Abstract
Background and Objective
Sarilumab binds to the interleukin-6 receptor with high affinity, inhibiting cis and trans signaling by interleukin-6. Sarilumab has demonstrated efficacy and safety in patients with rheumatoid arthritis. The objective of this study was to develop a population-pharmacokinetic model using data from 1770 patients with rheumatoid arthritis across phase I–III studies.
Methods
Potential covariates were identified using a stepwise forward-addition and backward-deletion strategy, and the final model was evaluated by visual predictive check and bootstrap methods.
Results
Sarilumab pharmacokinetics is described by a two-compartment model with first-order absorption and parallel linear and nonlinear Michaelis–Menten elimination. A subcutaneous dose of sarilumab 200 mg every 2 weeks resulted in more pronounced saturation of the nonlinear clearance pathway over the dosing interval than 150 mg every 2 weeks. Steady-state exposure (area under the plasma concentration–time curve from day 0 to day 14) increased twofold with dose escalation from 150 to 200 mg every 2 weeks. Body weight, anti-drug antibody status, sarilumab drug product, sex, creatinine clearance, albumin, and baseline C-reactive protein levels were identified as significant covariates according to the predefined statistical significance criteria in stepwise covariate searches. The main intrinsic source of pharmacokinetic variability in exposure was body weight. Compared with a typical 71-kg patient, the area under the plasma concentration–time curve from day 0 to day 14 was 20–23% lower for an 83-kg patient and 20–25% higher for a 62-kg patient.
Conclusions
These findings, combined with the safety and efficacy data, indicated limited clinical relevance of body-weight effect on sarilumab exposure. No adjustment in sarilumab dose is required for body weight or any other demographics assessed.
Similar content being viewed by others
Using data from 1770 patients with rheumatoid arthritis treated with sarilumab in clinical trials, a population-pharmacokinetic model was developed to describe the pharmacokinetics of sarilumab and impact of patient characteristics on pharmacokinetic variability |
The pharmacokinetics of sarilumab is described by a two-compartment model with first-order absorption and parallel linear and nonlinear Michaelis–Menten elimination |
We found limited clinical relevance of body weight on sarilumab exposure; no adjustment in sarilumab dose is required for body weight or any other patient characteristics assessed |
1 Introduction
Interleukin-6 (IL-6) is a multifunctional cytokine that acts as a critical signaling node in the complex pro-inflammatory cytokine network [1], which underpins rheumatoid arthritis (RA) [2]. In RA, IL-6 acts both locally, to promote joint inflammation and destruction, and systemically in the circulation, to mediate systemic manifestations of RA [2, 3].
Sarilumab is a recombinant, human, immunoglobulin isotype G1 monoclonal antibody that binds to the alpha subunit of membrane-bound and soluble IL-6 receptors and inhibits IL-6 signaling through these receptors [4]. Inhibition of IL-6 signaling interrupts the cytokine-mediated signaling cascade thought to be one of the underlying causes of inflammation and joint destruction in RA [2, 3, 5]. Sarilumab is approved for the treatment of adult patients with moderate-to-severe RA who show an inadequate response to other disease-modifying anti-rheumatic drugs, and it can be used in combination with a conventional disease-modifying anti-rheumatic drug or as monotherapy [6, 7]. In clinical studies, the sarilumab safety profile was consistent with IL-6 receptor blockade [8, 9].
Sarilumab exhibits nonlinear pharmacokinetics with target-mediated clearance [6, 7, 10]. Sarilumab binds to its biologic target; subsequent turnover of the sarilumab-IL-6 receptor complex is saturable and results in nonlinear pharmacokinetics [11]. Sarilumab is eliminated by parallel linear and nonlinear pathways, where at higher concentrations, the elimination is predominantly through the linear nonsaturable proteolytic pathway, and at lower concentrations, nonlinear, saturable target-mediated elimination predominates [7].
Pharmacokinetics for drugs with saturable clearance is usually described by the Michaelis–Menten (M–M) equation. Where saturable clearance and/or the distribution processes are controlled by a drug target, such as a receptor, mechanistic target-mediated drug disposition models have been applied [12]. The aim of this study was to develop a population-pharmacokinetic (Pop-PK) model for sarilumab in patients with RA and to identify covariates that are potential sources of variability in exposure using concentration data combined from phase I, II, and III studies.
2 Methods
2.1 Participants and Design of Clinical Studies
Sarilumab concentration data from 1935 patients with RA who participated in seven phase I studies, one phase II study, and four phase III studies were included in the analysis (Table 1). Sarilumab was administered as a single subcutaneous dose of 50–200 mg or as repeated subcutaneous doses of 50–200 mg once every week or every 2 weeks (q2w). All available concentration–time data (12,567 concentration–time points) up to a predefined cut-off date (October 31, 2014) were included in the analysis, and at least 80% of patients were from two phase III open-label studies (NCT02121210 and NCT02057250). Table 2 reports baseline patient demographics. All clinical studies were performed in accordance with the principles of the Declaration of Helsinki and all applicable amendments by the World Medical Assemblies and the International Conference on Harmonisation Guidelines for Good Clinical Practice. Details on patient-level data are provided in Appendix 1 of the Electronic Supplementary Material (ESM).
2.2 Bioanalysis
Concentrations of functional sarilumab in serum (i.e., sarilumab with one or two sites available for target binding), representing the pharmacologically active form of the drug, were measured using a validated enzyme-linked immunosorbent assay with a lower limit of quantification (LLOQ) of 0.294 mg/L (NCT01055899, NCT01026519, NCT01011959, NCT01328522, NCT01850680, and NCT01061736) or 0.313 mg/L (NCT02097524, NCT02017639, NCT01768572, NCT02057250, and NCT02121210) [13]. Immunogenicity was assessed using an electrochemiluminescence-based bridging assay. Neutralizing antibodies (NAbs) were assessed in phase III study samples that were positive for anti-drug antibodies (ADAs) using a validated competitive ligand-binding assay.
2.3 Data Handling
Patients who received placebo only were excluded from the analysis. For sarilumab-treated patients, complete patient data or specific serum concentration data could be excluded for any of the following: predose sarilumab concentration greater than LLOQ before treatment initiation; date or time of blood sample missing; concentration below LLOQ; obvious inconsistencies between sampling and dosing information; and outliers. Outliers and missing data were handled as described in Appendix 1 of the ESM.
2.4 Population-Pharmacokinetic Analysis
The Pop-PK analysis was performed using NONMEM 7.2.0 (ICON plc, Dublin, Ireland) software on a Linux cluster of multiprocessor computers. Parameter estimations were performed using the first-order conditional estimation method with the interaction option.
2.5 Base Model Development
Based on prior pharmacokinetic knowledge of sarilumab, an M–M model was evaluated [14]. Population parameters [fixed effects: maximum elimination rate (Vm), M–M constant (Km), apparent linear clearance (CLO/F), apparent volume of distribution for central compartment (Vc/F), apparent intercompartment rate constant (Q/F); random effects: inter-individual variability (η), and residual intra-individual variability (ε)] together with the individual estimates were computed assuming no dependency existed between pharmacokinetic parameters and covariates. Different combinations of residual (proportional and combined error) and structural models were tested, and the best structural model was chosen based on examination of objective functional value and visual inspection of goodness-of-fit (GOF) plots. Models were fitted to the data using a log-transformed two-sided approach. Sarilumab concentrations were log-transformed in the final model.
2.6 Covariate Screening and Inclusion
The following covariates were evaluated: demographic characteristics (age, sex, body weight, body mass index, and race); renal function (serum creatinine and creatinine clearance [CrCl]); liver function (aspartate aminotransferase, alanine aminotransferase, albumin, and total bilirubin); baseline C-reactive protein (CRP); baseline 28-joint Disease Activity Score with CRP (DAS28-CRP); prior and concomitant treatments (methotrexate and biologics); ADAs; NAbs; and sarilumab drug product (DP1, used in phase I studies; DP2, used in some phase I studies and the dose-ranging phase II study; and DP3, used in phase III studies and the commercial product). Except baseline DAS28-CRP and baseline CRP, all continuous covariates (age, body weight, body mass index, and renal and liver function tests) were assessed as time-varying covariates.
As the individual laboratories defined their own upper limit of normal (ULN), values of alanine aminotransferase, aspartate aminotransferase, albumin, creatinine, and total bilirubin were normalized to their respective ULN. ADAs in the present analysis were categorized either as a time-varying covariate (primary assessment in the covariate model) or at the patient level (ADA positive or negative; as a sensitivity analysis). Baseline DAS28-CRP, prior biologic treatment, and NAbs were not available as covariates for all studies, thus their effects on sarilumab exposure were tested only by comparison of post hoc predicted pharmacokinetic parameters; exposures were compared at different ranked levels of the specific covariate (i.e., univariate analysis), and other covariates across the ranks of this covariate were not necessarily balanced. Relationships between individual pharmacokinetic estimates and the above-mentioned covariates were investigated by graphical inspection of posterior individual estimates or inter-individual variability (ɳs) values vs. covariates using Xpose [15].
A covariate regression model was constructed using a forward-addition and backward-deletion method. During forward selection, covariates were added individually to the model; those providing statistically significant changes (p < 0.01, log likelihood ratio test) in the objective functional value were retained. During backward deletion, only covariates associated with significant changes in the objective functional value (p < 0.001) were retained in the final model. Model selection was also guided by visual inspection of GOF plots and model performance. Statistically significant covariates were assessed in terms of biologic plausibility and clinical implications.
2.7 Model Verification
The quality of the Pop-PK model was extensively evaluated at base model and final model stages using standard GOF criteria, as well as by the condition, number and estimation of η and residual intra-individual variability shrinkage. The final Pop-PK model was evaluated by visual predictive check (VPC), and a bootstrap method was used to test robustness of the model and accuracy of parameter estimates. A VPC was conducted according to Monte Carlo simulation to generate > 200 replicates that reflected the demographics of patients included in the Pop-PK analysis. Observations below the LLOQ (BQL) were retained in the final dataset.
One thousand bootstrap sets were resampled on patient identification with replacement. For each bootstrap set, the Pop-PK parameters were re-estimated and summarized by mean, median, and 2.5th and 97.5th percentiles. Model stability was evaluated by computation of the percentage of successful runs.
2.8 Visualization of Covariate Effects
The impact of a covariate on steady-state exposure over a range of values in the current patient dataset was assessed using a simulation approach, where all covariates were kept the same (except for the one being assessed) to deduce true covariate effects. For continuous covariates, the exposures for the 25th and 75th percentiles of the covariate were compared to exposure for the median value. For categorical covariates, exposure for one category of the covariate was compared to another category. The impact of ADA on sarilumab was assessed in patients who were ADA negative before initiation of sarilumab treatment. The impact of each covariate on sarilumab was graphically compared to the typical patient by incorporating the parameter’s uncertainty (derived from a bootstrap of the final model).
3 Results
3.1 Patient Characteristics
Sarilumab serum concentration data were obtained from 1935 adult patients (12,567 concentration–time points). The initial dataset for the Pop-PK analysis consisted of sarilumab data from 1770 patients (7676 concentration–time points) after excluding outliers (0.1%) and the placebo (6.1%), pre-dose BQL (12.0%), pre-dose > LLOQ (0.1%), and post-dose BQL (19.7%) samples. The final data set was constructed following exclusion of outlier concentrations. Overall, data for 16 sarilumab concentrations, consistently associated with large conditional weighted residuals (> 4) in different base models, were identified as outliers and excluded from the initial dataset. The final model was re-run to ensure that removal of outliers did not bias the final model parameter estimates. Baseline patient demographics in the final dataset are shown in Table 2.
3.2 Base Model
The base model used for covariate screening was a two-compartment model with first-order absorption from the depot to the central compartment with parallel linear and nonlinear (i.e., M–M) elimination from the central compartment and with η in CLO/F, Vc/F, absorption rate constant (Ka), and Vm (Fig. 1). Values for base-model estimated Pop-PK parameters Vm, M–M constant, Vc/F, CLO/F, Ka, apparent intercompartment rate constant, and apparent volume of distribution for the peripheral compartment are shown in Table 3. The final model used log-transformed sarilumab concentrations and an additive residual error model for residual (intra-individual) variability (σ2).
Scheme of the base model. C sarilumab concentration, CLO apparent clearance, Ka absorption rate constant from depot, Km Michaelis–Menten constant, Q apparent distribution, SC subcutaneous, Vc apparent volume of central compartment, Vm maximum elimination rate, Vp apparent volume of peripheral compartment
3.3 Covariate Model
The distributions of and correlations between continuous baseline covariate values in the final dataset, as well as major co-linearity (correlation coefficient > 0.5), are shown in Fig. 1 of the ESM. Covariates included in the forward inclusion steps were: body weight, albumin, ADA, DP2, sex, and CrCl on CLO/F; body weight, albumin, CrCl, and baseline CRP on Vm; DP2 on Ka; and body weight on Vc/F. Of these, albumin and CrCl on CLO/F and body weight on Vc/F were not statistically significant in the backward elimination step and were therefore eliminated.
3.4 Final Population Model
The final Pop-PK model parameters are shown in Table 3. Equations for the final model were as follows, in which parameters of fixed effect are denoted as θ, and η refers to inter-individual random effects that were parameterized using an exponential variance model:
where ADA is the anti-drug antibody, ALBR is albumin normalized to the ULN, BLCRP is baseline C-reactive protein, BSA is body surface area, CrCl is BSA-normalized creatinine clearance, DP2 is sarilumab drug product 2, and WT is body weight; detailed descriptions of all abbreviations are given in Table 3. The typical patient was female with a body weight of 71 kg, with serum albumin of 38 g/L (or albumin of 0.78 g/L normalized to ULN), CrCl of 100 mL/min, baseline CRP of 14.2 mg/L, ADA negative, and drug product administered (DP1 or DP3).
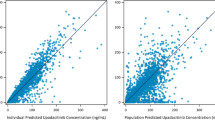
The basic GOF plots (Fig. 2) indicate that the final model was consistent with observed data, and no systematic bias was evident. No apparent bias was observed across different studies (data not shown). Remaining trends of random effects (ηs) related to covariate factors were also explored (data not shown), with no obvious trends observed.
The VPC plots for the final model are presented for the pharmacokinetic profiles after single (Fig. 3a) or repeated (Fig. 3b) doses. The majority of observed sarilumab concentrations fell within the 95% confidence intervals of the predicted concentrations, suggesting that the model accurately captured the variability in observed data. The VPC suggested that the model slightly over-predicted the low concentrations, possibly owing to exclusion of BQL data during model fitting.
Final model visual predictive check after a single dose of sarilumab 150 or 200 mg and multiple doses of sarilumab 150 or 200 mg every 2 weeks (q2w) in linear scale (a, b) and log scale (c, d). In these figures, solid and dashed red lines connect the median and bounds (i.e., 2.5th and 97.5th percentiles) of observed concentrations at each time point. Red and blue rectangles represent the median and bounds, respectively, of predicted concentrations at each time point. For each simulated dataset (out of a large number of simulated datasets, e.g., 500), the percentiles (i.e., the median and 2.5th and 97.5th percentiles) of simulated concentrations for each time point were computed, then the percentiles obtained from 500 simulated datasets were pooled, and 95% confidence intervals for each percentile were computed and represented as rectangles
The model’s performance for predicting BQL data was also assessed by VPC. The fraction of BQL data within one time bin after simulation was computed taking the LLOQ as 0.3 mg/L; this was then compared against the fraction of BQL data in the observed data in that specific time bin. Using this approach, the predicted fraction of BQL data was lower than the observed (e.g., the fraction of BQL data after 3 and 12 months’ repeated sarilumab 200-mg q2w treatment was approximately 10%, but the predicted fraction was slightly above 5%). When a doubling of the LLOQ (i.e., 0.6 mg/L) was used as the threshold to categorize BQL data in the simulated data, the fraction of predicted BQL data was in good agreement with the observed value, suggesting that the over-prediction of low concentrations was minimal. Analysis of the model’s performance using normalized prediction distribution errors provided similar conclusions to those derived from the VPC and bootstrap analyses (data not shown).
3.5 Impact of Covariates on Sarilumab Exposure
Body weight, ADA status, drug product, albumin, sex, CrCl, and baseline CRP were identified as significant covariates influencing sarilumab pharmacokinetics. The impact of covariates on steady-state sarilumab exposure over the range of 25th–75th percentiles of covariates vs. the median (or ‘with’ vs. ‘without’ one specific categorical covariate) is described below and shown in Fig. 4 and Table 1 of the ESM.
Impact of covariates on sarilumab steady-state exposure for (a) sarilumab 150 mg every 2 weeks (q2w) and (b) sarilumab 200 mg q2w displayed as ratio (open circle) with 90% confidence interval (short horizontal line) relative to a typical patient. AUC area under the plasma concentration–time curve, BSA body surface area, Cmax maximum serum concentration, Ctrough serum concentration observed before drug administration during repeated dose administration
The main source of intrinsic pharmacokinetic variability in sarilumab exposure in serum was body weight. Compared with a typical 71-kg (median) patient receiving sarilumab 150 or 200 mg q2w, respectively, the area under the plasma concentration–time curve over the 14-day dosing interval (AUC0–14d) for an 83-kg patient was 23% and 20% lower, while for a 62-kg patient it was 25% and 20% higher. Sarilumab exposure was also lower in male patients than in female patients by 12% and 14% (AUC0–14d) with the 150-mg q2w and 200-mg q2w doses, respectively. Sarilumab exposure decreased as the serum albumin level decreased over the analysis range of 25–53 g/L (median 38 g/L). With a decrease in the albumin level from 38 g/L to 34.8 g/L, the decrease in AUC0–14d was 15% for sarilumab 150 mg q2w and 9% for sarilumab 200 mg q2w; with an increase in the albumin level from 38 g/L to 40.2 g/L, the increase in AUC0–14d was 8% and 5% with 150 mg q2w and 200 mg q2w, respectively. Sarilumab AUC0–14d increased with a decline in CrCl; a decrease in CrCl from 100 mL/min to 83.0 mL/min increased AUC0–14d by 8% at 150 mg q2w and 4% at 200 mg q2w. In contrast, an increase in CrCl from 100 mL/min to 120 mL/min resulted in a decrease in AUC0–14d of 7% and 5% at 150 mg q2w and 200 mg q2w, respectively. Sarilumab exposure increased with a decline in baseline CRP. When CRP decreased from the baseline value of 14.2 mg/L (median) to 6.63 mg/L or increased from 14.2 mg/L to 28.3 mg/L, the change in AUC0–14d was < 5% for either drug dose. Sarilumab exposure was lower by < 22% for the DP2 drug product compared with the other drug products, but this is of no relevance, as DP2 was neither used in the pivotal phase III studies nor is it the commercial formulation.
When sarilumab exposure was evaluated with respect to patient ADA status, exposure was lower in ADA-positive patients than in ADA-negative patients by 24% and 28% (AUC0–14d) at 150-mg q2w and 200-mg q2w dosages, respectively (Fig. 5). Additionally, sarilumab exposure in NAb-positive patients was lower than in NAb-negative patients based on post hoc graphical inspection of predicted exposure data (Fig. 2 of the ESM). Post hoc graphical inspection of sarilumab exposure after repeated dosing showed baseline DAS28-CRP and prior use of biologics for RA treatment had no appreciable impact on sarilumab pharmacokinetics (Fig. 5).
Box plot of sarilumab area under the plasma concentration–time curve from day 0 to day 14 (AUC0–14d) in patients with rheumatoid arthritis in phase III studies by covariates of general interest. The number above each box plot represents the number of patients in the corresponding group. ADA anti-drug antibody, coadmin, coadministration, q2w every 2 weeks
3.6 Pharmacokinetics of Sarilumab
Parameters for model-estimated individual sarilumab steady-state exposure after repeated dosing of sarilumab 150 mg q2w and 200 mg q2w in the two pivotal phase III studies are shown in Table 4. The simulated time course of population mean sarilumab serum concentrations over 48 weeks following 150- and 200-mg q2w dosing suggested steady-state exposure was reached at 14–16 weeks with a two- to threefold accumulation. Steady-state exposure (AUC0–14d) increased twofold with a 1.3-fold increase in dose (150–200 mg q2w) [6]. Sarilumab serum concentrations after the last steady-state dose were measurable up to a median time of 28 and 43 days for 150 mg and 200 mg q2w, respectively.
Over the dosing interval at the therapeutic doses investigated, target-mediated clearance represented a large portion of total clearance (Fig. 6). Compared with sarilumab 150 mg q2w, the 200-mg q2w dose resulted in more pronounced saturation of the nonlinear clearance pathway over the dosing interval. The absolute bioavailability of a sarilumab subcutaneous injection was estimated to be 80% using the final dataset including the limited intravenous pharmacokinetic data (four patients who received sarilumab 0.6 mg/kg intravenously; two patients who received sarilumab 2.0 mg/kg intravenously).
4 Discussion
The pharmacokinetics of sarilumab in patients with RA was adequately described by a two-compartment model with first-order absorption and parallel linear and nonlinear M–M elimination. This analysis showed that the pharmacokinetics of sarilumab can be accurately predicted using this model, allowing characterization of sarilumab pharmacokinetics in the target population of patients with RA, and prediction of individual exposure of sarilumab in serum.
Of the two parallel clearance pathways (nonlinear, target-mediated pathway and nonspecific, linear pathways), target-mediated clearance represents a large portion of total clearance in the range of serum concentrations achieved over the dosing interval at therapeutic sarilumab doses. Notably, there is more pronounced saturation of the nonlinear clearance pathway over the dosing interval at 200 mg q2w than at 150 mg q2w, consistent with sarilumab exposure in serum increasing in a greater than dose-proportional manner from 150 to 200 mg q2w. As steady-state exposure of sarilumab increased twofold from 150 to 200 mg q2w dosing, it is important to understand that a 1.3-fold reduction in sarilumab dose from 200 to 150 mg q2w in patients with RA actually reduces sarilumab exposure in serum by half.
This model enabled the identification of covariates that explained part of the inter-individual variability of sarilumab. Of these, body weight was the main source of intrinsic pharmacokinetic variability of sarilumab. In the Pop-PK analysis across patients with RA weighing 32–177 kg, body weight significantly influenced CLO/F and Vm, resulting in increased exposure of sarilumab in serum with decreased body weight. The impact of body weight on sarilumab clearance was translated into an impact on sarilumab steady-state exposure. The greater effect of body weight on sarilumab steady-state exposure at 150 mg q2w vs. 200 mg q2w is consistent with the impact of body weight on nonlinear clearance, which is predominant at lower concentrations, in addition to the impact on linear clearance. Notably, the effect of body weight was more prominent on minimum concentration at steady state after repeated dosing than on AUC0–14d and maximum concentration; this is owing to nonlinear pharmacokinetics exhibited by sarilumab and greater effects of covariates at lower drug concentrations.
The effect of body weight on sarilumab pharmacokinetics is consistent with clinical efficacy findings, which showed a trend for lower American College of Rheumatology 20% improvement criteria response rates for sarilumab vs. placebo at 150 mg q2w in patients weighing ≥ 100 kg vs. those weighing 60 to < 100 kg [14]. Subgroup analyses for treatment-emergent adverse events, infections, serious adverse events, and treatment-emergent adverse events leading to discontinuation showed no consistent trend with increasing weight or body mass index. While absolute neutrophil count < 1.0 G/L was observed more frequently in patients weighing < 60 kg than in heavier patients, this was not associated with increased serious infections [14]. Therefore, despite the influence of body weight on sarilumab pharmacokinetics, no dose adjustment is recommended based on body weight [6]. The recommended dose of sarilumab is 200 mg q2w; dose reduction to 150 mg q2w is recommended for the management of neutropenia, thrombocytopenia, and liver enzyme elevations [6, 7].
Other statistically significant covariates included ADA status, drug product, albumin, sex, CrCl, and baseline CRP. Except for drug product and ADA status, the magnitude of impact of most of these covariates was approximately within ± 10% change and less than the effect of body weight (Table 1 of the ESM). For example, sarilumab exposure decreased with increased baseline CRP; however, at both sarilumab doses, a change from median baseline CRP (14.2 mg/L) to 6.63 mg/L (25th percentile) or 28.3 mg/L (75th percentile) resulted in < 5% change in AUC0–14d. The impact of the drug product (lower sarilumab exposure for DP2 vs. DP1 and DP3) is not relevant as only DP3 is marketed. ADA status influenced CLO/F, with a resultant trend toward higher apparent clearance of sarilumab and lower exposure in the presence of ADAs. ADA status was included in the final model as a time-varying covariate; however, the assumption that the ADA was positive from treatment initiation for the simulations that assessed the effect of the ADA on sarilumab exposure in serum may have overestimated the magnitude of this covariate’s impact. This may represent the worst-case scenario of ADA effect. Thus, none of the statistically significant covariates identified in the model are clinically meaningful and hence, no dose adjustment is required for these patients’ characteristics.
Age, race, concomitant methotrexate, total bilirubin, creatinine clearance, aspartate aminotransferase, and alanine aminotransferase did not have a statistically significant effect on the pharmacokinetics of sarilumab in the final model. The lack of effect on laboratory parameters is also consistent with the limited involvement of the kidneys and liver in monoclonal antibody clearance. Furthermore, post hoc assessment of sarilumab exposure in serum after repeated dosing showed baseline DAS28-CRP and prior use of biologics had no appreciable impact on sarilumab pharmacokinetics.
The recommended dosage of sarilumab is 200 mg q2w, which can be reduced to 150 mg q2w to manage neutropenia, thrombocytopenia, and liver enzyme elevations [6, 7]. This recommendation is supported by the exposure–response analyses conducted using data from phase III studies over the dose range of 150–200 mg q2w and ensures a greater likelihood of achieving a clinical response while being able to manage the only dose-related safety endpoint of absolute neutrophil count [16].
5 Conclusion
The pharmacokinetics of sarilumab was described by a two-compartment model with first-order absorption and parallel linear and nonlinear (M–M) elimination, which allowed for characterization of sarilumab pharmacokinetics in the target patient population of patients with RA, and estimation of exposure in individual patients. Compared with sarilumab 150 mg q2w, the 200-mg q2w dose resulted in more pronounced saturation of the nonlinear clearance pathway over the dosing interval and, hence, a greater than dose-proportional increase in exposure over this dose range. Increasing the sarilumab dose by one-third from 150 mg q2w to 200 mg q2w resulted in a twofold increase in AUC0–14d. Body weight, ADA status, sarilumab drug product, albumin, sex, CrCl, and baseline CRP, but not age, race, concomitant treatments, or liver function tests, were identified as significant covariates influencing sarilumab pharmacokinetics. Evidence indicates that the impact of these covariates on exposure does not translate into clinically meaningful differences in efficacy or safety. Therefore, no adjustment in the sarilumab dose is required for body weight or any of the other patient characteristics assessed, including age, sex, and race.
References
Kishimoto T. The biology of interleukin-6. Blood. 1989;74(1):1–10.
Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122(4):143–59.
Yoshida Y, Tanaka T. Interleukin 6 and rheumatoid arthritis. Biomed Res Int. 2014;2014:698313.
Kim GW, Lee NR, Pi RH, Lim YS, Lee YM, Lee JM, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res. 2015;38(5):575–84.
Dayer JM, Choy E. Therapeutic targets in rheumatoid arthritis: the interleukin-6 receptor. Rheumatology (Oxford). 2010;49(1):15–24.
Kevzara. US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf. Accessed 6 Apr 2019.
Kevzara. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004254/WC500230068.pdf. Accessed 6 Apr 2019.
Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–50.
Emery P, Rondon J, Garg A, van Hoogstraten H, Graham N, Liu M, et al. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with RA [abstract no. 971]. Arthritis Rheumatol. 2015;67(Suppl. 10):971.
Belomestnov P, Hamilton JD, DiCioccio AT, Jasson M, Radin AR. Sarilumab, a subcutaneously administered fully human monoclonal antibody inhibitor of the IL-6 receptor: pharmacokinetic profile and its relationship to changes in pharmacodynamic markers in patients with rheumatoid arthritis [abstract no. 1337]. ACR/ARHP Annual Meeting; 9–14 Nov, 2012; Washington, DC.
Dua P, Hawkins E, van der Graaf PH. A tutorial on target-mediated drug disposition (TMDD) models. CPT Pharmacometrics Syst Pharmacol. 2015;4(6):324–37.
Yan X, Mager DE, Krzyzanski W. Selection between Michaelis–Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2010;37(1):25–47.
Andisik M, DeStefano L, Stefan C, Gathani M, Laurino K, Lai V, et al. Effects of blood processing and sample storage on the stability of biotherapeutics and anti-drug antibodies. Bioanalysis. 2015;7(11):1325–35.
US Food and Drug Administration. Center for drug evaluation and research. Clin Pharmacol Biopharm. 2015;351:37.
Jonsson EN, Karlsson MO. Xpose: an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51–64.
Zhaoling Meng Z, Sebastien B, Sun S, Rigaux-Lampe C, Paccaly A, Xu C, et al., editors. Exposure-response modeling for sarilumab dose regimens [abstract no. 7285]. Population Approach Group in Europe; 6–9 June, 2017; Budapest.
Acknowledgments
The authors acknowledge the contribution of all study investigators and patients. In particular, the authors acknowledge Vicky C.H. Lai for providing support in the bioanalytical analyses of samples, and Pascale Boittet for providing programming support for the population-pharmacokinetic analysis. Editorial support for the development of this manuscript was provided by Vicki Cronin, PhD, Adelphi Group, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Conflict of interest
Christine Xu and Vanaja Kanamaluru are employees of Sanofi Genzyme, and may hold stock and/or stock options in the company. Yaming Su is a former employee of Sanofi Genzyme, and may hold stock and/or stock options in the company. Anne Paccaly is an employee of Regeneron Pharmaceuticals, Inc., and may hold stock and/or stock options in the company. None of the authors have any non-financial conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xu, C., Su, Y., Paccaly, A. et al. Population Pharmacokinetics of Sarilumab in Patients with Rheumatoid Arthritis. Clin Pharmacokinet 58, 1455–1467 (2019). https://doi.org/10.1007/s40262-019-00765-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00765-1