Abstract
Introduction
Elevated interleukin (IL)-6 occurs in patients with active rheumatoid arthritis (RA), which has been shown to lead to a decrease in cytochrome P450 (CYP) enzyme activity and alterations in drug concentrations metabolized by CYP. IL-6 signaling blockade by IL-6 receptor (IL-6R) antagonists may reverse this effect of IL-6 and restore CYP activity. This study evaluated the pharmacokinetic profile of simvastatin (a CYP3A4 substrate) before and 1 week after a single dose of sarilumab (a human monoclonal antibody [mAb] blocking the IL-6Rα) in patients with RA, to assess potential interaction.
Methods
Nineteen patients with active RA received oral simvastatin 40 mg 1 day before and 7 days after subcutaneous injection of sarilumab 200 mg. The pharmacokinetic parameters of simvastatin and its primary metabolite, β-hydroxy-simvastatin acid, were calculated using noncompartmental analysis.
Results
Compared with simvastatin alone, single-dose simvastatin administration 7 days after single-dose sarilumab administration in patients with RA resulted in reduced simvastatin and β-hydroxy-simvastatin acid exposure in plasma. Mean effect ratios (90 % confidence interval) for simvastatin peak plasma concentration (C max) and area under the concentration–time curve extrapolated to infinity (AUC∞) were 54.1 % (42.2–69.4 %) and 54.7 % (47.2–63.3 %), respectively. No changes occurred in time to C max or half-life for either simvastatin or β-hydroxy-simvastatin acid after sarilumab administration.
Conclusions
Sarilumab treatment resulted in a reduction in exposure of simvastatin, consistent with reversal of IL-6-mediated CYP3A4 suppression in patients with active RA, as was reported for tocilizumab with simvastatin and for sirukumab with midazolam.
Clinical trial registration number
NCT02017639.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Interleukin (IL)-6 levels are elevated in inflammatory conditions, including rheumatoid arthritis (RA), leading to reduced cytochrome P450 (CYP) 3A4 activity and higher CYP3A4 substrate concentrations. |
Sarilumab, a human monoclonal antibody blocking the IL-6 receptor-α (IL-6Rα), restores CYP3A4 activity, which results in decreased exposure of the sensitive CYP3A4 substrate simvastatin and its active metabolite β-hydroxy-simvastatin acid in patients with active RA. |
These findings are consistent with those reported for the IL-6R antagonists tocilizumab and sirukumab. |
1 Introduction
Interleukin (IL)-6 is a key cytokine involved in the pathogenesis of inflammation and joint damage occurring in rheumatoid arthritis (RA) [1–3]. Patients with RA exhibit elevated levels of IL-6 in synovial joints and the systemic circulation. Mean baseline plasma IL-6 levels in patients with RA have been measured at 50–80 pg/mL compared with 5 pg/mL in healthy individuals [4]. The consequences of the pleiotropic effects of elevated IL-6 include B cell maturation, T-cell proliferation and differentiation, direct stimulation of C-reactive protein (CRP) from hepatocytes, and monocyte recruitment, implicating IL-6 in the shift from acute to chronic inflammation [2]. In addition to modulating inflammation, IL-6 levels have also been shown to modify the expression and activities of specific cytochrome P450 (CYP) enzymes, both in vitro and in vivo [5–8]. IL-6 was found to suppress activities of CYP enzymes in vitro, such as CYP3A, CYP2C19, and CYP1A2, by pan-suppression of messenger RNA (mRNA) of CYP enzymes [9–11].
CYP enzymes are critical proteins that metabolize endogenous and exogenous chemicals [12]. Elevated levels of IL-6, such as those seen in patients with RA, have been shown to modulate CYP enzyme activity in vitro and in vivo. In particular, such elevation has been shown to decrease CYP3A4 activity. Consequently, reduced CYP3A4 enzyme activity is expected to increase the exposure of concomitantly administered drugs that are substrates of CYP3A4 enzymes, resulting in a disease–drug interaction [9, 13]. In patients with RA, elevated levels of IL-6 led to higher concentrations of both simvastatin and its primary active metabolite β-hydroxy-simvastatin acid [6], both of which undergo extensive metabolism by CYP3A4, with high liver extraction and short half-life (t½z), and are therefore good indicators of CYP3A4 activity [14]. In addition, simvastatin has a low oral bioavailability, with extensive first-pass metabolism.
The effects of IL-6 in mediating both acute and chronic damage in RA make it an attractive target for therapeutic strategies [15]. Approaches that diminish inflammation via reduced IL-6 signaling may thus restore CYP3A4 activity in patients with RA toward that observed in healthy individuals, thereby decreasing CYP3A4 substrate concentrations in plasma. Tocilizumab is a humanized, monoclonal, IL-6 receptor-α (IL-6Rα) antagonist that inhibits binding of IL-6 to membrane-expressed IL-6R and soluble IL-6R (sIL-6R) [6]. Decreased IL-6 activity due to tocilizumab restores CYP3A4 activity and thus reduces CYP3A4 substrate concentrations. Indeed, in vivo, tocilizumab significantly reduced peak plasma concentration (C max) and area under the plasma concentration–time curve extrapolated to infinity (AUC∞) for both simvastatin and β-hydroxy-simvastatin acid, indicating that inhibition of IL-6 signaling increases apparent oral clearance (CL/F) of drugs metabolized by CYP3A4, potentially altering their therapeutic efficacy in patients with RA [6]. Sirukumab is an anti-IL-6 antibody that has also been shown to alter the pharmacokinetics of CYP substrates. In particular, sirukumab reverses the IL-6-mediated suppression of CYP3A4 activity in patients with RA [7]. Thus, the reduction of IL-6 signaling by an IL-6R antagonist, rather than the drug concentration itself, is the driving factor in this CYP substrate disease–drug interaction.
The investigational drug sarilumab is a human monoclonal antibody (mAb) that blocks IL-6 from binding to both membrane-bound and soluble IL-6Rα [16]. Sarilumab improved signs and symptoms of RA, including inhibition of IL-6 signaling and reduction of CRP concentration by more than 90 % relative to baseline, over 12 weeks in patients with moderate-to-severe RA, with a safety profile similar to other IL-6 inhibitors [16]. The objective of this study was to evaluate the effect of a single subcutaneous (SC) injection of sarilumab 200 mg on the pharmacokinetics of simvastatin, a sensitive CYP3A4 substrate used as a probe for CYP3A4 activity, in plasma of patients with active RA.
2 Methods
2.1 Study Design
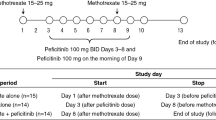
This was an open-label, single-sequence, nonrandomized, phase I study in patients with active RA (ClinicalTrials.gov identifier NCT02017639). The study was conducted at five sites in three countries: US, Moldova, and South Korea. Patients received a single oral dose of simvastatin 40 mg (two 20-mg tablets) 1 day before and 7 days after SC injection of sarilumab 200 mg.
Study duration included up to 28 days for screening before initial dosing, and the treatment period extending for up to 20 days, which comprised the first simvastatin dose and pharmacokinetic assessment (period 1), followed by period 2 with sarilumab administration on day 1, the second simvastatin dose and pharmacokinetic assessment on day 8, and end of treatment on day 15 (Fig. 1). Twenty-four-hour blood sampling was used to evaluate the pharmacokinetic profile of simvastatin on day 1 of period 1 and day 8 of period 2.
The study was conducted in compliance with Institutional Review Board regulations, International Conference on Harmonisation Good Clinical Practice guidelines, and the Declaration of Helsinki. After completion of this study, patients were given the option to participate in an ongoing open-label extension study of sarilumab in patients with RA (ClinicalTrials.gov identifier NCT01146652).
The sample size calculation indicated that 14 patients were adequate to estimate the effect of sarilumab on the pharmacokinetics of simvastatin and its active metabolite with 90 % confidence intervals (CIs) based on within-patient standard deviation (SDwithin) for log-transformed AUC from time zero to the last quantifiable concentration (AUClast) and AUC∞, assuming the true SDwithin was 0.325.
2.2 Patient Inclusion and Exclusion Criteria
Patients aged 18–75 years with a body weight between 40 and 110 kg (female) or 50 and 120 kg (male) were included in the study. The potential effect of inhibition of IL-6 by sarilumab on CYP3A4 activity, as measured by simvastatin exposure in plasma, was to be evaluated in patients with active RA with elevated IL-6 levels. As such, patients fulfilling the American College of Rheumatology (ACR) revised criteria for the diagnosis of moderate-to-severe RA [17] with ≥3 months’ disease duration and ACR class I–III functional status despite stable background methotrexate, 10–25 mg/week for at least 12 consecutive weeks before inclusion, were enrolled in the study. Moderate-to-severe RA was defined as ≥4 of 68 tender joints, ≥4 of 66 swollen joints, and CRP ≥6 mg/L. Concomitant medications affecting the activity of CYP were not allowed.
Patients were excluded if they had prior or current uncontrolled concomitant diseases, significant extra-articular manifestations of RA, other inflammatory diseases, current/recurrent infections, or were receiving prednisone (or equivalent) >10 mg/day.
2.3 Safety and Tolerability
Safety assessments included incidence of treatment-emergent adverse events (TEAEs), serious treatment-emergent AEs (SAEs), and laboratory tests. TEAEs, SAEs, and AEs of special interest were reported by investigators, and laboratory parameters were measured. Adverse events were described at the Medical Dictionary for Regulatory Activities (MedDRA; version 17.1) preferred-term level, whereas AEs of special interest were identified using prespecified search criteria. Antidrug antibody (ADA) positivity at two or more consecutive samplings during the TEAE period was classified as persistent; the number of patients experiencing TEAEs was summarized by treatment (simvastatin alone, sarilumab alone, and simvastatin after sarilumab administration).
Individual laboratory data (biochemistry and hematology) were noted when outside of laboratory reference ranges or when exceeding the cut-off value defined for each potentially clinically significant abnormality criterion.
2.4 Sample Collection and Analysis
Blood samples for measurement of concentrations of simvastatin and its metabolite β-hydroxy-simvastatin acid in plasma were collected at baseline and at 0.5, 1, 1.5, 2, 4, 6, 8, 10, 12, and 24 h post-dose on day 1 (period 1) and day 8 (period 2). Blood samples were collected in sodium heparin tubes and immediately centrifuged at 4 °C. Plasma samples were then frozen at −70 °C and stored at approximately −60 to −80 °C before analysis. Concentrations of simvastatin and β-hydroxy-simvastatin acid in plasma were determined using a validated liquid chromatography–tandem mass spectrometry method with a lower limit of quantification (LLOQ) of 0.05 and 0.1 ng/mL, respectively (Covance Bioanalytical Services, Indianapolis, IN, USA). All simvastatin and β-hydroxy-simvastatin acid concentrations measured with this assay were used for the pharmacokinetic analysis; however, simvastatin and β-hydroxy-simvastatin acid concentrations in pharmacokinetic samples from two patients that were analyzed using an earlier assay were not reported because of bioanalytical stability issues. For a calibration curve to have been considered acceptable, a minimum of six calibration levels and 75 % of all calibration standards must have fallen within ±15.0 % (±20.0 % at the LLOQ) of nominal. Concentrations of functional sarilumab were analyzed using a validated enzyme-linked immunosorbent assay (ELISA) method with an LLOQ of 312.5 ng/mL at pre-dose day 1 (period 2) and days 7, 9, and 15 (period 2). Immunogenicity was assessed by the presence of anti-sarilumab antibodies in serum. Anti-sarilumab antibody levels in serum were listed as negative (below the detection limit) or positive with titer by patient and visit. The number (%) of patients with ADA positive or negative response was summarized by scheduled visit. Sampling times for total sIL-6R, IL-6, and CRP in serum included day 1 in period 1, and day 8 and day 15 (window between days 15 and 18, at the end of treatment) of period 2. Concentrations of total sIL-6R and IL-6 in serum were measured using validated quantitative sandwich ELISA methods; CRP was measured by a validated immunonephelometry assay (Covance Bioanalytical Services).
2.5 Pharmacokinetic Analysis
Simvastatin and β-hydroxy-simvastatin acid concentrations in plasma, obtained as real-time values after single-dose administration, were used to calculate the following pharmacokinetic parameters using noncompartmental analysis with validated software (WinNonlin® software [Pharmacokinetic Data Management Services, version 2.1, incorporating WinNonlin® Professional, version 5.2.1, Pharsight Corporation, Mountain View, CA, USA]): C max, time to C max (t max), AUClast, AUC∞, and t ½z . The C max and t max values of simvastatin and β-hydroxy-simvastatin acid were obtained directly from experimental observations, whereas the remaining pharmacokinetic parameters were calculated as described. Any AUC∞ values with >30 % of area extrapolation were not reported.
2.6 Statistical Analysis
Statistical analyses were performed using Pharmacokinetic Data Management Services, version 2.1, with WinNonlin Professional, version 5.2.1 (Pharsight Corporation) and SAS® (SAS/UNIX version 9.4, SAS Institute, Cary, NC, USA). Simvastatin and β-hydroxy-simvastatin acid plasma concentrations and pharmacokinetic parameters were summarized using descriptive statistics for each treatment. The effect of a single SC dose of sarilumab on single-dose simvastatin and β-hydroxy-simvastatin acid pharmacokinetic parameters was analyzed using a linear mixed-effects model on log-transformed C max, AUClast, and AUC∞ of simvastatin and β-hydroxy-simvastatin acid to obtain estimates and 90 % CIs for the ratio of geometric means of simvastatin and β-hydroxy-simvastatin acid after sarilumab administration versus simvastatin alone.
Pharmacodynamic variables consisted of total sIL-6R, IL-6, and CRP. Summary statistics of the raw data and percentage change from baseline for each pharmacodynamic variable were calculated for each scheduled time for continuous pharmacodynamic variables.
3 Results
3.1 Patient Demographics
A total of 19 patients were enrolled in, and completed, the study. At baseline, mean patient age (±SD) was 52.8 ± 11.4 years (range 27–71) and mean body weight was 71.5 ± 18.0 kg (range 48.0–108.5) (Table 1). The majority of patients were female (79 %) and Caucasian (63 %). All patients had active moderate-to-severe disease activity per study inclusion criteria, and mean tender and swollen joint counts (±SD) were 29.4 ± 15.3 and 16.2 ± 8.8, respectively. Mean duration of RA at baseline (±SD) was 8.6 ± 6.1 years. Most patients (89 %) received concomitant methotrexate (10–25 mg/week) and folic acid.
3.2 Pharmacokinetics
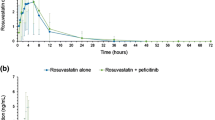
Administration of a single oral dose of simvastatin 40 mg to patients with RA 1 week after a single SC dose of sarilumab 200 mg resulted in reducing simvastatin exposure in plasma by 45 % and β-hydroxy-simvastatin acid exposure in plasma by 36 %, compared with administration of simvastatin 40 mg alone (Table 2; Fig. 2). Mean effect ratios (90 % CI) for simvastatin C max, AUClast, and AUC∞ were 54.1 % (42.2–69.4 %), 54.0 % (46.5–62.8 %), and 54.7 % (47.2–63.3 %), respectively. Corresponding ratios for β-hydroxy-simvastatin acid were 64.1 % (55.5–74.1 %), 62.8 % (53.6–73.7 %), and 64.1 % (54.1–75.8 %), respectively. Because of sample stability issues during the bioanalysis, pharmacokinetic parameters could not be calculated for two patients.
Mean [+SD] a simvastatin and b β-hydroxy-simvastatin acid plasma concentration–time profiles after administration of simvastatin 40 mg 1 day before and 7 days after subcutaneous injection of sarilumab 200 mg (N = 19). Pharmacokinetic parameters for two patients were not calculated because of sample stability issues during bioanalysis. SD standard deviation
Concentrations of sarilumab in serum after a single 200-mg dose were comparable with historical data in patients with RA [18].
3.3 Pharmacodynamics
After sarilumab administration, mean total sIL-6R levels in serum increased as expected, from 52.5 ng/mL at baseline (day 1) to 209.6 ng/mL on day 8. Total sIL-6R reached 286.4 ng/mL approximately 2 weeks (day 15) after sarilumab administration (Fig. 3a). Mean IL-6 levels in serum increased from 47.5 pg/mL at baseline to 219.9 pg/mL on day 8, and then decreased to 138.5 pg/mL on day 15 (Fig. 3b).
Pharmacodynamic response data corresponding to baseline, 1 week after sarilumab administration alone (day 8), and approximately 2 weeks after sarilumab administration and 1 week after simvastatin administration (day 15) for a sIL-6R, b IL-6, and c CRP. N = 19, N = 17, and N = 19 for baseline, sarilumab, and sarilumab plus simvastatin, respectively. Data are expressed as mean ± SEM. CRP C-reactive protein, IL-6 interleukin 6, SEM standard error of the mean, sIL-6R soluble IL-6 receptor
CRP in serum levels (mean ± standard error [SE]; median) were elevated at baseline (22.1 ± 5.60 mg/L; 14.5 mg/L), consistent with active RA (Fig. 3c), decreased after sarilumab administration on day 8 (1.9 ± 0.2 mg/L; 2.0 mg/L), and remained low on day 15 (5.9 ± 1.5 mg/L; 2.2 mg/L). This pharmacodynamic profile indicated IL-6 inhibition by sarilumab [19].
3.4 Safety
The safety population included all 19 enrolled and treated patients (Electronic Supplementary Table 1). There were no SAEs, deaths, or AEs leading to permanent treatment discontinuation. Two patients experienced TEAEs after administration of simvastatin alone (period 1), seven patients experienced TEAEs after administration of sarilumab alone (period 2), and four patients experienced TEAEs after administration of sarilumab and simvastatin (period 2). Most TEAEs were mild or moderate in intensity.
The most frequently reported TEAE was neutropenia, which was reported in four patients after sarilumab administration, and in one patient after sarilumab and simvastatin administration. Grade 3 neutropenia (0.5–1 Giga/L) was observed in three patients. Decreases in neutrophil counts after treatment with sarilumab were not associated with any sequelae, including infection, although patient numbers were small.
One patient with an observed increase in alanine aminotransferase (ALT) >3 × the upper limit of normal (onset 6 days after the second administration of simvastatin; period 2) did not show significant changes in aspartate aminotransferase, total bilirubin, or alkaline phosphatase. The patient spontaneously recovered from the event and remained asymptomatic, with no trigger factors noted.
All 19 patients were ADA-negative at baseline, and one patient had a positive result for ADA during the TEAE period.
4 Discussion
In patients with RA, elevated levels of IL-6 reduce CYP3A4 enzyme activity [7], and treatment with a single dose of the IL-6Rα antagonist sarilumab led to restoration of CYP3A4 enzyme activity to non-disease levels, as measured by decreases in simvastatin and its primary active metabolite β-hydroxy-simvastatin acid concentrations, presumably due to the reduction in IL-6 signaling by sarilumab. After oral administration, simvastatin undergoes extensive first-pass metabolism and is readily hydrolyzed by esterases into β-hydroxy-simvastatin acid, the pharmacologically active metabolite. Both simvastatin and its active metabolite β-hydroxy-simvastatin acid are CYP3A4 substrates yielding inactive metabolites; simvastatin is therefore used as a probe to measure CYP3A4 enzyme activity in drug–drug interaction studies with assessment of both simvastatin and β-hydroxy-simvastatin acid concentrations in plasma. The conversion of β-hydroxy-simvastatin acid is also, to a lesser extent, CYP2C8-mediated [20]. In the present study, a single SC dose of sarilumab 200 mg decreased the C max of simvastatin by 46 %, and that of β-hydroxy-simvastatin acid by 35 %. The AUC∞ of simvastatin was decreased by 45 %, and that of β-hydroxy-simvastatin acid exposure was decreased by 36 %. Sarilumab did not affect the t max or t ½z of simvastatin and β-hydroxy-simvastatin acid. The t ½z of simvastatin did not appear to change after sarilumab administration, suggesting an increase of the first-pass metabolism of simvastatin and less alteration of the elimination. Additionally, as sarilumab had a similar effect on both simvastatin and β-hydroxy-simvastatin acid, the effect of sarilumab on simvastatin exposure is consistent with reduced IL-6 signaling by sarilumab and subsequent restoration of the reduced CYP3A4 enzyme activity in patients with RA [14]. A similar effect in patients with RA was also described after a single dose of the IL-6R antagonists tocilizumab, with both simvastatin and β-hydroxy-simvastatin acid as CYP3A4 substrates, and sirukumab, using midazolam as a CYP3A4 substrate [6, 7]. This effect has been related to their IL-6 inhibitory effect and subsequent restoration of decreased CYP3A4 activity in this patient population. It is important to note that in a study of sirukumab that investigated a cocktail of CYP substrates composed of midazolam (CYP3A4), omeprazole (CYP2C19), S-warfarin (CYP2C9), and caffeine (CYP1A2) in patients with active RA, a nonequivalent decrease in AUC was observed for midazolam (30–35 %), omeprazole (37–45 %), and S-warfarin (18–19 %), while an increase in AUC was observed for caffeine (20–34 %). These results suggest that sirukumab may reverse IL-6-mediated suppression of CYP3A4, CYP2C9, and CYP2C19 enzyme activity in patients with active RA treated with sirukumab [7]. As the same effect was observed with the IL-6 inhibitor sarilumab on a CYP3A4 substrate, it is likely that sarilumab would show similar suppression of CYP2C9 and CYP2C19 enzyme activity as that observed with sirukumab.
Statins are one of the most commonly prescribed agents for the treatment of hyperlipidemia because of their efficacy in reducing low-density lipoprotein (LDL) cholesterol levels [21]. This LDL-lowering effect is the primary treatment for patients with multiple risk factors or established coronary heart disease [22]. Of the statins on the market, simvastatin and lovastatin are known to be the most sensitive to changes in CYP3A4 enzyme activity [20]. A meta-analysis in patients with hyperlipidemia reported a reduction in LDL (treated placebo) of 1.08 mmol/L (23 %) to 2.01 mmol/L (42 %) over a simvastatin daily dose ranging from 5 to 80 mg, and of 1.78 mmol/L (37 %) to 2.01 mmol/L (42 %) over a simvastatin dose ranging from 40 to 80 mg [23, 24]. The simvastatin label reports an LDL-lowering effect of 41–47 % over a simvastatin daily dose ranging from 40 to 80 mg [25]. Hence, the decrease in simvastatin exposure (46 %) observed with sarilumab in this study, based on the exposure (dose)-response relationships for the LDL-lowering effect of simvastatin, would translate into a 5–6 % difference in LDL-lowering effect, e.g. LDL-lowering effect of simvastatin would change from 42 to 47 % (without sarilumab) to 37 to 41 % (with sarilumab). Therefore, the disease–drug interaction of sarilumab on simvastatin and its active metabolite β-hydroxy-simvastatin acid is believed to have limited clinical relevance. However, for other statins with a narrow therapeutic index, the modulation of IL-6 effect on CYP enzymes may be clinically relevant, and individual dose adjustment may be recommended.
The levels of CRP in serum are indicative of systemic inflammation and have been found to be elevated in many patients with RA [26, 27]. Although normal CRP values vary among different laboratories, healthy individuals have low values of CRP in serum (median ≤1 mg/L). The patients with active RA in this study had a median baseline CRP value of 14.5 mg/L, suggesting the presence of significant systemic inflammation. Approximately 1 week after a single dose of sarilumab, median CRP decreased more than sevenfold to 2.0 mg/L, and remained low even at follow-up 2 weeks after sarilumab treatment (2.2 mg/L). These CRP values, measured after a single dose of sarilumab during the assessment of simvastatin exposure, are indicative of the anticipated inhibitory effect on IL-6 signaling and support this single-dose disease–drug interaction study design, previously used for similar drug interaction assessments with the IL-6 inhibitors tocilizumab and sirukumab [6, 7].
Both total sIL-6R and IL-6 in serum increased after administration of sarilumab over baseline. Transient increases in IL-6 have also been observed with another IL-6Rα blocker, tocilizumab [6, 28]. Increased totals of IL-6R and IL-6 are related to reduced receptor-mediated clearance of IL-6 in the presence of an IL-6Rα inhibitor, such as sarilumab or tocilizumab. When the IL-6 clearance rate is reduced, total sIL-6R and IL-6 concentrations rise until a steady state is reached, where the endogenous production rate of IL-6 matches the degradation rate [28]. In this study, the decrease in CRP suggests that IL-6 signaling was effectively inhibited with sarilumab; therefore, the IL-6-mediated effects would be diminished. Thus, the pharmacodynamic profile of sarilumab was consistent with effects of IL-6 inhibition observed in previous studies of this drug [6, 7].
A limitation of this study, as with other in vivo drug–drug interaction studies with IL-6 inhibitors, is that IL-6 signaling and CYP3A4 activity were both indirectly measured through surrogate markers (i.e. CRP and simvastatin, respectively). The results of this disease–drug interaction study of sarilumab were consistent with those of other monoclonal antibodies against IL-6/IL-6R, i.e. tocilizumab and sirukumab [6, 7], suggesting that IL-6-mediated suppression of CYP3A4 may last as long as IL-6 signaling is inhibited by IL-6R blockade.
No serious AEs were reported in the present study, and, in general, AE occurrence was limited and consistent with the anticipated effects of IL-6Rα inhibition and the known safety profile of sarilumab.
Overall, the findings in this study are consistent with the hypothesis that IL-6Rα blockade with sarilumab decreases IL-6 signaling in patients with RA, leading to restoration of CYP3A4 enzyme activity. The modulation of IL-6 effect on CYP enzymes by sarilumab may be clinically relevant for CYP substrates with a narrow therapeutic index, where the dose is individually adjusted. Upon initiation or discontinuation of sarilumab in patients being treated with CYP substrate medicinal products, therapeutic monitoring of effect or drug concentration should be performed, and the individual dose of the medicinal product may need to be adjusted, as needed. Caution should be exercised when sarilumab is coadministered with CYP3A4 substrates (e.g. oral contraceptives or statins) as there may be a reduction in exposure, which may reduce the activity of these CYP3A4 substrates.
5 Conclusion
Administration of a single SC dose of sarilumab 200 mg with a single oral dose of simvastatin 40 mg to patients with moderate-to-severe RA resulted in reduced exposure to simvastatin and its metabolite β-hydroxy-simvastatin acid compared with simvastatin alone. Because sarilumab inhibits IL-6 signaling, care should be taken with concomitant use of other drugs that are metabolized by CYP3A4 enzymes.
References
Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–4.
Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2010;2:247–56.
Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–74.
Robak T, Gladalska A, Stepień H, Robak E. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediat Inflamm. 1998;7:347–53.
Jover R, Bort R, Gómez-Lechón MJ, Castell JV. Down-regulation of human CYP3A4 by the inflammatory signal interleukin 6: molecular mechanism and transcription factors involved. FASEB J. 2002;16:1799–801.
Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease–drug–drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89:735–40.
Zhuang Y, de Vries DE, Xu Z, Marciniak SJ Jr, Chen D, Leon F, et al. Evaluation of disease-mediated therapeutic protein–drug interactions between an anti–interleukin-6 monoclonal antibody (sirukumab) and cytochrome P450 activities in a phase I study in patients with rheumatoid arthritis using a cocktail approach. J Clin Pharmacol. 2015;55:186–94.
Xu Y, Hijazi Y, Wolf A, Wu B, Sun Y-N, Zhu M. Physiologically based pharmacokinetic model to assess the influence of blinatumomab-mediated cytokine elevations on cytochrome P450 enzyme activity. CPT Pharmacometrics Syst Pharmacol. 2015;4:507–15.
Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–22.
Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993;44:707–15.
Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–93.
Riddick DS, Lee C, Bhathena A, Timsit YE, Cheng PY, Morgan ET, et al. Transcriptional suppression of cytochrome P450 genes by endogenous and exogenous chemicals. Drug Metab Dispos. 2004;32:367–75.
Machavaram KK, Almond LM, Rostami-Hodjegan A, Gardner I, Jamei M, Tay S, et al. A physiologically based pharmacokinetic modeling approach to predict disease–drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther. 2013;94:260–8.
Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003;56:120–4.
Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2:288–94.
Huizinga TW, Fleischmann RM, Jasson M, Radin AR, van Adelsberg J, Fiore S, et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. 2014;73:1626–34.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
Belomestnov P, Hamilton J, DiCioccio AT, Jasson M, Radin AR. Sarilumab, a subcutaneously-administered, fully-human monoclonal antibody inhibitor of the IL-6 receptor: pharmacokinetic profile and its relationship to changes in pharmacodynamic markers in patients with rheumatoid arthritis [ACR abstract 1337]. Arthritis Rheum. 2012;64(Suppl 10):S576.
Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122:143–59.
Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81.
IMS Institute for Healthcare Informatics. The use of medicines in the United States: review of 2010. 2011. https://www.imshealth.com/files/web/IMSH%20Institute/Reports/The%20Use%20of%20Medicines%20in%20the%20United%20States%202010/Use_of_Meds_in_the_U.S._Review_of_2010.pdf. Accessed 5 Jun 2016.
Superko HR, King S III. Lipid management to reduce cardiovascular risk: a new strategy is required. Circulation. 2008;117:560–8.
Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423.
Kim J, Ahn BJ, Chae HS, Han S, Doh K, Choi J, et al. A population pharmacokinetic-pharmacodynamic model for simvastatin that predicts low-density lipoprotein-cholesterol reduction in patients with primary hyperlipidaemia. Basic Clin Pharmacol Toxicol. 2011;109:156–63.
Zocor [package insert]. Whitehouse Station: Merck Sharp & Dohme Corporation; 2015.
Tishler M, Caspi D, Yaron M. C-reactive protein levels in patients with rheumatoid arthritis: the impact of therapy. Clin Rheumatol. 1985;4:321–4.
Shadick NA, Cook NR, Karlson EW, Ridker PM, Maher NE, Manson JE, et al. C-reactive protein in the prediction of rheumatoid arthritis in women. Arch Intern Med. 2006;166:2490–4.
Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti–IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–64.
Acknowledgments
The authors would like to thank Andrea Eckhart, PhD, MedThink SciCom, for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc. Andrea Eckhart, PhD, an employee of MedThink SciCom, provided editorial assistance, which was funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Conflicts of interest
Eun Bong Lee has acted as a consultant to Pfizer. Nikki Daskalakis, Christine Xu, and Barry Miller are employees of Sanofi Genzyme and may hold stock and/or stock options in the company. Anne Paccaly is an employee of Regeneron Pharmaceuticals, Inc. and may hold stock and/or stock options in the company. Roy Fleischmann has received research grants from AbbVie, Amgen, Ardea, AstraZeneca, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Pfizer, Roche, Sanofi, and UCB; and has received consulting fees from AbbVie, Akros, Amgen, AstraZeneca, Bristol-Myers Squibb, Janssen, Eli Lilly, Pfizer, Roche, and UCB. Inga Bodrug was an investigator for this study. Alan Kivitz has received research grants from, and holds stock in, Sanofi and Regeneron Pharmaceuticals, Inc.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lee, E.B., Daskalakis, N., Xu, C. et al. Disease–Drug Interaction of Sarilumab and Simvastatin in Patients with Rheumatoid Arthritis. Clin Pharmacokinet 56, 607–615 (2017). https://doi.org/10.1007/s40262-016-0462-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0462-8