Abstract
Background and Objectives
Durvalumab and atezolizumab are approved as first-line therapy in extensive-stage small-cell lung cancer. Although cost-effectiveness analyses compared these immunotherapy drugs with standard chemotherapy-alone regimens, no head-to-head cost-effectiveness comparisons for these treatments exist. The aim of the present analysis is to determine the cost-effectiveness of durvalumab and atezolizumab as first-line therapy for extensive-stage small-cell lung cancer from the US payers’ perspective.
Methods
This study is based on two placebo-controlled, phase 3 clinical trials: CASPIAN and IMpower133. A Markov model was developed to simulate the three health states: progression-free survival, progressed disease, and death in patients with extensive-stage small-cell lung cancer. Transition probabilities were estimated from the clinical trial survival curves and extended with life-time modelling. Health utilities and direct costs of adverse event treatment were included. Main outcome was the incremental cost-effectiveness ratio (ICER) using quality-adjusted life-years saved (QALYS). Sensitivity analysis was performed to assess the impact of variables on the ICER.
Results
Durvalumab group has a cost of $187,503 with an effectiveness of 1.08 while atezolizumab has a cost of $160,219 and an effectiveness of 0.932. Durvalumab is not cost-effective compared to atezolizumab with an ICER of $165,182 QALYS, which is over the willingness-to-pay threshold of $150,000. The model was most sensitive to durvalumab cost and the cost of treating durvalumab adverse effects.
Conclusions
With the ICER of durvalumab treatment group being very close to $150,000, setting a higher willingness-to-pay threshold or decreasing the drug cost through contract pricing can increase the cost-effectiveness of durvalumab compared to atezolizumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The present cost-effectiveness analysis indicates that atezolizumab plus chemotherapy is the more cost-effective treatment compared with durvalumab plus chemotherapy in the treatment of small cell lung cancer. |
However, the durvalumab plus chemotherapy ICER of the durvalumab combination treatment is very close to the willingness-to-pay of $150,000. |
Since this is the first head-to-head comparison between first-line agents, this analysis provides value for clinical decision-making in determining which treatment to initiate or add to the formulary or if both can be added. |
1 Introduction
Small-cell lung cancer (SCLC) accounts for 15% of all lung cancers and is the leading cancer death among men and second among women worldwide [1]. The 5-year survival rate for patients diagnosed with SCLC is 5%, with only 2–4 months survival if untreated [1, 2]. About 60% of SCLC patients are diagnosed with extensive-stage SCLC due to poor detection and rapid tumor growth [3].
Chemotherapy plus immunotherapy is the preferred therapy for patients with extensive-stage SCLC with a median overall survival of 10 months [4]. The immunotherapy agents primarily used to treat extensive-stage SCLC are immune checkpoint inhibitors including first-line atezolizumab and durvalumab, which work by inhibiting PD-L1 to decrease tumor growth [4]. First-line treatment is a combination of chemotherapy, etoposide with a platinum agent, plus either atezolizumab or durvalumab checkpoint inhibitor; if patients fail first-line treatment, standard second-line therapy is topotecan [4]. Addition of immunotherapy in the setting of SCLC has shown promising results with regards to duration of response and survival benefit compared to chemotherapy alone [4].
A double-blind, placebo-controlled, phase 3 trial (IMpower133) comparing atezolizumab plus chemotherapy versus chemotherapy alone revealed that the combination treatment increased overall survival (OS) (12.3 vs. 10.3 months; HR = 0.70, p = 0.007) and progression-free survival (PFS) (5.2 vs. 4.3 months; HR = 0.77, p = 0.02) compared to carboplatin alone [5]. The CASPIAN open-label, randomized, multicenter, phase 3 clinical trial (CASPIAN) comparing durvalumab plus chemotherapy versus chemotherapy alone demonstrated that overall survival in the durvalumab plus chemotherapy combination group was increased to 13.0 months, compared with 10.3 months in the chemotherapy alone group (HR = 0.73, p = 0.0047) [6].
While both atezolizumab and durvalumab have increased the overall survival for patients, there is no head-to-head comparison of these two drugs. Additionally, the treatment cost for the combination regimen has increased significantly compared to the cost of chemotherapy alone. With the rise of immunotherapy use for SCLC, a cost-effectiveness analysis comparing each of these immunotherapies can guide clinical decision-making for the most efficient treatment. Although other studies have compared cost-effectiveness of each treatment and treatment combination with a chemotherapy alone, none have compared these two treatment combinations directly. The present study aims to determine the cost-effectiveness of durvalumab plus platinum-etoposide versus atezolizumab plus platinum-etoposide as first-line treatment of extensive-stage SCLC from an American payer perspective.
2 Patients and Methods
2.1 Patient Population
The patient population for our two treatment groups was treatment-naïve patients 18 years and older, with measurable and confirmed extensive-stage SCLC, following that of the IMpower133 and CASPIAN trials. Following IMpower133, patients in our model received four cycles of a 1200 mg dose of atezolizumab intravenously on day 1 of each 21-day cycle with carboplatin and etoposide, followed by maintenance therapy of atezolizumab 1200 mg alone every 21 days with a median treatment duration for atezolizumab of 4.7 months and a median seven doses administered. Following the CASPIAN trial treatment regimen, patients in our model received four 21-day cycles of etoposide and platinum plus durvalumab 1500 mg intravenously, followed by maintenance durvalumab 1500 mg every 4 weeks, for a median duration of 7 months and a median of seven durvalumab doses.
Since the patient population for our analysis is from two different trials, the distribution of patient characteristics in the groups receiving chemotherapy-alone of the two trials were reviewed carefully. These groups in both trials received the same chemotherapy agents—etoposide with a platinum agent (carboplatin in IMpower133 and either carboplatin or cisplatin in CASPIAN). They were also very similar across multiple factors including median age, sex, ECOG score, and presence of brain or CNS metastases. Progression-free survival and overall survival data are also very similar between the two groups receiving chemotherapy-alone (Fig. 1). The similarities of these groups allowed a one-to-one comparative cost-effectiveness analysis of the two immune checkpoint inhibitor treatments without using placebo differences to adjust treatment survival comparisons across the two trials [7].
2.2 Model Structure
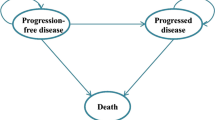
A Markov model using TreeAge Pro 2021 software was used to simulate the disease states of extensive-stage SCLC [8]. A Markov model is used to represent stochastic processes or random processes where a patient’s future states depend on the current state. Three health states were included in the model: progression-free survival (PFS), progressed disease (PD), and death (Fig. 2). Each state is associated with corresponding estimated cost and a transition probability, or the probability of moving from one state to another after each 1 month simulated cycle. The three-state Markov model was simulated with two treatment branch options: durvalumab plus etoposide/platinum and atezolizumab plus etoposide/platinum. All patients were initially placed in progression-free survival state and moved to a different state based on transition probabilities after one simulated cycle. The simulation continued for 360 months or until all entered the death state. The model’s main outcome was the incremental cost-effectiveness ratio (ICER) using quality-adjusted life-years (QALY) gained. The ICER is calculated as the difference in each comparator’s costs divided by the difference in each treatment’s QALYs.
2.3 Transition Probabilities
Overall survival and progression-free survival data was extracted from the published Kaplan–Meier curves from IMpower133 and CASPIAN trials using Engauge Digitizer software [9]. The digitization of data was done four times and the values were then averaged. Digitized Kaplan-Meier curves for each treatment were then extended to lifetime duration using Hoyle Henley approximations in R studio to find the best possible distribution [10]. The best distribution was chosen using a combination of the lowest Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) value and the best visual comparison as suggested by Gibson et al. [11]. Although the Weibull distribution produced the optimal AIC for atezolizumab and durvalumab, the log-logistic distributions were used for both treatment groups to produce survival curves that better matched published Kaplan-Meier curves and to provide the best possible comparison of data among the treatment groups (Fig. 3). Transition probabilities were calculated for “alive to alive,” “alive to progressed,” “progressed to progressed,” and “progressed to dead” states that determined the rate at which patients moved from state to state. To account for background mortality in patients who transition from the alive state directly to the dead state without disease progression, the probability of dying as reported in 2018 Social Security Mixed Life Tables was used starting with the mean patient age of 63 years [12].
2.4 Measurement of Costs
Total cost for two treatment groups included the cost of the two treatment drugs, the cost of follow-up drugs after progression, and the costs of treating grade 3 and 4 adverse events (Table 1). Medication treatment costs were calculated from wholesale acquisition cost obtained from Red Book [13]. The costs were adjusted to 2020 US dollars using the US Bureau of Labor Statistics CPI inflation calculator to keep prices consistent [14]. Cost calculations of weight-based dosing were based on an average weight of 70 kg and with the assumption that there was no drug wastage. Our analysis assumes that patients were on drug treatment until they entered the progressed disease state. The price of follow-up adjunctive chemotherapy treatment was adjusted by the percentage of participants on each etoposide/platinum regimen. Costs of secondary drug treatments for patients who transitioned to the progressed disease state were included as well.
Costs for grade 3 and 4 adverse events included adverse event-related treatment medications, diagnostic lab tests, monitoring lab tests, follow-up visits, outpatient visits, and hospitalization costs that were associated with a particular adverse event. The total cost for adverse events was calculated as a product of the cost of each adverse event and the proportion of patients who experienced the event. For instance, only 9% of patients had anemia; therefore, the weighted cost was derived by multiplying the total cost times 0.09. Grade 1 and 2 adverse events were not included in the analysis due to small cost impacts as they require minimal medical services. Patients who had neutropenia were assumed to have febrile neutropenia. Clinical visit costs, lab costs, and hospitalization costs were based on average length of stay reported in the AMA RBRVS Data Manager, the CMS clinical laboratory services, and Healthcare Cost and Utilization Project (HCUP) database, respectively [15,16,17].
2.5 Utilities
To our knowledge, data of utility values for SCLC are limited [18]. Therefore, health utility values for each health state and each adverse event were from previously published studies evaluating non-SCLC (NSCLC) [19,20,21,22]. This approach is consistent with other published cost-effectiveness studies for SCLC treatment [22, 23]. The health state utilities for PFS and PD are 0.673 and 0.473, respectively [19]. The disutility of adverse effects was determined by subtracting the utility of adverse effects from the PFS utility. Disutility of atezolizumab and durvalumab was calculated by determining the prevalence of each adverse effect in our respective patient populations. The proportion of patients experiencing each adverse effect was totaled to give an estimated disutility of our treatment drugs (Table 2) [19,20,21]. Disutilities associated with grade 3 and 4 anemia, thrombocytopenia, neutropenia, leukopenia, nausea, and diarrhea were based on the proportion of patients experiencing these adverse events in the atezolizumab and durvalumab groups. Disutilities associated with grade 3 and 4 pneumonia, pulmonary embolism, back pain, headache, hyponatremia, and hypertension were based on patients in the durvalumab group only, as grade 3 and 4 of these adverse events were not reported by patients receiving atezolizumab.
2.6 Sensitivity Analysis
One-way sensitivity analysis was performed to investigate potential threshold analyses and to identify variables that influence our cost-effectiveness analysis. The analysis was run using low-high ranges of ± 25% of our values.
A probabilistic sensitivity analysis using Monte Carlo simulations was also performed to explore factors that can influence the ICER. The Monte Carlo model assigns a randomly selected value from a chosen distribution for each parameter in the Markov model and runs these iterations 10,000 times. We determined an acceptability curve for each treatment using the Monte Carlo simulations.
3 Results
3.1 Base-Case Outcomes
The total cost for durvalumab treatment group is $187,503 with 1.08 QALYs gained. The total cost for atezolizumab treatment group is $160,219 with 0.91 QALYs gained. Thus, the durvalumab treatment group compared to the atezolizumab treatment group has an incremental cost of $27,284 and an incremental effectiveness of 0.17. Compared to the atezolizumab group, the durvalumab group had an ICER of $165,182 per QALY, which crosses the threshold of the willingness to pay (WTP) set at $150,000, as just slightly not cost-effective (Table 3).
3.2 Sensitivity Analysis
The results of the one-way sensitivity analysis are demonstrated in the tornado diagram in Fig. 4 to determine which factors affect the ICER values in the base case. Cost of durvalumab treatment, cost of atezolizumab treatment, and cost of durvalumab adverse events were the top three factors that impacted the ICER values. The disutility associated with durvalumab and atezolizumab adverse effects had the least impact on ICER (Fig. 4). Adjusting the drug cost of durvalumab downward by 13% would bring down the ICER to reach the WTP of $150,000 and become cost-effective.
The acceptability curve demonstrates that durvalumab does not consistently become cost-effective over atezolizumab unless the WTP is over the threshold of around $165,000 (Fig. 5). At this threshold, durvalumab starts to become more cost-effective for more than 50% of iterations, while atezolizumab is cost-effective at less than 50% of the iterations. Raising the WTP threshold shifts durvalumab to become cost-effective. For instance, at the WTP threshold of $200,000, durvalumab is more cost-effective 57% of the time, while atezolizumab is cost-effective for 43% of the iterations. Compared to the WTP threshold of $150,000, atezolizumab is cost-effective 54% iterations, while durvalumab is cost-effective at 46% iterations.
Cost-effectiveness acceptability curves (CEACs). CEAC is a curve used to indicate the probability of a drug being economical. The curves show the percentage of iterations the treatment protocol is cost effective based on the value of willingness to pay (WTP). The percentage of cost-effectiveness is produced by simulation of different treatment options
The cost-effective scatter plot demonstrates that at a cost lower than $160,000, there are more instances in which atezolizumab is more effective with effectiveness values between 0.6 and 1.4. At higher costs at around $180,000, there are more instances in which durvalumab is more effective with effectiveness values between 1.0 and 1.6 (Fig. 6).
4 Discussion
Our study is the first to directly compare the cost-effectiveness of atezolizumab plus chemotherapy versus durvalumab plus chemotherapy. In recent years, immunotherapy has shown clinical efficacy and been approved as first-line use for SCLC. Positive survival results from both the Impower133 trial and the CASPIAN trial provide clear evidence that different immune checkpoint therapies provide potentially better options for patients than chemotherapy agents [5, 6]. With the emergence of these immunotherapy options, it is important to compare cost-effectiveness of these therapies to chemotherapy alone and to each other from different healthcare system perspectives.
Cost-effectiveness analyses comparing checkpoint inhibitors to chemotherapy alone from an American perspective showed that both atezolizumab and durvalumab were less cost-effective for the WTP threshold than chemotherapy alone, most likely due to the high cost of immunotherapy compared to standard chemotherapy regimen [22,23,24,25]. Zhou et al. estimated the ICER of $528,810 per QALY for atezolizumab plus chemotherapy versus chemotherapy alone [22]. Three different studies evaluating the cost-effectiveness of durvalumab plus chemotherapy versus chemotherapy alone from a US healthcare system perspective calculated ICERs ranging from $216,953 to $355,448 to $464,712 per QALY, respectively [23,24,25]. The estimated ICER for atezolizumab is thus considerably higher than three different ICERs calculated for durvalumab, suggesting that durvalumab might be the more cost-effective strategy. Both therapy strategies require considerable reductions in cost to match cost-effectiveness of chemotherapy at WTP threshold of $150,000 per QALY [22,23,24,25].
A separate model by Liu et al. comparing the cost-effectiveness of three treatments—atezolizumab versus durvalumab versus chemotherapy alone—calculated ICERs of $382,469 and $464,593 per QALY for atezolizumab plus chemotherapy and durvalumab plus chemotherapy versus chemotherapy alone, respectively [26]. Comparing the higher ICER for durvalumab to a lower ICER for atezolizumab, researchers concluded that atezolizumab was more cost-effective compared to durvalumab [26]. However, such a comparison does not provide an ICER for durvalumab in relation to atezolizumab, nor does it assess a change in the cost of treatment that would shift the cost-effectiveness pendulum toward durvalumab. In addition, the results of the Liu et al. study are inconsistent with earlier studies that estimated durvalumab rather than atezolizumab to have a lower ICER [22,23,24,25]. All these studies compare atezolizumab and durvalumab to standard chemotherapy regimen; however, the preferred treatment is immunotherapy with chemotherapy. Additional long-term studies comparing these specific checkpoint inhibitors to each other are needed to contribute to best evidence-based decision making. Unlike previous studies, our study compares cost-effectiveness of two preferred treatments for extensive-stage SCLC.
Based on our pre-determined WTP limit of $150,000 per QALY, our cost-effectiveness analysis showed that durvalumab plus chemotherapy as first-line treatment of extensive-stage SCLC is not cost-effective compared to atezolizumab plus chemotherapy with an ICER of $165,182 from a US payer’s perspective. Therefore, atezolizumab plus chemotherapy is the more cost-effectiveness checkpoint inhibitor. Other health economists have argued for a higher threshold of $200,000–$300,000 based on increased healthcare spending over time, safety risks, and expensive oncology drugs [27]. The World Health Organization suggests a threshold of two to three times the per capita annual income [28]. Tripling $56,490 US per capita personal income in 2019 results in $169,470 WTP, which could also be used as a reasonable WTP threshold. For durvalumab to be more cost-effective, cost must be reduced by 13% to reach an ICER of $150,000 per QALY. Certain factors were most sensitive and had considerable effect on our study, although the cost of durvalumab has the greatest impact on the ICER.
Our study has several limitations that should be considered. First, the one-to-one comparative analysis of two different studies based on the similarities in patient population characteristics introduces potential variability in clinical efficacy of each treatment combination regimen. However, the closeness of the chemotherapy-alone outcomes and patient populations across trials shows that this comparison is likely sound. Secondly, the adverse effect costs were calculated based on the median duration of treatment, but there was no certainty that these side effects only lasted the estimated times. It was also assumed that patients only had the side effects once, which does not reflect the possibility of experiencing the side effect multiple times. Additionally, utility value data published for extensive-stage SCLC patients is limited, leading us to choose values from previously published literature on NSCLC [18, 22, 23]. This choice could lead to some variability in ICER values. Lastly, though our distribution curves had the best AIC score with Weibull distribution, the log-logistic curve was chosen in order to formulate survival curves. These curves had the best fit visually to both the Kaplan-Meier curves in the clinical trials and as suggested by Gibson et al., it is best to include visual inspection as well as statistical tests when choosing a best fit curve [11].
5 Conclusion
The present cost-effectiveness analysis indicates that atezolizumab plus chemotherapy is the more cost-effective treatment compared with durvalumab plus chemotherapy in the treatment of SCLC. However, the durvalumab plus chemotherapy ICER of the durvalumab combination treatment is very close to the WTP of $150,000. There are some instances of oncologic drug comparisons that set a WTP to a higher value as costs of healthcare and safety risk have increased. Additionally, changes in drug costs through contract pricing can alter the cost-effectiveness of our durvalumab versus atezolizumab comparison. Since this is the first head-to-head comparison between first-line agents, this analysis provides value for clinical decision-making in determining which treatment to initiate or add to the formulary or if both can be added.
References
Basumallik N, Agarwal M. Small cell lung cancer. StatPearls 2020. Updated: 2021 Jul 17. https://www.ncbi.nlm.nih.gov/books/NBK482458/.
Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol. 2009;471:469–86.
Pakkala S, Owonikoko TK. Immune checkpoint inhibitors in small cell lung cancer. J Thorac Dis. 2018;10(Suppl 3):S460–7.
Pacheco JM. Immunotherapy for extensive stage small cell lung cancer. J Thorac Dis. 2020;12(10):6212–24.
Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9.
Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39.
Watson TR, Gao X, Reynolds KL, et al. Cost-effectiveness of pembrolizumab plus axitinib vs nivolumab plus ipilimumab as first-line treatment of advanced renal cell carcinoma in the US. JAMA Netw Open. 2020;3(10): e2016144. https://doi.org/10.1001/jamanetworkopen.2020.16144.
TreeAge Software Pro 2021, R1. TreeAge Software, Williamstown, MA; software available at http://www.treeage.com.
Mitchell M, Muftakhidinov B, Winchen T, et al. Engauge digitizer software. http://markummitchell.github.io/engauge-digitizer. Accessed 13 June 2021.
Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139.
Gibson E, Koblbauer I, Begum N, et al. Modelling the survival outcomes of immuno-oncology drugs in economic evaluations: a systematic approach to data analysis and extrapolation. Pharmacoeconomics. 2017;35(12):1257–70.
Arias E, Xu JQ. United States life tables, 2018. National Vital Statistics Reports. 2020; 69(12).
RED BOOK search—MICROMEDEX. https://www-micromedexsolutions-com.ucsf.idm.oclc.org/micromedex2/librarian/PFDefaultActionId/redbook.ModifyRedBookSearch. Accessed 13 June 2021.
CPI Inflation Calculator. https://www.bls.gov/data/inflation_calculator.htm. Accessed 13 June 2021.
RBRVS DataManager | RBRVS Data Manager.title. https://ocm.ama-assn.org/OCM/DataManager/AccessDenied.do?url=https%3a%2f%2focm.ama-assn.org%2fOCM%2fWEB-INF%2fpages%2fDataManager%2fGeneralInformation.jsp. Accessed 13 June 2021.
Clinical Laboratory Fee Schedule. Center for Medicare and Medicaid Services (CMS). U.S. Centers for Medicare & Medicaid Services, Baltimore, MD. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files. Accessed 13 June 2021.
HCUPnet. Healthcare Cost and Utilization Project (HCUP). 206-2009. Agency for Healthcare and Research and Quality, Rockville, MD. http://hcupnet.ahrq.gov/. Accessed 13 June 2021.
Dasari A, Pathak S, Aggarwal A, Singh R, Goyal R. PCN59 health utility values in small cell lung cancer: a literature review. Value Health. 2021;24(Supp 1):S30. https://doi.org/10.1016/j.jval.2021.04.151.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. https://doi.org/10.1186/1477-7525-6-84.
Doyle S, Lloyd A, Walker M. Health state utility scores in advanced non-small cell lung cancer. Lung cancer (Amst, Neth). 2008;62(3):374–80. https://doi.org/10.1016/j.lungcan.2008.03.019.
Handorf EA, McElligott S, Vachani A, Langer CJ, Bristol Demeter M, Armstrong K, Asch DA. Cost-effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncol Pract. 2012;8(5):267–74. https://doi.org/10.1200/JOP.2011.000502.
Zhou K, Zhou J, Huang J, Zhang N, Bai L, Yang Y, Li Q. Cost-effectiveness analysis of atezolizumab plus chemotherapy in the first-line treatment of extensive-stage small-cell lung cancer. Lung Cancer. 2019;130:1–4. https://doi.org/10.1016/j.lungcan.2019.01.019.
Zhang L, Hang Y, Liu M, Li N, Cai H. First-line durvalumab plus platinum-etoposide versus platinum-etoposide for extensive-stage small-cell lung cancer: a cost-effectiveness analysis. Front Oncol. 2020;10: 602185. https://doi.org/10.3389/fonc.2020.602185.
Ding D, et al. Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J Natl Compr Cancer Netw. 2021;19(10):1141–7. https://doi.org/10.6004/jnccn.2020.7796.
Lin, et al. First-line durvalumab in addition to etoposide and platinum for extensive-stage small cell lung cancer: a U.S.-based cost-effectiveness analysis. Oncologist. 2021;26(11):e2030–e2020. https://doi.org/10.1002/onco.13954.
Liu, et al. First-line chemo-immunotherapy for extensive-stage small cell lung cancer: a United States-based cost-effectiveness analysis. Front Oncol. 2021;11: 699781. https://doi.org/10.3389/fonc.2021.699781.
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. https://doi.org/10.1056/NEJMp1405158.
McDougall JA, Furnback WE, Wang B, Mahlich J. Understanding the global measurement of willingness to pay in health. J Mark Access Health Policy. 2020;8(1):1717030. https://doi.org/10.1080/20016689.2020.1717030.
Acknowledgements
We thank the reviewers of the manuscript and the editors for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was used for the study.
Conflict of interest
Yelena Ionova, Wilson Vuong, Omar Sandoval, Jodie Fong, Vincent Vu, Lixian Zhong, and Leslie Wilson declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Available upon request.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Wilson Vuong, Omar Sandoval, Jodie Fong, Vincent Vu, and Lixian Zhong. The first draft of the manuscript was written by Yelena Ionova and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ionova, Y., Vuong, W., Sandoval, O. et al. Cost-Effectiveness Analysis of Atezolizumab Versus Durvalumab as First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer in the USA. Clin Drug Investig 42, 491–500 (2022). https://doi.org/10.1007/s40261-022-01157-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01157-3