tacrolimus CL/F (L/h) immediately after transplantation:
The required dose can be calculated using the estimated tacrolimus CL/F and the desired target C 0. To calculate this, AUC0–12h were determined for all the available pharmacokinetic time profiles. A C 0 of 10 ng/mL corresponded with an AUC0–12h of approximately 177 ng h/mL, 12.5 ng/mL with 209 ng h/mL, 15 ng/mL with 241 ng h/mL, 17.5 ng/mL with 274 ng h/mL and 20 ng/mL with 306 ng h/mL. Based on the formula: dose = AUC * CL/F, it leads to the following formula for a target C 0 of 12.5 ng/mL based on a twice-daily dose:
This formula can be used when dose adjustments are based on AUC; however, most hospitals base dose adjustments on C 0. Therefore, dosing guidelines were developed using the above formula, which was fine tuned by simulating a 1000 doses in different patients. Table 4 shows the dosing guideline including the simulated median C 0 with the interquartile range at steady state. Dosing guidelines range from 0.27 mg/kg/day for a CYP3A5 non-expresser weighing 80 kg receiving a kidney from a living donor, to 1.33 mg/kg/day for a CYP3A5 expresser weighing 10 kg and receiving a kidney from a deceased donor.
4 Discussion
This is the second population pharmacokinetic study of tacrolimus in pediatric renal transplant recipients covering the first 6 weeks after transplantation. Our results showed that a two-compartment model with first-order absorption, lag time, allometric scaling, IIV on CL, V 1, V 2, and k a, and IOV on CL and V 2 was optimal for data modeling. Besides bodyweight, we also demonstrated that CYP3A5 genotype, hematocrit, eGFR, and donor type all significantly influence tacrolimus CL. Together these covariates explained 41% of the variability in tacrolimus CL. Calculation of tacrolimus dosing guidelines for the initial dose after transplantation showed a factor four difference in daily dose, ranging from 0.27 to 1.33 mg/kg. This is vastly different from the 0.3 mg/kg/day currently used in clinical practice [40]. At first steady state, only 26.1% of patients were within the tacrolimus target C 0 range, 67.4% had subtherapeutic tacrolimus concentrations, and 6.5% had supratherapeutic concentrations. After a median of 9.8 days (range 1.7–23.8 days), patients were within the tacrolimus target C 0 range.
Genetic polymorphisms in CYP3A5 partly explain the IIV in tacrolimus pharmacokinetics. In this study, 25.9% of the included children of whom the genotype was known were CYP3A5 expressers. In agreement with previously published data, we report a significantly lower CL/F in children having the CYP3A5*3/*3 genotype compared with children carrying at least one CYP3A5*1 allele. Given the wide availability of TDM, genetic testing is most useful prior to initiation of tacrolimus to more rapidly reach the target concentration [41]. In adult kidney transplant recipients, two randomized controlled trials concluded that optimization of the initial tacrolimus dose using CYP3A5 genetic testing does not improve clinical outcomes when TDM is performed [26, 42]. As the pharmacokinetics of tacrolimus differs between children and adults, these findings cannot be extrapolated. Moreover, our model is more sophisticated than dosing based on the CYP3A5 genotype only. Therefore, the question remains if genotyping in pediatric transplant recipients prior to the start of tacrolimus therapy adds to adequate dosing of tacrolimus and improvement of clinical outcomes.
Children with a lower bodyweight had a higher weight-normalized tacrolimus dose requirement than children with a higher bodyweight. This is in line with previous findings [10, 11, 20, 30]. Allometric scaling significantly improved the model, substantiating findings from previous published pediatric models [10, 20]. It has been previously reported that younger children have significantly higher weight-normalized dose requirements than older recipients, suggesting the dose should be based on age rather than bodyweight [12, 13]. Incorporation of age in our model did not improve it further. This is in line with previously developed pharmacokinetic models in this population [10, 20, 30].
Contrary to what we expected, kidneys from a deceased donor had a higher tacrolimus CL than kidneys from a living donor. This finding was confirmed in the external validation of the model. A literature search was performed and showed no previous reports substantiating this observation. Dialysis prior to kidney transplantation or pre-emptive kidney transplantation was not significantly associated with tacrolimus CL, and neither was the number of human leukocyte antigen mismatches. As tacrolimus undergoes hepatic metabolism, higher tacrolimus CL in kidneys from a deceased donor seems highly unlikely. All patients received the same immunosuppressive protocol, regardless of the donor status. Only if there was a slow graft function, was the start of tacrolimus treatment postponed and patients continued treatment with glucocorticoids. This occurred in four patients, but none of them experienced delayed graft function in sensu stricto (i.e., the need for dialysis in the first week after transplantation). However, no correlation between slow graft function and tacrolimus CL was found in these patients. Furthermore, glucocorticoid use was tested as a covariate and did not significantly influence tacrolimus CL. Higher tacrolimus CL in kidneys from a deceased donor is probably caused by other unknown parameters that could not be tested as covariates and therefore cannot be corrected for. One of these parameters could be the interaction between donor and age. Recipients of a deceased donor kidney tended to be younger than recipients of a living donor kidney transplant, although this was not statistically significant. Another explanation could be other non-investigated parameters such as serum albumin, anemia, and metabolic acidosis. Recipients of deceased donors are usually more catabolic and might therefore have a higher free fraction of tacrolimus. However, this is not supported by our data showing the difference in CL between living and deceased donors does not decrease during the first 6 weeks post-transplantation.
Approximately 70–80% of tacrolimus is distributed in erythrocytes [43]. This indicates that low hematocrit concentrations will reduce the whole-blood concentrations of tacrolimus. In our cohort, children with a hematocrit level <0.30 had an increased CL/F and thus required a higher tacrolimus dose. This is in line with previous findings in pediatric kidney recipients [10, 20]. More than 98% of tacrolimus in the plasma is bound to plasma proteins, and despite measuring the tacrolimus concentration in whole blood, it is actually the unbound concentration that is pharmacologically active [44]. Hematocrit levels do not seem to influence the unbound fraction of tacrolimus in plasma [45]. Low albumin concentrations will increase the tacrolimus unbound fraction. Unfortunately, there were not enough albumin measurements available to determine if there was a relationship between albumin and tacrolimus CL/F. A recently published study reported the validation of an assay specifically developed for measurement of unbound tacrolimus concentrations [46].
In our cohort, children with higher eGFR had higher tacrolimus CL/F. As an increase in eGFR from 30 to 90 mL/min resulted in only 19% higher CL/F, eGFR-based dosage adjustment of tacrolimus seems unnecessary. Tacrolimus undergoes almost no renal elimination; therefore, the explanation for the observed association between tacrolimus CL and eGFR remains unclear. To our knowledge, this is the first pediatric model to include eGFR. Serum creatinine was tested in one model, but was not significantly correlated with tacrolimus CL [29]. In adults, some studies have reported no significant correlation between serum creatinine and tacrolimus CL [47,48,49], whereas others did find an effect [50, 51]. Recent data showed that the CYP3A5*1 genotype is associated with a greater extent of renal tacrolimus metabolism and a lower apparent urinary tacrolimus CL as compared with subjects expressing CYP3A5*3/*3. This is highly indicative of intra-renal CYP3A5-dependent tacrolimus metabolism and could possibly explain the influence of eGFR on tacrolimus CL/F [52].
There were no patients expressing CYP3A4*22, and therefore we could not confirm the reported relationship between CYP3A4*22 and tacrolimus CL [22]. An association between the glucocorticoid dose and tacrolimus CL has been reported [53, 54]. We could not substantiate this finding, probably because all children were treated with a glucocorticoid-minimization regimen and prednisolone was only prescribed the first 5 days in a relatively low dose.
The main strength of this study was the extensive evaluation of the final model; bootstrap analyses, VPCs, and an NPDE were performed, and the model was also externally validated. Another strength of the study is the large amount of blood samples per patient, including abbreviated tacrolimus pharmacokinetic curves. An extensive literature search was performed and all covariates known to influence the CL of tacrolimus, were tested. The population pharmacokinetic model was developed in patients treated according to the same immunosuppressive protocol. Other studies included children with different regimens, for example, with azathioprine instead of mycophenolic acid or glucocorticoids in different doses, making it difficult to determine the effect of tacrolimus. The final strength of the study is that dosing guidelines for the starting dose of tacrolimus after renal transplantation were developed. These dosing guidelines will be prospectively tested.
The main limitation of this study is that it is a retrospective analysis and therefore we had to rely on data available in the medical records. Because of this limitation we did not include a patient expressing CYP3A4*22, nor did we have enough CYP3A5 expressers in the validation cohort. Another limitation is that during the study period the tacrolimus analysis changed from the immunoassay to an LC–MS/MS. However, this difference was built into the residual error model. Furthermore, the relatively large proportion of Caucasian patients in our center is a limitation. Finally, the developed population pharmacokinetic model is only suitable for children aged between 2 and 18 years receiving immediate-release formulations of tacrolimus, as the once-daily preparation was not tested.
5 Conclusions
The population pharmacokinetics of tacrolimus during the first 6 weeks after renal transplantation can be adequately described using the model presented in this article. Higher bodyweight, lower eGFR, and higher hematocrit levels resulted in lower tacrolimus CL. CYP3A5 expressers and recipients who received a kidney from a deceased donor had higher tacrolimus CL. The tacrolimus weight-normalized starting dose should be higher in patients with lower bodyweight, in those who are CYP3A5 expressers, and in patients receiving a kidney from a deceased donor. By combining these parameters, an individualized tacrolimus dosing regimen has been developed that adequately predicts the target C 0 and hopefully will improve patient outcome.
References
Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605–12.
Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transpl. 2006;6(5 Pt 2):1111–31.
Burckart GJ, Liu XI. Pharmacogenetics in transplant patients: can it predict pharmacokinetics and pharmacodynamics? Ther Drug Monit. 2006;28(1):23–30.
Hesselink DA, van Schaik RH, van Agteren M, de Fijter JW, Hartmann A, Zeier M, et al. CYP3A5 genotype is not associated with a higher risk of acute rejection in tacrolimus-treated renal transplant recipients. Pharmacogenet Genom. 2008;18(4):339–48.
Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508.
Hesselink DA, Hoorn EJ. Improving long-term outcomes of kidney transplantation: the pressure is on. Neth J Med. 2014;72(5):248–50.
Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transpl. 2011;11(3):450–62.
Passey C, Birnbaum AK, Brundage RC, Oetting WS, Israni AK, Jacobson PA. Dosing equation for tacrolimus using genetic variants and clinical factors. Br J Clin Pharmacol. 2011;72(6):948–57.
Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–53.
Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86(6):609–18.
Kausman JY, Patel B, Marks SD. Standard dosing of tacrolimus leads to overexposure in pediatric renal transplantation recipients. Pediatr Transpl. 2008;12(3):329–35.
de Wildt SN, van Schaik RH, Soldin OP, Soldin SJ, Brojeni PY, van der Heiden IP, et al. The interactions of age, genetics, and disease severity on tacrolimus dosing requirements after pediatric kidney and liver transplantation. Eur J Clin Pharmacol. 2011;67(12):1231–41.
Naesens M, Salvatierra O, Li L, Kambham N, Concepcion W, Sarwal M. Maturation of dose-corrected tacrolimus predose trough levels in pediatric kidney allograft recipients. Transplantation. 2008;85(8):1139–45.
van Gelder T. Drug interactions with tacrolimus. Drug Saf. 2002;25(10):707–12.
Staatz CE, Willis C, Taylor PJ, Tett SE. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin Pharmacol Ther. 2002;72(6):660–9.
Tang JT, Andrews LM, van Gelder T, Shi YY, van Schaik RH, Wang LL, et al. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol. 2016;12(5):555–65.
Hesselink DA, Ngyuen H, Wabbijn M, Gregoor PJ, Steyerberg EW, van Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. Br J Clin Pharmacol. 2003;56(3):327–30.
Picard N, Bergan S, Marquet P, van Gelder T, Wallemacq P, Hesselink DA, et al. Pharmacogenetic biomarkers predictive of the pharmacokinetics and pharmacodynamics of immunosuppressive drugs. Ther Drug Monitor. 2016;38(Suppl. 1):S57–69.
Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus cosing. Clin Pharmacol Ther. 2015;98(1):19–24.
Prytula AA, Cransberg K, Bouts AH, van Schaik RH, de Jong H, de Wildt SN, et al. The effect of weight and CYP3A5 genotype on the population pharmacokinetics of tacrolimus in stable paediatric renal transplant recipients. Clin Pharmacokinet. 2016;55(9):1129–43.
Macphee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74(11):1486–9.
Elens L, van Schaik RH, Panin N, de Meyer M, Wallemacq P, Lison D, et al. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors’ dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics. 2011;12(10):1383–96.
Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53(2):123–39.
Andreu F, Colom H, Elens L, van Gelder T, van Schaik RH, Hesselink DA, et al. A new CYP3A5*3 and CYP3A4*22 cluster influencing tacrolimus target concentrations: a population approach. Clin Pharmacokinet. 2017. doi:10.1007/s40262-016-0491-3 (Epub ahead of print).
Miura M, Satoh S, Kagaya H, Saito M, Numakura K, Tsuchiya N, et al. Impact of the CYP3A4*1G polymorphism and its combination with CYP3A5 genotypes on tacrolimus pharmacokinetics in renal transplant patients. Pharmacogenomics. 2011;12(7):977–84.
Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87(6):721–6.
Brooks E, Tett SE, Isbel NM, Staatz CE. Population pharmacokinetic modelling and Bayesian estimation of tacrolimus exposure: is this clinically useful for dosage prediction yet? Clin Pharmacokinet. 2016;55(11):1295–335.
Andrews LM, Riva N, de Winter BC, Hesselink DA, de Wildt SN, Cransberg K, et al. Dosing algorithms for initiation of immunosuppressive drugs in solid organ transplant recipients. Expert Opin Drug Metab Toxicol. 2015;11(6):921–36.
Jacobo-Cabral CO, Garcia-Roca P, Romero-Tejeda EM, Reyes H, Medeiros M, Castaneda-Hernandez G, et al. Population pharmacokinetic analysis of tacrolimus in Mexican paediatric renal transplant patients: role of CYP3A5 genotype and formulation. Br J Clin Pharmacol. 2015;80(4):630–41.
Zhao W, Fakhoury M, Baudouin V, Storme T, Maisin A, Deschenes G, et al. Population pharmacokinetics and pharmacogenetics of once daily prolonged-release formulation of tacrolimus in pediatric and adolescent kidney transplant recipients. Eur J Clin Pharmacol. 2013;69(2):189–95.
Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, Fitzpatrick M, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transpl. 2010;10(4):828–36.
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37.
Vroling AB, Dorresteijn EM, Cransberg K, de Rijke YB. The impact of estimated glomerular filtration rate equations on chronic kidney disease staging in pediatric renal or heart transplant recipients. Pediatr Nephrol. 2016;31(7):1145–55.
van Schaik RH, van der Heiden IP, van den Anker JN, Lindemans J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48(10):1668–71.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17–20.
Jonsson EN, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res. 1998;15(9):1463–8.
Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997;37(6):486–95.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Progr Biomed. 2008;90(2):154–66.
Lancia P, Jacqz-Aigrain E, Zhao W. Choosing the right dose of tacrolimus. Arch Dis Child. 2015;100(4):406–13.
van Gelder T, Hesselink DA. Dosing tacrolimus based on CYP3A5 genotype: will it improve clinical outcome? Clin Pharmacol Ther. 2010;87(6):640–1.
Shuker N, Bouamar R, van Schaik RH, Clahsen-van Groningen MC, Damman J, Baan CC, et al. A randomized controlled trial comparing the efficacy of CYP3A5 genotype-based with bodyweight-based tacrolimus dosing after living donor kidney transplantation. Am J Transplant. 2016;16(7):2085–96.
Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29(6):404–30.
Nagase K, Iwasaki K, Nozaki K, Noda K. Distribution and protein binding of FK506, a potent immunosuppressive macrolide lactone, in human blood and its uptake by erythrocytes. J Pharm Pharmacol. 1994;46(2):113–7.
Størset E, Holford N, Hennig S, Bergmann TK, Bergan S, Bremer S, et al. Improved prediction of tacrolimus concentrations early after kidney transplantation using theory-based pharmacokinetic modelling. Br J Clin Pharmacol. 2014;78(3):509–23.
Stienstra NA, Sikma MA, van Dapperen AL, de Lange DW, van Maarseveen EM. Development of a simple and rapid method to measure the free fraction of tacrolimus in plasma using ultrafiltration and LC–MS/MS. Ther Drug Monit. 2016;38(6):722–7.
Gruber SA, Hewitt JM, Sorenson AL, Barber DL, Bowers L, Rynders G, et al. Pharmacokinetics of FK506 after intravenous and oral administration in patients awaiting renal transplantation. J Clin Pharmacol. 1994;34(8):859–64.
Sam WJ, Tham LS, Holmes MJ, Aw M, Quak SH, Lee KH, et al. Population pharmacokinetics of tacrolimus in whole blood and plasma in asian liver transplant patients. Clin Pharmacokinet. 2006;45(1):59–75.
Staatz CE, Willis C, Taylor PJ, Lynch SV, Tett SE. Toward better outcomes with tacrolimus therapy: population pharmacokinetics and individualized dosage prediction in adult liver transplantation. Liver Transplant. 2003;9(2):130–7.
Fukatsu S, Yano I, Igarashi T, Hashida T, Takayanagi K, Saito H, et al. Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur J Clin Pharmacol. 2001;57(6–7):479–84.
Jacobson P, Ng J, Ratanatharathorn V, Uberti J, Brundage RC. Factors affecting the pharmacokinetics of tacrolimus (FK506) in hematopoietic cell transplant (HCT) patients. Bone Marrow Transpl. 2001;28(8):753–8.
Zheng S, Tasnif Y, Hebert MF, Davis CL, Shitara Y, Calamia JC, et al. Measurement and compartmental modeling of the effect of CYP3A5 gene variation on systemic and intrarenal tacrolimus disposition. Clin Pharmacol Ther. 2012;92(6):737–45.
Kim JS, Aviles DH, Silverstein DM, Leblanc PL, Matti Vehaskari V. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatr Transpl. 2005;9(2):162–9.
van Duijnhoven EM, Boots JM, Christiaans MH, Stolk LM, Undre NA, van Hooff JP. Increase in tacrolimus trough levels after steroid withdrawal. Transpl Int. 2003;16(10):721–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used in the preparation of this article.
Conflict of interest
Teun van Gelder has received a study Grant from Chiesi Pharmaceuticals, lecture fees from Chiesi Pharmaceuticals, Astellas Pharma, Roche Pharma, and Novartis Pharma, and consulting fees from Astellas Pharma, Novartis Pharma, and Teva Pharma. Dennis A. Hesselink has received grant support and lecture and consulting fees from Astellas Pharma and Chiesi Pharmaceuticals, as well as a lecture fee from Hikma Pharma. Brenda C. M. de Winter has received travel support from Astellas Pharma. Saskia N. de Wildt has received funding from The Netherlands Organisation for Health Research and Development (Grant No. 90700304). Louise M. Andrews, Roger J. M. Brüggemann, Elisabeth A. M. Cornelissen, Karlien Cransberg, Birgit C. P. Koch, and Ron H. N. van Schaik have no conflicts of interest directly relevant to the content of this article.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Andrews, L.M., Hesselink, D.A., van Gelder, T. et al. A Population Pharmacokinetic Model to Predict the Individual Starting Dose of Tacrolimus Following Pediatric Renal Transplantation. Clin Pharmacokinet 57, 475–489 (2018). https://doi.org/10.1007/s40262-017-0567-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0567-8