Abstract
Background and Objective
Technosphere® Insulin (TI), a human insulin powder for inhalation (Afrezza®; MannKind Corporation, Westlake Village, CA, USA), is an ultra-rapid-acting inhaled insulin indicated to improve postprandial glycemic control in patients with type 1 or type 2 diabetes mellitus (T1DM or T2DM). Because TI is absorbed across the alveolar membrane, the objective of this analysis was to characterize its pulmonary safety.
Methods
Pooled data from 13 phase 2/3 clinical studies in 5505 patients with T1DM or T2DM treated with TI, Technosphere inhalation powder without insulin (TP; placebo), or active-comparator treatment were analyzed for incidences of respiratory treatment-emergent adverse events (TEAEs), changes in pulmonary function, and lung malignancies. Radiographic changes in the lungs were monitored in a subset of 229 patients.
Results
Among 3017 patients receiving TI, the median duration of TI exposure was 168 days; median active-comparator and TP exposure durations were 363 and 149 days for 2198 and 290 patients, respectively. Respiratory TEAEs were comparable across treatments, except for a higher incidence of mild cough with TI in active-comparator studies (28.0% vs. 5.2%). Slight reversible declines in pulmonary function from baseline were observed for TI versus TP and active-comparator treatments, including in a subpopulation of patients with retrospectively identified lung dysfunction. Lung malignancies were reported in two patients on active TI therapy with a smoking history. No clinically significant changes from baseline were observed in radiographic images.
Conclusions
Pulmonary safety assessment of the TI inhalation system did not identify any safety issues in individuals with either T1DM or T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pulmonary safety of Technosphere Insulin, an ultra-rapid-acting prandial insulin that is inhaled through the mouth and absorbed via the alveoli, is of clinical interest, and detailed safety information is reviewed here. |
In this pulmonary safety analysis, data are assessed from 13 pooled phase 2/3 clinical trials with Technosphere Insulin and demonstrate that respiratory treatment-emergent adverse events, changes in pulmonary function from baseline, and rates of lung malignancies with inhaled Technosphere Insulin, active-comparator, and placebo treatment groups are similar. These data support that inhaled Technosphere Insulin is not associated with an excess of pulmonary safety events in the short term when compared with usual insulin therapy. |
1 Introduction
Insulin therapy is an important component of standard care of patients with diabetes mellitus (DM) and may be administered as a combination of basal (long-acting) insulin and prandial (mealtime, short-acting) insulin [1]. Prandial insulin, traditionally self-administered via subcutaneous injection or infusion from an insulin pump, can also be delivered via pulmonary inhalation. The first inhaled insulin to be approved by the US Food and Drug Administration (FDA) was EXUBERA® (insulin human [rDNA origin] inhalation powder; Pfizer Inc, New York, NY, USA) in 2006, although its use raised concerns about its possible effects on lung function and theoretical risk of lung malignancies [2,3,4]. EXUBERA was ultimately withdrawn from the market by the manufacturer [2].
In 2014, Technosphere® Insulin (TI; Afrezza®; MannKind Corporation, Westlake Village, CA, USA) was approved for the treatment of both type 1 DM (T1DM) and type 2 DM (T2DM) [5]. Technosphere Insulin, a dry powder formulation of recombinant human insulin adsorbed onto Technosphere microparticles for oral inhalation, is an ultra-rapid-acting prandial inhaled insulin that has a faster onset (approximately 12 min) and shorter duration (approximately 3 h) of action than currently available subcutaneously injected insulins [5, 6].
Given the pulmonary administration route, it is clinically important to assess and understand effects of TI on pulmonary safety/function. Technosphere Insulin has been investigated in numerous clinical studies in patients with T1DM or T2DM, affording the opportunity to assess its pulmonary safety profile in this combined cohort and in subpopulations of those patients with underlying abnormalities in lung function. The current study presents an integrated analysis of incidences of respiratory treatment-emergent adverse events (TEAEs) and lung malignancies, changes in measures of pulmonary function, and radiographic changes from baseline using serial high-resolution computed tomographic (CT) or magnetic resonance imaging (MRI) scans of patients over a treatment period of up to 4 years.
2 Methods
2.1 Studies and Study Populations
Data from all 13 controlled phase 2/3 clinical studies of TI for treatment of T1DM or T2DM with a planned continuous study-treatment exposure of > 14 days that were completed by 31 July 2013 were pooled for analysis (see Electronic Supplementary Material). Data from the pooled safety population were separated by placebo-controlled and active-comparator trials to allow for direct comparison between TI and placebo or TI and active comparators. Additionally, data were pooled and summarized for two inhaler types (MedTone® and Gen2®; MannKind Corporation, Westlake Village, CA, USA), and comparative data demonstrated that overall pulmonary safety profiles were similar between TI Gen2- and TI MedTone-treated groups. For pooled studies, the analysis population was the safety population, which was defined as all randomized patients who received one or more doses of study medication. Although there were variations in study designs between trials, patients generally were excluded if they had renal dysfunction, renal disease, or serum creatinine levels between 1.5 and 2.0 mg/dL for males or 1.3 and 1.8 mg/dL for females. Patients with a history of chronic obstructive pulmonary disease (COPD), asthma, or other clinically important pulmonary diseases were also excluded from the studies. Although the inclusion/exclusion criteria were designed to exclude patients diagnosed with asthma or COPD, subpopulations of patients with mild subclinical lung dysfunction from the pooled phase 2/3 studies were retrospectively identified to assess the safety of TI in patients with underlying lung disease.
For studies evaluating pulmonary function, data were presented as individual studies because of differing study designs and treatment durations. These studies included MKC-TI-171 (open-label study in T1DM with 24-week treatment period), MKC-TI-162 and substudy MKC-TI-164 (open-label studies in T2DM with 16-week treatment periods), and MKC-TI-175 (placebo-controlled, double-blind study in T2DM with 24-week treatment period).
High-resolution CT or MRI scans of the lungs were performed to monitor the development of lung abnormalities in a population of 229 patients with T2DM from MKC-TI-010, a 4-year, open-label extension study designed to evaluate safety and tolerability of TI.
All studies were approved by appropriate independent ethics committees or institutional review boards and monitored by independent data-safety-monitoring boards. All patients provided written informed consent.
2.2 Treatments and Exposure
Treatments included TI, Technosphere inhalation powder without insulin (TP; placebo), or active-comparator treatment, which included subcutaneous insulin (e.g., insulin aspart, insulin lispro), oral glucose-lowering drugs, or usual care.
2.3 Pulmonary Safety
Primary pulmonary safety endpoints were respiratory TEAEs, including cough. A TEAE was defined as an AE (except for hypoglycemia) that worsened in severity on or after study day 1 and within 30 days of the last treatment dose. Respiratory TEAEs in the pooled studies, including cough, were summarized by preferred term and coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.1.
Cough is an anticipated side effect of dry powder inhalation and therefore was also prespecified as an expected event of special interest. Cough events as TEAEs of special interest were monitored and reported on a separate cough case-report form (CRF). Information collected included cough frequency (single defined, intermittent, continuous), relationship to treatment, and absence or presence of sputum production. In cases of mild cough, the event may not have been recorded as an AE. Events collected on cough CRFs were excluded from respiratory TEAEs summarized by preferred term.
To evaluate changes from baseline in pulmonary function for the individual studies (MKC-TI-171, MKC-TI-162/164, and MKC-TI-175; Electronic Supplementary Material), formal spirometry testing was employed as previously described by Miller et al. [7]. Pulmonary endpoints included forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). All studies strictly adhered to American Thoracic Society or European Respiratory Society guidelines [7].
High-resolution CT or MRI lung scans were performed in 229 patients with T2DM (study MKC-TI-010). Of these patients, 206 had a baseline image and ≥ 1 subsequent image taken, allowing for assessment and inclusion in the analysis population. Images of the lungs were taken at baseline (visit 1) and annually thereafter over the 4-year treatment period. Scans were reviewed for abnormalities by an independent radiologist who was blinded to patient identity, investigator site, examination dates, sequence of examinations, and reason for imaging. Qualitative assessment criteria included interlobular septal thickening, ground-glass opacities, tree-in-bud opacities, parenchymal abnormalities, consolidation, nodules or masses, bronchial abnormalities, pleural abnormalities, and pleural effusions. Patients found to be absent of all criteria for both lungs were deemed normal. Any result other than normal was submitted for additional review with clinical data to an independent board-certified radiologist and an independent board-certified pulmonologist. Secondary interpretations, based on the images and clinical data, included whether the patients’ images were normal; abnormal, not clinically significant; or abnormal, clinically significant. Study investigators reviewed high-resolution CT or MRI reports and assessed the clinical significance of abnormalities.
2.4 Statistical Analysis
For TEAE reports, event rates were calculated by dividing number of events by patient exposure time. For pulmonary function tests, a mixed-effect model repeat-measurement analysis was performed to assess mean changes in FEV1 and FVC from baseline values. For change from baseline calculations, baseline values were defined as the last measurements collected on or before study day 1 (date the first dose of study medication was administered).
3 Results
3.1 Patient Disposition and Baseline Characteristics
A total of 5505 patients were included in the overall safety population. Of these, 3017 received TI (413 in placebo-controlled studies and 2604 in active-comparator studies), 290 received TP and 2198 received active-comparator treatment. Patients in the three treatment cohorts had similar demographic characteristics, duration of diabetes, and smoking histories (Table 1).
Among 3017 patients receiving TI, 2023 (67.1%) completed treatment. More patients discontinued prematurely in the TI (32.9%) versus TP (20.0%) and active-comparator (22.0%) cohorts, with the most common reasons for discontinuation being patient withdrawal of consent for personal reasons, lack of efficacy, and dissatisfaction with therapy.
3.2 Exposure to Treatment
Median (range) duration of exposure to treatment was 168 (1–764), 149 (2–205), and 363 (0–810) days for patients receiving TI, TP, and active-comparator treatment, respectively. The 3017 patients receiving TI had varying treatment exposure times (TI exposure: 0–3 months, n = 896; > 3–6 months, n = 978; > 6–12 months, n = 419; > 12 months, n = 724).
3.3 Respiratory Treatment-Emergent Adverse Events
The overall incidence of respiratory TEAEs occurring in ≥ 2% of each cohort was higher for TI (45.2%) than for TP (35.9%) and active-comparator (31.0%) treatments. The most common respiratory TEAE summarized by MedDRA preferred term was cough. Cough incidence was similar between patients receiving TI (20.3%) and TP (19.7%) in placebo-controlled studies and higher for patients receiving TI (28.0%) versus active comparator (5.2%) in active-comparator studies (Table 2). When recorded as a TEAE of special interest on a separate CRF, the incidence of patient-reported cough was also similar between TI (21.5%) and TP (20.3%) in placebo-controlled studies and higher for TI (27.0%) vs active comparator (5.1%) in active-comparator studies (Table 3).
Cough was most commonly described as mild, intermittent, and limited to a single defined event that occurred within 10 min after TI inhalation. Cough was not persistent, as incidence was highest within the first week of TI treatment and declined over the following 4–8 weeks. Cough was generally nonproductive and not associated with chronic sputum production. Treatment discontinuation due to cough was uncommon, occurring in a small proportion (2.8%) of patients receiving TI. Other respiratory TEAEs of special interest, such as asthma, wheezing, bronchospasm, and bronchial reactivity, were uncommon (incidence, < 0.5%) for patients receiving TI.
Upper respiratory tract infection (URTI) and nasopharyngitis were the next most common respiratory TEAEs summarized by preferred term (Table 2). Incidences of URTIs were comparable between cohorts in placebo-controlled (TI 4.6%; TP 4.8%) and active-comparator (TI 10.2%; active comparator 10.9%) studies. Similarly, incidences of nasopharyngitis were comparable across cohorts in placebo-controlled (TI 7.7%; TP 8.3%) and active-comparator (TI 7.2%; active comparator 7.8%) studies.
In the pooled, controlled phase 2/3 studies, there were no deaths due to a primary respiratory TEAE. Respiratory TEAEs were noted as the cause of early discontinuation in 4.6% of patients treated with TI, compared with 0.1% of patients in the active-comparator cohorts, respectively. This difference was predominantly driven by cough (TI 2.8%; active comparator 0%), followed by dyspnea (TI 0.5%; active comparator 0%).
3.4 Pulmonary Function
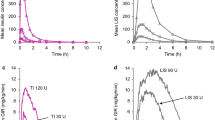
In study MKC-TI-171, there was a reduction in FEV1 from baseline to week 24 in patients with T1DM. A greater overall reduction was seen in patients receiving TI (Gen2 inhaler − 0.07 L; MedTone inhaler − 0.08 L) than in patients receiving insulin aspart (− 0.03 L; Fig. 1a), with absolute differences in FEV1 between TI and active-comparator treatment of − 0.04 L (Gen2 inhaler) and − 0.05 L (MedTone inhaler). Differences were no longer present at the 28-week follow-up visit (i.e., 4 weeks after TI treatment completion). Additionally, there was a small decline in mean change from baseline in FVC for patients receiving either TI or insulin aspart over the 24-week treatment period, with less of a decline in the insulin aspart cohort (Fig. 1b). These changes were not observed at week 28 of treatment.
In study MKC-TI-171 of patients with T1DM in which TI was administered with either the MedTone or Gen2 inhaler, changes from baseline in a FEV1 and b FVC. In study MKC-TI-175 of patients with T2DM (safety population), changes from baseline in c FEV1 and d FVC. Median baseline values are included in graph legends. FEV1 forced expiratory volume in 1 second, FVC forced vital capacity, SE standard error, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, TI Technosphere Insulin, TP Technosphere inhalation powder without insulin (placebo)
In study MKC-TI-175, patients with T2DM receiving TI experienced greater declines in FEV1 from baseline to week 12 (− 0.08 L) and week 24 (− 0.13 L) than patients receiving TP (− 0.04 L at week 12 and week 24; Fig. 1c), with a treatment difference at week 24 of − 0.09 L (95% confidence interval [CI], − 0.12, − 0.05). There were also small declines in mean FVC from baseline to week 12 and week 24 for both TI and TP cohorts. The FVC declines in the TI cohort (week 12, − 0.07 L; week 24, − 0.12 L) were greater than those of the TP cohort (week 12, − 0.04 L; week 24, − 0.03 L; Fig. 1d), with a treatment difference at week 24 of − 0.09 L (95% CI, − 0.13, − 0.04). At week 28, 4 weeks after stopping treatment, mean FEV1 and FVC values for patients receiving TI returned to levels similar to those of patients receiving TP.
Overall, a high percentage of patients across all treatment cohorts in the MKC-TI-171 and MKC-TI-175 studies had a small (0–5%) decline from baseline to the last study measurement in FEV1 (Fig. 2a, b) and FVC (data not shown). In both studies, a majority of TI-treated patients had observed declines in pulmonary function at time of last measurement. This distribution was similar at week 12 and week 24 of treatment.
Distribution of percentage change from baseline to last measurement in FEV1 in a study MKC-TI-171 (patients with T1DM in which TI was administered with either the MedTone or Gen2 inhaler) and b study MKC-TI-175 (patients with T2DM). FEV1 forced expiratory volume in 1 second, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, TI Technosphere Insulin, TP Technosphere inhalation powder without insulin (placebo)
Consistent with results observed in MKC-TI-171 and MKC-TI-175, differences in FEV1 and FVC changes from baseline between patients with T2DM receiving either TI plus insulin glargine or insulin aspart plus insulin glargine in study MKC-TI-162 were small (differences in mean changes from baseline [TI cohort minus active-comparator cohort]: FEV1 at week 6, − 0.07 L; FEV1 at week 16, − 0.07 L; FVC at week 6, − 0.07 L; FVC at week 16, − 0.06).
3.5 Pulmonary Safety in Special Populations
Among the pooled safety population, 101 patients were retrospectively identified with subclinical reversible airway dysfunction, defined as patients with a > 12% and > 0.2 L improvement in FEV1 pre- to post-bronchodilator at the screening visit (TI, n = 50; TP, n = 2; active comparator, n = 49). Among these patients, cough was the most common respiratory TEAE and was reported in 30.0% of patients in the TI cohort, compared with 12.2% in the TP/active-comparator cohort. In lung function analyses, mean changes in FEV1 values from baseline over 24 months slightly decreased over time (− 0.05 L) for the TI cohort and increased over time for the TP/active-comparator cohort (0.10 L). Similarly, the TI cohort demonstrated a greater decline from baseline in FVC over 24 months (− 0.16 L), whereas the TI/active-comparator cohort demonstrated an improvement from baseline (0.09 L).
Among the pooled safety population, 61 patients (TI, n = 38; TP, n = 2; active comparator, n = 21) were retrospectively identified as having subclinical fixed obstructive lung dysfunction, defined as patients aged > 40 years with a history of smoking and a post-bronchodilator FEV1/FVC ratio of < 70%. Cough was observed in 21.5% of these patients receiving TI, compared with 0% of patients receiving TP or active-comparator treatment. Over 24 months, mean changes in FEV1 from baseline slightly decreased for patients receiving TI (− 0.11 L) and slightly increased for patients receiving TP or active-comparator treatment (0.09 L). At 24 months, the TI cohort also demonstrated a decline from baseline in mean FVC (− 0.16 L), whereas the TP/active-comparator cohort demonstrated an improvement (0.13 L). In both retrospectively defined subpopulations, no bronchospasm AEs were reported.
3.6 Lung Malignancies
During the clinical trial program, there were two reported lung malignancies in 3283 patients (2747 patient-years of follow-up) receiving TI treatment and no cases of lung malignancy in 2205 patients (2169 patient-years of follow-up) receiving active-comparator treatment. One case was a neuroendocrine tumor involving the lung, which was diagnosed 71 days after the last TI dose; the second case was a non–small-cell bronchogenic carcinoma, which was diagnosed 2 months after TI discontinuation. In both cases, the patients had a history of cigarette exposure of approximately 40 pack-years. Additionally, two cases of non–small-cell lung cancer in nonsmoking patients were diagnosed after completion of participation in clinical trials (more than 2 years after completion of TI treatment). One of these tumors was later reclassified as a pulmonary blastoma. Longer-term follow-up of individuals receiving usual care was not routinely available.
3.7 High-Resolution CT and MRI
A total of 206 (N = 229) patients in the analysis population of study MKC-TI-010 received TI and had a baseline image plus one or more subsequent high-resolution CT or MRI image taken. At the 12-month follow-up, of 184 patients with baseline and follow-up images taken, 70.7% had normal radiologic images; 28.3% had abnormal, not clinically significant images; and 1.1% had abnormal, clinically significant images. Of 137 patients with images considered normal at baseline, 136 had normal or abnormal, not clinically significant images at the 12-month follow-up; of the four patients with abnormal, clinically significant radiologic images at baseline, one had abnormal, clinically significant images and three had abnormal, not clinically significant images at the 12-month follow-up.
Over the study course, six (2.6%) patients had local readings of lung nodules on high-resolution CTs. Although causality ultimately cannot be defined on the basis of these radiologic reviews, five were judged by the investigator to be unlikely related to TI exposure.
4 Discussion
Inhaled insulin offers numerous potential advantages over subcutaneously injected insulin, including faster insulin absorption rates, more rapid onset and offset of action, improved postprandial glucose control, and a lower risk of latent hypoglycemia [8]. Given the novel delivery system of inhaled insulin, pulmonary safety is of particular clinical interest, and the incidence of AEs and drug effects on the respiratory system are important to understand.
The most common respiratory AEs associated with inhaled medications such as inhaled corticosteroids for asthma or inhaled antibiotics for airway infections include bronchoconstriction, dysphonia, throat irritation, and cough [9, 10]. In the current pooled safety analysis, the most common respiratory TEAE associated with TI was cough. Cough was generally mild and nonproductive, occurred within 10 min of inhalation, was observed early in the first week of treatment, and diminished over time, consistent with previously published studies of TI [11,12,13]. Incidence of cough was similar between TI and TP in placebo-controlled trials but higher for TI in active-comparator trials, suggesting that the TI excipient is associated with cough. As expected, cough was noted as the cause of early discontinuation in more patients receiving TI than in patients receiving active-comparator treatments, although this occurred in a minority of patients (2.8%) and discontinuation may have been affected by the open-label design of the controlled studies. Patients who experienced a respiratory TEAE may have been more likely to discontinue treatment than if they were receiving comparator subcutaneous insulin in this open-label design, as TI is inhaled. The current analysis does not allow for assessment of potential bias. Incidences of other respiratory TEAEs, including asthma, wheezing, bronchospasm, and bronchial reactivity, were low, occurring in < 0.5% of patients receiving TI.
Potential concerns with inhaled medications include lung toxicity, inflammation, and anatomical injury resulting from the active drug product or the powder constituents. In the high-resolution CT/MRI analysis (MKC-TI-010), a small proportion (n = 2 [1.1%]) of patients had radiologic findings that were considered “abnormal, clinically significant” at the time of their 12-month CT/MRI measurement, and one of these patients had “abnormal, clinically significant” CT/MRI scans before treatment began. Overall, the observed radiologic findings are not suggestive of a safety signal in patients treated with TI for at least 12 months. Published results from high-resolution CT/MRI analyses with the inhaled insulin EXUBERA also showed no significant changes in scans from baseline, further indicating that insulin delivery via pulmonary inhalation does not appear to substantially alter lung anatomy [3]. These findings support the conclusion that decreased pulmonary function is not related to drug-induced lung abnormalities or inflammation but may be attributable to other factors associated with deep-lung inhalation systems.
Diabetes is associated with a progressive decline in pulmonary function that exceeds the decline observed in populations without diabetes [14]. This decline may be due to several factors, including overall higher body mass index (BMI) and chronic hyperglycemia effects, as indicated by higher hemoglobin A1C levels [14, 15]. The main factor accounting for the relationship between glycemic control and lung function may be the metabolic pathways related to hyperglycemia; however, several other mechanisms have been proposed that also consider BMI, including decreased respiratory muscle strength, impaired lung elasticity, defects in pulmonary surfactants, and low-grade chronic inflammation [16]. In the current study, pulmonary function was evaluated in three individual studies: MKC-TI-171, MKC-TI-175, and MKC-TI-162/164. These studies reported that there were greater (though small) declines in mean FEV1 and FVC from baseline for the TI versus TP and active-comparator cohorts. When looking at the distribution of percentage change from baseline, the observed changes in mean FEV1 and FVC for TI-treated patients were driven by slight declines in pulmonary function (as demonstrated by a slight shift to the left in distribution in Fig. 2) among a high percentage of TI-treated patients and not by significant declines among a low percentage of TI-treated patients (i.e., outliers). For all trials assessed in the current study, such declines resolved 4 weeks after the end-of-treatment period, regardless of exposure duration. Lung function decline also occurs in healthy, aging individuals, with normal age-related declines in pulmonary function reported as ranging from 0.02 to 0.05 L per year [17].
In the retrospectively defined subpopulation of patients with mild, subclinical lung dysfunction, cough was the most frequent respiratory TEAE, with an incidence similar to that of the overall safety population without underlying lung disease. These results support those from phase 1 studies in prospectively defined populations with asthma and COPD in which cough was the most frequent respiratory TEAE [2, 18]. Although acute bronchospasm was observed after TI administration in five of 17 asthmatic patients in one of the aforementioned phase 1 studies, it could be prevented by pretreatment with bronchodilators [2]. Bronchospasm was not reported in phase 2/3 studies in patients with either reversible airway disease or fixed obstructive lung dysfunction. In the retrospective analyses presented here, there was a greater decline in pulmonary function in patients with underlying lung dysfunction receiving TI compared with those receiving TP or active-comparator treatments. This is also consistent with phase 1 studies in prospectively defined asthma and COPD populations, in which a moderate decline in pulmonary function was seen immediately (15–18 min) after TI inhalation [2, 18]. However, the retrospectively defined studies presented here are limited by a small patient population evaluated at the completion of the study (month 24); therefore, additional studies are necessary to better establish the efficacy and safety of TI in these populations.
Chronic hyperinsulinemia has been proposed to play a role in the growth of non-small-cell lung cancer, with data suggesting that chronic stimulation of the insulin-like growth factor-1 signaling pathway may promote the rapid growth of existing neoplasms [19, 20]. This observation is supported by data demonstrating an overall increased risk of any malignancy in the population of patients with long-standing diabetes. Comparisons between lung cancer incidences in the referenced TI clinical studies and published incidences in other studies or in the general diabetes population are limited by differences in demographics (age), study populations (smokers, ex-smokers, nonsmokers), and the low rate of population events. For example, comparative incidences assessed from studies involving nonsmokers in the general diabetes population most likely underestimated the expected incidence of lung malignancies in the TI population because ex-smokers were included in the TI studies. However, the overall incidence of lung malignancies in the TI study population (0.8 cases per 1000 patient-years) was similar to that of the general population of patients with diabetes, both for smokers and for nonsmokers (incidence of 0.5–2.0 cases per 1000 patient-years) [21, 22]. There were four cases of lung malignancies reported among TI-treated patients: two were reported during the program in patients who were ex-smokers with a long history of cigarette exposure, and two were reported ≥ 2 years after trial completion in nonsmokers. Similarly, in a 2-year observational follow-up study (FUSE) in patients previously treated with the inhaled insulin EXUBERA, it was reported that the increased risk of lung cancer-related mortality in EXUBERA-treated patients including nonsmokers and those with a history of smoking was low (0.5 cases per 1000 patient-years) and was similar to the estimated lung cancer mortality rate in the general population of patients with diabetes [4]. Additionally, most (7/8) patients with lung cancer-related mortality (EXUBERA group, n = 5; active-comparator group, n = 2) were prior smokers [4]. These data support the limited risk of malignancy observed in patients using inhaled insulins as compared with the general population of patients with diabetes. However, the relatively low total exposure and short exposure duration in the studies included in the current analysis support the need for further long-term follow-up of patients to better inform conclusions regarding the risk of malignancy in patients receiving inhaled TI treatment.
The patients included in this pooled pulmonary safety analysis were participants in randomized controlled trials that were part of the TI phase 2/3 clinical program. Randomized controlled trials aim to reduce bias through strict inclusion and exclusion criteria; however, these criteria may exclude many patients typically seen in clinical care, including patients with multiple co-morbidities, potentially limiting the generalizability of the results [23]. Real-world studies and longer-term pharmacovigilance will be needed to further assess the long-term safety of TI in a more heterogenous patient population to broaden the applicability of the results found here.
Another potential limitation of this analysis is the duration of TI exposure for each study, with a maximum time of TI exposure of 764 days for the pooled analysis and 4 years for MKC-TI-010. These relatively short exposure durations limit the ability to draw conclusions regarding any long-term effects of TI treatment (beyond 4 years) on pulmonary safety, particularly risk of malignancy; additional long-term and follow-up studies will be helpful to further clarify the risk profile.
5 Conclusions
This analysis of long-term TI use in clinical studies provides a pulmonary safety assessment of inhaled insulin using the Technosphere powder inhalation platform. No significant or persistent pulmonary safety concerns were identified across the 13 clinical trials included in this analysis, providing further support for the use of TI treatment in adults with T1DM or T2DM.
References
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102.
Heinemann L, Parkin CG. Rethinking the viability and utility of inhaled insulin in clinical practice. J Diabetes Res. 2018;2018:4568903.
Rosenstock J, Cerfalu WT, Hollander PA, et al. Two-year pulmonary safety and efficacy of inhaled human insulin (Exubera) in adult patients with type 2 diabetes. Diabetes Care. 2008;31:1723–8.
Gatto NM, Koralek DO, Bracken MB, et al. Lung cancer-related mortality with inhaled insulin or a comparator: follow-up study of patients enrolled in Exubera-controlled clinical trials (FUSE) final results. Diabetes Care. 2019;42:1708–15.
Afrezza [package insert]. Danbury, CT: MannKind Corporation; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022472s018lbl.pdf. Accessed 4 June 2020.
FIASP [package insert]. Plainsboro, NJ: Novo Nordisk Inc.; 2018.
Miller MR, Hankinson J, Brusasco V, The ATS/ERS Task Force, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Seaquist ER, Blonde L, McGill JB, et al. Hypoglycaemia is reduced with use of inhaled Technosphere® Insulin relative to insulin aspart in type 1 diabetes mellitus. Diabetes Med. 2019;37:752–9.
Quon BS, Goss CH, Ramsey BW. Inhaled antibiotics for lower airway infections. Ann Am Thorac Soc. 2014;11:425–34.
Hanania NA, Chapman KR, Kesten S. Adverse effects of inhaled corticosteroids. Am J Med. 1995;98:196–208.
Bode BW, McGill JB, Lorber DL, The Affinty 1 Study Group Inhaled, et al. Technosphere Insulin compared with injected prandial insulin in type 1 diabetes: a randomized 24-week trial. Diabetes Care. 2015;38:2266–73.
Rosenstock J, Lorber DL, Gnudi L, et al. Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicentre randomised trial. Lancet. 2010;375:2244–53.
Rave K, Heise T, Heinemann L, Boss AH. Inhaled Technosphere insulin in comparison to subcutaneous regular human insulin: time action profile and variability in subjects with type 2 diabetes. J Diabetes Sci Technol. 2008;2:205–12.
Davis WA, Knuiman M, Kendall P, Grange V, Davis TME. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27:752–7.
Banerjee J, Roy A, Singhamahapatra A, Dey PK, Ghosal A, Das A. Association of body mass index (BMI) with lung function parameters in non-asthmatics identified by spirometric protocols. J Clin Diagn Res. 2014;8:12–4.
Lecube A, Sampol G, Muñoz X, Hernández C, Mesa J, Simó. Type 2 diabetes impairs pulmonary function in morbidly obese women: a case-control study. Diabetologia. 2010;53:1210–6.
Thomas ET, Guppy M, Straus SE, Bell KJL, Glasziou P. Rate of normal lung function decline in ageing adults: a systematic review of prospective cohort studies. BMJ Open. 2019;9:e028150.
Potocka E, Amin N, Cassidy J, et al. Insulin pharmacokinetics following dosing with Technosphere insulin in subjects with chronic obstructive pulmonary disease. Curr Med Res Opin. 2010;26:2347–53.
Frisch CM, Zimmermann K, Zilleßen P, Pfeifer A, Racké K, Mayer P. Non-small cell lung cancer cell survival crucially depends on functional insulin receptors. Endocr Relat Cancer. 2015;22:609–21.
Piper AJ, Clark JL, Mercado-Matos J, et al. Insulin receptor substrate-1 (IRS-1) and IRS-2 expression levels are associated with prognosis in non-small cell lung cancer (NSCLC). PLoS One. 2019;14:e0220567.
Ehrlich SF, Quesenberry CP Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33:55–60.
Hall GC, Roberts CM, Boulis M, Mo J, MacRae KD. Diabetes and the risk of lung cancer. Diabetes Care. 2005;28:590–4.
Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–74.
Acknowledgements
The authors would like to thank Byron J. Hoogwerf, MD, FACP, FACE, for his contributions to this work. Editorial assistance was funded by MannKind Corporation and provided under the direction of the authors by Bethany Reinecke, PhD, Elizabeth A. Harvie, PhD, ELS, and Jenna Lewis, MA, ELS, MedThink SciCom.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Financial support for this study was provided by MannKind Corporation, Westlake Village, CA, USA.
Conflict of interest
JBM has received research funding from Dexcom, Medtronic, and Novo Nordisk; she has served as a consultant or on advisory boards for Bayer, Boehringer Ingelheim, Dexcom, Metavant, Novo Nordisk, and Valeritas; and she has served as a member of the Speakers Bureau for Janssen Pharmaceuticals Inc. AP has served on advisory boards for Abbott Diabetes Care, Bigfoot, Eli Lilly, MannKind Corporation, Novo Nordisk, Pendulum Therapeutics, and Sanofi; she has received research funding from Dexcom and vTv Therapeutics; and she holds stock options in Livongo, Mellitus Health, Omada Health, Pendulum Therapeutics, and Stability Health. JBB has received contracted consulting fees and grant support paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Eli Lilly, MannKind Corporation, NovaTarg, Novo Nordisk, Sanofi, Senseonics, Tolerion, vTv Therapeutics, and Zafgen; he has served as a consultant to Cirius Therapeutics Inc, CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, and Stability Health; he holds stock options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health; and he is supported by grants from the National Institutes of Health (UL1TR002489, U01DK098246, UC4DK108612, U54DK118612, P30DK124723), Patient-Centered Outcomes Research Institute, and American Diabetes Association. SS, TT, FMP, and DMK are paid employees of MannKind Corporation and may hold stock with the company.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical standards
All procedures performed in studies involving human participants were in accordance with ethical standards that were approved by appropriate independent ethics committees or institutional review boards and were monitored by independent data-safety-monitoring boards. All patients provided written informed consent.
Author contributions
JMG assisted in data collection. All authors contributed to the study conception, design, and data review; commented on previous versions of the manuscript; and read, edited, and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McGill, J.B., Peters, A., Buse, J.B. et al. Comprehensive Pulmonary Safety Review of Inhaled Technosphere® Insulin in Patients with Diabetes Mellitus. Clin Drug Investig 40, 973–983 (2020). https://doi.org/10.1007/s40261-020-00958-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00958-8