Abstract
Background
The aim of this study was to develop a population pharmacokinetic model of tacrolimus in paediatric patients at least 1 year after renal transplantation and simulate individualised dosage regimens.
Patients and methods
We included 54 children with median age of 11.1 years (range 3.8–18.4 years) with 120 pharmacokinetic profiles performed over 2 to 4 h. The pharmacokinetic analysis was performed using the non-linear mixed-effects modelling software (NONMEM®). The impact of covariates including concomitant medications, age, the cytochrome P450 (CYP) CYP3A5*3 gene and the adenosine triphosphate binding cassette protein B1 (ABCB1) 3435 C→T gene polymorphism on tacrolimus pharmacokinetics was analysed. The final model was externally validated on an independent dataset and dosing regimens were simulated.
Results
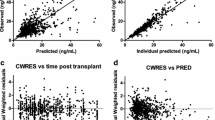
A two-compartment model adequately described tacrolimus pharmacokinetics. Apparent oral clearance (CL/F) was associated with weight (allometric scaling) but not age. Children with lower weight and CYP3A5 expressers required higher weight-normalised tacrolimus doses. CL/F was inversely associated with haematocrit (P < 0.05) and γ-glutamyl transpeptidase (γGT) (P < 0.001) and was increased by 45 % in carriers of the CYP3A5*1 allele (P < 0.001). CL/F was not associated with concomitant medications. Dose simulations show that a daily tacrolimus dose of 0.2 mg/kg generates a pre-dose concentration (C 0) ranging from 5 to 10 µg/L depending on the weight and CYP3A5 polymorphism. The median area under the plasma concentration–time curve (AUC) corresponding with a tacrolimus C 0 of 4–8 µg/L was 97 h·µg/L (interquartile range 80–120).
Conclusions
In patients beyond the first year after transplantation, there is a cumulative effect of CYP3A5*1 polymorphism and weight on the tacrolimus C 0. Children with lower weight and carriers of the CYP3A5*1 allele have higher weight-normalised tacrolimus dose requirements.




Similar content being viewed by others
References
Claeys T, Van Dyck M, Van Damme-Lombaerts R. Pharmacokinetics of tacrolimus in stable paediatric renal transplant recipients. Pediatr Nephrol. 2010;25(2):335–42. doi:10.1007/s00467-009-1331-6.
Zhao W, Fakhoury M, Baudouin V, Maisin A, Deschenes G, Jacqz-Aigrain E. Limited sampling strategy for estimating individual exposure of tacrolimus in pediatric kidney transplant patients. Ther Drug Monit. 2011;33(6):681–7. doi:10.1097/FTD.0b013e318235d067.
Armendariz Y, Pou L, Cantarell C, Lopez R, Perello M, Capdevila L. Evaluation of a limited sampling strategy to estimate area under the curve of tacrolimus in adult renal transplant patients. Ther Drug Monit. 2005;27(4):431–4.
Scholten EM, Cremers SC, Schoemaker RC, Rowshani AT, van Kan EJ, den Hartigh J, et al. AUC-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005;67(6):2440–7. doi:10.1111/j.1523-1755.2005.00352.x.
Uchida K, Tominaga Y, Haba T, Katayama T, Matsuoka S, Sato T, et al. Usefulness of monitoring of AUC(0–4 h) during the induction period of immunosuppressive therapy with tacrolimus after renal transplantation. Transplant Proc. 2002;34(5):1736–7.
Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31(2):139–52. doi:10.1097/FTD.0b013e318198d092.
Naesens M, Salvatierra O, Li L, Kambham N, Concepcion W, Sarwal M. Maturation of dose-corrected tacrolimus predose trough levels in pediatric kidney allograft recipients. Transplantation. 2008;85(8):1139–45. doi:10.1097/TP.0b013e31816b431a.
Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86(6):609–18. doi:10.1038/clpt.2009.210.
Hesselink DA, Ngyuen H, Wabbijn M, Gregoor PJ, Steyerberg EW, van Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. Br J Clin Pharmacol. 2003;56(3):327–30.
van Gelder T. Drug interactions with tacrolimus. Drug Saf. 2002;25(10):707–12.
Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74(3):245–54. doi:10.1016/S0009-9236(03)00168-1.
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4):383–91. doi:10.1038/86882.
Anglicheau D, Verstuyft C, Laurent-Puig P, Becquemont L, Schlageter MH, Cassinat B, et al. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14(7):1889–96.
Kurzawski M, Dabrowska J, Dziewanowski K, Domanski L, Peruzynska M, Drozdzik M. CYP3A5 and CYP3A4, but not ABCB1 polymorphisms affect tacrolimus dose-adjusted trough concentrations in kidney transplant recipients. Pharmacogenomics. 2014;15(2):179–88. doi:10.2217/pgs.13.199.
Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 2010;49(3):141–75. doi:10.2165/11317350-000000000-00000.
Benkali K, Premaud A, Picard N, Rerolle JP, Toupance O, Hoizey G, et al. Tacrolimus population pharmacokinetic-pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin Pharmacokinet. 2009;48(12):805–16. doi:10.2165/11318080-000000000-00000.
Antignac M, Barrou B, Farinotti R, Lechat P, Urien S. Population pharmacokinetics and bioavailability of tacrolimus in kidney transplant patients. Br J Clin Pharmacol. 2007;64(6):750–7. doi:10.1111/j.1365-2125.2007.02895.x.
Zhao CY, Jiao Z, Mao JJ, Qiu XY. External evaluation of published population pharmacokinetic models of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol. doi:10.1111/bcp.12830. Epub 2015 Nov 17.
Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet. 2002;41(11):813–51. doi:10.2165/00003088-200241110-00003.
Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, Fitzpatrick M, Murer L, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant. 2010;10(4):828–36. doi:10.1111/j.1600-6143.2010.03047.x.
van Rossum LK, Mathot RA, Cransberg K, Zietse R, Vulto AG. Estimation of the glomerular filtration rate in children: which algorithm should be used? Pediatr Nephrol. 2005;20(12):1769–75. doi:10.1007/s00467-005-2001-y.
van Schaik RH, van der Heiden IP, van den Anker JN, Lindemans J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48(10):1668–71.
Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36.
Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51–64.
Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101(1):72–9. doi:10.1016/j.cmpb.2010.04.018.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17–20. doi:10.1038/sj.clpt.6100241.
Savic RM, Karlsson MO. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11(3):558–69. doi:10.1208/s12248-009-9133-0.
Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic–pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20(5):511–28.
Jonsson EN, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res. 1998;15(9):1463–8.
Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997;37(6):486–95.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241–57. doi:10.1016/j.cmpb.2005.04.005.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51. doi:10.1208/s12248-011-9255-z.
Zhao W, Fakhoury M, Baudouin V, Storme T, Maisin A, Deschenes G, et al. Population pharmacokinetics and pharmacogenetics of once daily prolonged-release formulation of tacrolimus in pediatric and adolescent kidney transplant recipients. Eur J Clin Pharmacol. 2013;69(2):189–95. doi:10.1007/s00228-012-1330-6.
Musuamba FT, Guy-Viterbo V, Reding R, Verbeeck RK, Wallemacq P. Population pharmacokinetic analysis of tacrolimus early after pediatric liver transplantation. Ther Drug Monit. 2014;36(1):54–61. doi:10.1097/FTD.0b013e31829dcbcd.
Guy-Viterbo V, Scohy A, Verbeeck RK, Reding R, Wallemacq P, Musuamba FT. Population pharmacokinetic analysis of tacrolimus in the first year after pediatric liver transplantation. Eur J Clin Pharmacol. 2013;69(8):1533–42. doi:10.1007/s00228-013-1501-0.
Wallin JE, Bergstrand M, Wilczek HE, Nydert PS, Karlsson MO, Staatz CE. Population pharmacokinetics of tacrolimus in pediatric liver transplantation: early posttransplantation clearance. Ther Drug Monit. 2011;33(6):663–72. doi:10.1097/FTD.0b013e31823415cc.
Fukudo M, Yano I, Masuda S, Goto M, Uesugi M, Katsura T, et al. Population pharmacokinetic and pharmacogenomic analysis of tacrolimus in pediatric living-donor liver transplant recipients. Clin Pharmacol Ther. 2006;80(4):331–45. doi:10.1016/j.clpt.2006.06.008.
Bjorkman S. Prediction of cytochrome p450-mediated hepatic drug clearance in neonates, infants and children: how accurate are available scaling methods? Clin Pharmacokinet. 2006;45(1):1–11. doi:10.2165/00003088-200645010-00001.
Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–53.
Zhao W, Fakhoury M, Jacqz-Aigrain E. Developmental pharmacogenetics of immunosuppressants in pediatric organ transplantation. Ther Drug Monit. 2010;32(6):688–99. doi:10.1097/FTD.0b013e3181f6502d.
de Wildt SN, van Schaik RH, Soldin OP, Soldin SJ, Brojeni PY, van der Heiden IP, et al. The interactions of age, genetics, and disease severity on tacrolimus dosing requirements after pediatric kidney and liver transplantation. Eur J Clin Pharmacol. 2011;67(12):1231–41. doi:10.1007/s00228-011-1083-7.
Ferraris JR, Argibay PF, Costa L, Jimenez G, Coccia PA, Ghezzi LF, et al. Influence of CYP3A5 polymorphism on tacrolimus maintenance doses and serum levels after renal transplantation: age dependency and pharmacological interaction with steroids. Pediatr Transplant. 2011;15(5):525–32. doi:10.1111/j.1399-3046.2011.01513.x.
Lalan S, Abdel-Rahman S, Gaedigk A, Leeder JS, Warady BA, Dai H, et al. Effect of CYP3A5 genotype, steroids, and azoles on tacrolimus in a pediatric renal transplant population. Pediatr Nephrol. 2014;29(10):2039–49. doi:10.1007/s00467-014-2827-2.
Kausman JY, Patel B, Marks SD. Standard dosing of tacrolimus leads to overexposure in pediatric renal transplantation recipients. Pediatr Transplant. 2008;12(3):329–35. doi:10.1111/j.1399-3046.2007.00821.x.
Gijsen V, Mital S, van Schaik RH, Soldin OP, Soldin SJ, van der Heiden IP, et al. Age and CYP3A5 genotype affect tacrolimus dosing requirements after transplant in pediatric heart recipients. J Heart Lung Transplant. 2011;30(12):1352–9. doi:10.1016/j.healun.2011.08.001.
Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther. 2015;98(1):19–24. doi:10.1002/cpt.113.
MacPhee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant. 2004;4(6):914–9. doi:10.1111/j.1600-6143.2004.00435.x.
Kuypers DR, de Loor H, Naesens M, Coopmans T, de Jonge H. Combined effects of CYP3A5*1, POR*28, and CYP3A4*22 single nucleotide polymorphisms on early concentration-controlled tacrolimus exposure in de-novo renal recipients. Pharmacogenet Genomics. 2014;24(12):597–606. doi:10.1097/FPC.0000000000000095.
Zahir H, McCaughan G, Gleeson M, Nand RA, McLachlan AJ. Factors affecting variability in distribution of tacrolimus in liver transplant recipients. Br J Clin Pharmacol. 2004;57(3):298–309.
Hebert MF, Zheng S, Hays K, Shen DD, Davis CL, Umans JG, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95(7):908–15. doi:10.1097/TP.0b013e318278d367.
Storset E, Holford N, Midtvedt K, Bremer S, Bergan S, Asberg A. Importance of hematocrit for a tacrolimus target concentration strategy. Eur J Clin Pharmacol. 2014;70(1):65–77. doi:10.1007/s00228-013-1584-7.
Abu-Elmagd K, Fung JJ, Alessiani M, Jain A, Venkataramanan R, Warty VS, et al. The effect of graft function on FK506 plasma levels, dosages, and renal function, with particular reference to the liver. Transplantation. 1991;52(1):71–7.
Fung JJ, Todo S, Tzakis A, Demetris A, Jain A, Abu-Elmaged K, Alessiani M, et al. Conversion of liver allograft recipients from cyclosporine to FK 506-based immunosuppression: benefits and pitfalls. Transplant Proc. 1991;23(1 Pt 1):14–21.
Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27(1):127–33. doi:10.1161/01.ATV.0000251993.20372.40.
Kim JS, Aviles DH, Silverstein DM, Leblanc PL, Matti Vehaskari V. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatr Transplant. 2005;9(2):162–9. doi:10.1111/j.1399-3046.2005.00263.x.
van Duijnhoven EM, Boots JM, Christiaans MH, Stolk LM, Undre NA, van Hooff JP. Increase in tacrolimus trough levels after steroid withdrawal. Transplant Int. 2003;16(10):721–5. doi:10.1007/s00147-003-0615-1.
Cheung C, Yu AM, Chen CS, Krausz KW, Byrd LG, Feigenbaum L, et al. Growth hormone determines sexual dimorphism of hepatic cytochrome P450 3A4 expression in transgenic mice. J Pharmacol Exp Ther. 2006;316(3):1328–34. doi:10.1124/jpet.105.094367.
Tyden G, Berg U, Reinholt F. Acute renal graft rejection after treatment with human growth hormone. Lancet. 1990;336(8728):1455–6.
Guest G, Berard E, Crosnier H, Chevallier T, Rappaport R, Broyer M. Effects of growth hormone in short children after renal transplantation. French Society of Pediatric Nephrology. Pediatr Nephrol. 1998;12(6):437–46.
Hodson EM, Willis NS, Craig JC. Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev. 2012;2:CD003264. doi:10.1002/14651858.CD003264.pub3.
Filler G, Feber J, Lepage N, Weiler G, Mai I. Universal approach to pharmacokinetic monitoring of immunosuppressive agents in children. Pediatr Transplant. 2002;6(5):411–8.
van Boekel GA, Donders AR, Hoogtanders KE, Havenith TR, Hilbrands LB, Aarnoutse RE. Limited sampling strategy for prolonged-release tacrolimus in renal transplant patients by use of the dried blood spot technique. Eur J Clin Pharmacol. 2015;71(7):811–6. doi:10.1007/s00228-015-1863-6.
Montini G, Ujka F, Varagnolo C, Ghio L, Ginevri F, Murer L, et al. The pharmacokinetics and immunosuppressive response of tacrolimus in paediatric renal transplant recipients. Pediatr Nephrol. 2006;21(5):719–24. doi:10.1007/s00467-006-0014-9.
Barraclough KA, Isbel NM, Kirkpatrick CM, Lee KJ, Taylor PJ, Johnson DW, et al. Evaluation of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients. Br J Clin Pharmacol. 2011;71(2):207–23. doi:10.1111/j.1365-2125.2010.03815.x.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–12.
Lapeyraque AL, Kassir N, Theoret Y, Krajinovic M, Clermont MJ, Litalien C, et al. Conversion from twice- to once-daily tacrolimus in pediatric kidney recipients: a pharmacokinetic and bioequivalence study. Pediatr Nephrol. 2014;29(6):1081–8. doi:10.1007/s00467-013-2724-0.
Staatz CE, Tett SE. Clinical pharmacokinetics of once-daily tacrolimus in solid-organ transplant patients. Clin Pharmacokinet. 2015;54(10):993–1025. doi:10.1007/s40262-015-0282-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Agnieszka Prytuła has been supported by a Clinical Research Grant funded by the University of Ghent. Saskia de Wildt has been supported by a ZonMW Clinical Fellowship. Agnieszka Prytuła, Karlien Cransberg, Antonia Bouts, Ron van Schaik, Huib de Jong, Saskia de Wildt and Ron Mathôt have no conflicts of interest related to this work to declare.
Rights and permissions
About this article
Cite this article
Prytuła, A.A., Cransberg, K., Bouts, A.H.M. et al. The Effect of Weight and CYP3A5 Genotype on the Population Pharmacokinetics of Tacrolimus in Stable Paediatric Renal Transplant Recipients. Clin Pharmacokinet 55, 1129–1143 (2016). https://doi.org/10.1007/s40262-016-0390-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0390-7