Abstract
Background
Patients with chronic obstructive pulmonary disease (COPD) are at elevated risk of pneumococcal infection. A 13-valent pneumococcal conjugate vaccine (PCV13) was approved for protection against invasive disease and pneumonia caused by Streptococcus pneumoniae in adults. This study estimated the incremental cost-effectiveness ratio (ICER) of vaccinating COPD patients ≥50 years old with PCV13 compared with current vaccination policy (CVP) with 23-valent pneumococcal polysaccharide vaccine.
Methods
A Markov model accounting for the risks and costs for all-cause non-bacteremic pneumonia (NBP) and invasive pneumococcal disease (IPD) was developed. All parameters, such as disease incidence and costs (€; 2015 values), were based on published data. The perspective of the analysis was that of the Spanish National Healthcare System, and the horizon of evaluation was lifetime in the base case. Vaccine effectiveness considered waning effect over time. Outcomes and costs were both discounted by 3 % annually.
Results
Over a lifetime horizon and for a 629,747 COPD total population, PCV13 would prevent 2224 cases of inpatient NBP, 3134 cases of outpatient NBP, and 210 IPD extra cases in comparison with CVP. Additionally, 398 related deaths would be averted. The ICER was €1518 per quality-adjusted life-year (QALY) gained for PCV13 versus CVP. PCV13 was found to be cost effective versus CVP from a 5-year modelling horizon (1302 inpatient NBP and 1835 outpatient NBP cases together with 182 deaths would be prevented [ICER €25,573/QALY]). Univariate and probabilistic sensitivity analyses confirmed the robustness of the model.
Conclusions
At the commonly accepted willingness-to-pay threshold of €30,000/QALY gained, PCV13 vaccination in COPD patients aged ≥50 years was a cost-effective strategy compared with CVP from 5 years to lifetime horizon in Spain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The administration of 13-valent pneumococcal conjugate vaccine (PCV13) in a ≥50 years of age chronic obstructive pulmonary disease (COPD) cohort would have higher health benefits than the current vaccination policy with polysaccharide vaccine in Spain. |
The incremental costs of this vaccination strategy are counterbalanced in part by savings from averted pneumococcal disease cases. |
Vaccination with PCV13 in COPD patients aged ≥50 years was a cost-effective strategy in Spain. |
1 Introduction
Streptococcus pneumoniae is a major cause of morbidity, mortality, and associated costs in the adult population [1]. This bacterium causes different disease manifestations, including invasive pneumococcal disease (IPD) and non-invasive mucosal infections (non-IPD) and non-bacteremic pneumonia (NBP). Older adults and those with certain clinical conditions, such as those with immunocompromising conditions and immunocompetent patients with chronic diseases, are at increased risk of developing pneumococcal disease (PD), particularly pneumonia, along with having a higher risk of related mortality [2, 3]. In particular, one of the most relevant underlying conditions associated with increased risk for PD is chronic obstructive pulmonary disease (COPD) [4]. Adults with COPD run more than a four-fold increased risk of PD than patients without this condition [5, 6].
All-cause NBP constitutes between 5 and 12 % of all respiratory tract infections in the adult population [7], with an estimated annual incidence rate in Spain of 3 cases per 1000 adult habitants [8], which means 114,000 annually diagnosed pneumonias throughout the country (for an adult population estimated to be 38,162,985 in the year 2014 [9]). Approximately 41.5 % of all NBP cases require hospitalization [8], with an associated disease fatality rate of 17.4 % [10]. Gil-Prieto and colleagues [10] identified 75,932 deaths due to NBP among hospitalized patients aged 50 years or older in the period 2003–2007. The estimated cost per pneumonia management case was €568.43 [8] for outpatient NBP and €2465 and €5534 by series [8, 10], with a total annual cost due to these hospitalizations estimated to be more than €479 million. As the only health policy intervention that reduces the high burden of PD [11], vaccination strategies have been established in almost all European countries for those at high risk and/or the elderly population [12]. In Spain, current vaccination policy (CVP) recommends 23-valent pneumococcal polysaccharide (PPV23) or 13-valent pneumococcal conjugate (PCV13) vaccination for high-risk adults of all ages (immunocompromised patients and those with chronic diseases) and, depending on the region, for the population ≥60 or ≥65 years old [13]. A review of Spanish pneumococcal vaccination recommendations for adults performed by 16 scientific societies and published in 2013 concluded that non-conjugated polysaccharide vaccines are less immunogenic than conjugated vaccines and their efficacy decreases with time [4]. Several studies suggest that non-conjugated polysaccharide vaccines may not contribute to adequate protection against non-invasive pneumonia [14, 15], while their efficacy in preventing invasive pneumonia in elderly patients and adults with co-morbidities remains limited [14, 16, 17]. Conjugated vaccines are able to induce functional antibody response (T cell dependent) directed to the bacterial capsule, resulting in a robust initial response and in the establishment of immunological long-lived memory [18, 19]. In children, conjugated pneumococcal vaccines have been shown to be highly effective in preventing both IPD and pneumonia caused by the vaccine-related serotypes [20]; however, data concerning the impact in adults and high-risk populations are scarce [14].
After the introduction and widespread use of PCV13 in the infant population, on 3 March 2015 it was approved for the prevention of pneumonia and invasive disease caused by S. pneumoniae in the adult population [21]. At present, there is no evidence available regarding the cost effectiveness of pneumococcal immunization with conjugate vaccines in adult patients at increased risk of PD in Spain. The aim of this analysis was to evaluate the clinical and economic consequences of the use of a single dose of PCV13 among the COPD adult population aged ≥50 years compared with CVP based on PPV23.
2 Materials and Methods
2.1 Model Description
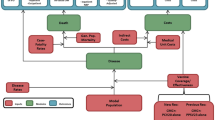
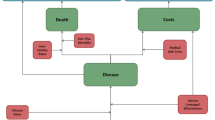
A model with a Markov process based on the following health states was developed in Microsoft Excel® 2007 to depict the risks and costs of IPD and all-cause NBP in a ≥50-year-old Spanish COPD population: alive without IPD or all-cause NBP; alive with IPD; alive with all-cause inpatient NBP; alive with all-cause outpatient NBP; and death (Fig. 1). All patients entered the model in the non-PD state. Therefore, the risks of developing IPD (such as meningitis and bacteremia, among others) and all-cause NBP (outpatient or inpatient) were modeled. An age-stratified cohort of patients with known underlying COPD was included in the model according to the known prevalence of disease in Spain [22].
The expected total number of IPD cases and all-cause NBP (by setting of care), expected number of deaths due to IPD and all-cause inpatient NBP, expected total costs of medical treatment for IPD and all-cause NBP, and total costs of vaccination were evaluated. All expected outcomes were evaluated on an annual basis, from model entry through the end of the modeling horizon. In each year, pneumococcal-related outcomes were projected for each person in the model population based on age, risk profile, and vaccination status.
Cost effectiveness was estimated based on the incremental cost-effectiveness ratio (ICER), which was calculated by dividing the differences in costs by the quality-adjusted life-years (QALYs) gained with a PCV13 strategy versus CVP with PPV23. A QALY takes into account both the quantity and quality of life generated by healthcare interventions. It is the arithmetic product of life expectancy and a measure of the quality of the remaining life-years.
2.2 Vaccination Strategies
Two vaccination strategies were compared: the CVP consisting of one dose of PPV23 at model entry, and an alternative vaccination strategy with one dose of PCV13. The pneumococcal vaccination strategy was estimated to reach an annual coverage of 59.5 % of COPD patients in the base-case scenario for subjects above 50 years old (50–64 years: 41.1 %; 65–74 years: 62.9 %; 75–84 years: 69.4 %; 85–99 years: 71.8 % [23]), and was considered to be equivalent for both vaccination strategies. The potential herd effect from vaccinating the pediatric population in any of the vaccination strategies analyzed was also considered (see Table 1 for the herd protection effect in percentages). Maximum values for herd effects were assumed to be obtained in year 1 of the modeling horizon [24–26].
2.3 Patient Population
Population estimations were based on national figures from the Spanish National Statistical Institute [9]. Stratification was considered by the following age groups: 50–64, 65–74, 75–84, and ≥85 years. The population modeled included 629,727 Spanish adults aged ≥50 years with COPD. This number considered the COPD prevalence by age group and the proportion (26.9 %) of diagnosed COPD patients in the Spanish population [22]. Subjects were assumed not to be previously vaccinated at model entry.
2.4 Clinical Data
Risk-specific incidence and case fatality rates associated with IPD were based on published data in chronic respiratory disease [3], which concluded that the annual IPD incidence for all age groups was 91 cases per 100,000 inhabitants. The all-cause NBP inpatient incidence rate was taken from a Spanish database that collected hospitalizations due to several diseases, including pneumonia [27]. The incidence rate of all-cause outpatient NBP was estimated by the proportion of outpatient pneumonia from the total all-cause NBP (58.5 %) data [8]. Due to a lack of local data, the division of NBP incidence by each of the age groups was performed based on the proportion of NBP (such as inpatient or outpatient) incidence by age group observed in a surveillance program carried out by the US Centers for Disease Control and Prevention [28]. The annual all-cause COPD population mortality rate was obtained from national statistics [9]. The IPD-related mortality was based on specific chronic respiratory disease published data [3]. The all-cause inpatient NBP mortality rate was estimated based on the data published by Merino-Sánchez et al. [29] and divided by age group. All clinical and epidemiology data are summarized in Table 1.
2.5 Vaccine Effectiveness
The effectiveness of both pneumococcal vaccines depended on immunization level, vaccine efficacy in IPD and non-IPD differentiated by age and risk groups, vaccine coverage, serotype coverage, and duration of protection [30]. As the COPD population were considered to be at-risk but immunocompetent individuals, the data for PPV23 effectiveness against IPD were obtained from an investigation that aimed to evaluate the epidemiological impact of the PPV23 vaccination program in the elderly in England and Wales [31]. The effectiveness of PPV23 against all-cause NBP was assumed to be zero based on published meta-analyses and systematic reviews [14]. For PCV13, effectiveness data for both IPD and pneumonia in the adult immunocompetent population was taken from CAPiTA (Community-Acquired Pneumonia Immunization Trial in Adults), which was performed in The Netherlands in adults aged ≥65 years not previously vaccinated against S. pneumoniae [32]. Effectiveness of the vaccine decreased with age in both strategies and a waning effect over time was considered for all age groups. All effectiveness data used in the model are given in Table 2 as they appeared in the mentioned sources [31, 32]. The coverage of vaccine serotypes for IPD was 76.5 and 70.0 % for PPV23 and PCV13, respectively [33] and for any-cause NBP either requiring inpatient or outpatient care was 19.4 % for PCV13 using the serotype-specific urinary antigen detection assay [34].
2.6 Utilities
Self-assessed health state (or utility) scores measure an individual’s preferences for specific outcomes and were used to calculate QALYs. Estimations of age-/risk-specific health-state utility and disutility values due to disease were based on published studies. Health-state utilities for the COPD general population by age group were 0.8101 (50–64 years old), 0.7542 (65–74 years old), 0.6792 (75–84 years old), and 0.5280 (85–99 years old) [35]. Also, reductions in health-state utility values due to disease were considered in the model: IPD (–0.076), inpatient NBP (–0.079), and outpatient NBP (–0.004) [36, 37].
2.7 Costs
Medical costs considered in this model were bacteremia, meningitis, and all-cause NBP (inpatient and outpatient) management costs. Management costs of PD were obtained from the literature [8, 38]. The pharmacy retail price [39] adjusted with a 7.5 % mandatory deduction [40] was used to estimate vaccine costs. Administration costs for both CVP and PCV13 were assumed to be zero as they were supposed to be administered together with the influenza vaccine. All costs are presented in euros (€) adjusted to 2015 prices, using appropriate price inflation rates when required (Table 3).
2.8 Time Horizon, Perspective, and Discount Rate
A lifetime horizon (82 years maximum) was adopted for the base case, following all patients until death. The analysis considered the perspective of the Spanish National Healthcare System (NHS); thus, only direct healthcare costs (cost of vaccines only) were included. Costs and health benefits were both discounted at 3.0 % annually, as counseled by the latest Spanish recommendations for development of economic evaluations [41].
2.9 Uncertainty Management and Sensitivity Analyses
Inherent variability in the population of interest was managed in the base case by applying a probabilistic sensitivity analysis (PSA), in order to get population-specific values based on probabilities for the previously described parameters. A Monte-Carlo simulation was used to assess the uncertainty of incidence and mortality rates, vaccine effectiveness, and medical costs. The distributions used to simulate these parameters were beta distribution for incidence and mortality rates and vaccine effectiveness, Log-normal distribution for cost and uniform for utility values and herd effect (Table 4). The PSA was run for 1000 iterations and a cost-effectiveness acceptability curve was plotted in terms of the probability of net monetary benefit is above 0 for different willingness-to-pay thresholds.
Additionally, one-way deterministic sensitivity analyses were also performed, involving modification of values for some specific parameters not related to inter-sample variation. Parameters that were modified were the time horizon (5, 10, and 20 years), discount rate (undiscounted and 5 % for both outcomes and costs), revaccination at 5 years for 56.4 % with CVP only, vaccination coverage based on an influenza vaccination program for the general population instead of COPD patients (based on the Spanish National Health Survey [23]), inclusion of only the COPD population ≥65 years, serotype coverage of immunocompromised subjects (64.2 % PPV23 and 44.1 % for PCV13; based on a Spanish publication [33]), waning effect (decreasing by an additional 15 %) only for PCV13, the cost of outpatient pneumonia including the healthcare component only (€349.74) and ±25 % variation in indirect effect, utility values, vaccine effectiveness, disease incidences, and mortality. Finally, the vaccine price for both PPV23 and PCV13 was reduced by 15 % separately.
3 Results
The administration of PCV13 to the Spanish COPD cohort ≥50 years, under base-case assumptions, would account for higher health benefits than CVP with PPV23 (Fig. 2). Overall, compared with CVP the inclusion of one dose of PCV13 would avoid 210 IPD cases, 2224 inpatient NBP cases, and 3134 outpatient NBP cases for a lifetime horizon. Additionally, 398 related deaths would be averted. Medical plus vaccination costs per patient obtained in the model would imply a cost of €682 and €686 for CVP and PCV13, respectively, for the NHS in a lifetime period. In addition, the total survival gain in terms of life-years gained (LYG) and QALYs would be slightly higher with PCV13 vaccination than with CVP per COPD-assessed patient, with a mean increase of 0.0036 LYG and 0.0024 QALYs. Costs and outcomes results per patient are shown in Table 5. The resulting ICER was €1245 per additional LYG and €1844 per QALY gained for PCV13 compared with CVP with PPV23. All ICERs obtained by age group were less than €10,000/QALY. For the 75–84 and 85–99 years age groups, PCV13 would be the dominant alternative versus CVP.
3.1 Sensitivity Analysis
Since some model variables, such as vaccine effectiveness, were inferred, different scenarios were assessed to further investigate the relationship between parameters and cost-effectiveness results and to confirm the robustness of the model. PSA results revealed that the PCV13 vaccination strategy was a cost-effective option in 100 % of 1000 simulations performed (Fig. 3). Univariate sensitivity analyses results are included in Fig. 3 and Table 6. Results from sensitivity analyses on previously mentioned parameters did not substantially affect the results, with all scenarios indicating that use of PCV13 in COPD patients aged ≥50 years was cost effective (Table 6). The most sensitive parameter was vaccine effectiveness, with a reduction in PCV13 effectiveness either in IPD or inpatient pneumonia producing the highest variation in ICER values. In particular, a 25 % decrease in PCV13 effectiveness in IPD would increase the ICER (€29,055) close to the willingness-to-pay threshold cost-effectiveness value that exists in Spain. In contrast, a 25 % reduction in PPV23 effectiveness in IPD would produce a dominant situation favoring PCV13. The rest of the modified parameters showed that the cost-effectiveness analysis results are robust to a variety of alternatives scenarios. Even increasing vaccination coverage to 100 % of the identified COPD cohort would also result in a cost-effective strategy reporting an ICER of €2499/QALY (Fig. 4). Another influential parameter was time horizon since the scenario of a 5-year horizon showed an ICER of €25,573/QALY. Nonetheless, in this scenario, PCV13 vaccination would still be associated with 1302 inpatient NBP cases, 1835 outpatient NBP cases, and 182 deaths prevented versus PPV23 vaccination (see Table 6). Finally, the vaccine price was sensitive to variation in cases where the PCV13 price was reduced by 15 %, as this scenario showed PCV13 to be a dominant option. However, the same reduction of price for PPV23 showed a 27 % increase in the ICER for PCV13, which could still be considered highly cost effective in Spain.
4 Discussion
In Spain, pneumococcal vaccination with PPV23 is currently funded by the NHS and recommended for adults with certain chronic conditions. The cost effectiveness of PCV13 vaccination of individuals aged ≥50 years with COPD was assessed in comparison with the existing policy. All scenarios analyzed in the model suggest that, from the Spanish NHS perspective, a routine pneumococcal adult vaccination scheme with PCV13 would be a highly cost-effective intervention. Under base-case analysis, a PCV13 policy would cost €1844 per additional QALY versus CVP. Other model parameters included in the deterministic sensitivity analysis impacted the final ICER, but in all cases, and assuming the common willingness-to-pay threshold in Spain of around €30,000 per additional QALY [42], provision of a single dose of PCV13 to the Spanish cohort with COPD aged ≥50 years would be considered a cost-effective strategy compared with CVP with PPV23 from the NHS perspective.
The most influential parameters were vaccine effectiveness on IPD, time horizon, and vaccine price. Herd effect, even considering high values that are out of the scope of the Spanish situation regarding vaccination policy in childhood (still very limited), was of little impact in the model, with only small differences found between the ±25 % variation and the value included in the base-case scenario. A 25 % reduction in PCV13 effectiveness on IPD would be accompanied by an ICER increment close to the willingness-to-pay threshold cost-effectiveness value existing in Spain of around €30,000/QALY [42]. On the other hand, a 25 % reduction of PPV23 effectiveness on IPD would produce a dominant situation (lower cost and higher clinical benefits) favoring PCV13. A PCV13 vaccination strategy versus CVP over a lifetime horizon resulted in an ICER of €1844 per QALY gained that is clearly below the commonly acceptable threshold used to determine the cost-effectiveness profile of a health technology in Spain [42]. It is worth highlighting that crucial outcomes for prophylactic measures are referred to using averted cases, and in all PD types assessed in this research a PCV13 vaccination strategy scenario compared with CVP would avoid 2224 inpatient NBP, 3134 outpatient NBP, and 210 IPD cases. In cost terms, for the lifetime horizon, the results of this study suggest that use of PCV13 would lead to a reduction in the total number of cases of PD as well as a cost reduction via avoided healthcare services that would partially offset the incremental costs related to PCV13 vaccination in adults aged ≥50 years. Finally, while a 15 % reduction in the PCV13 price was associated with a dominant situation in favor of this vaccination strategy, the same reduction for PPV23 had little effect on the ICER value.
To date, several economic PCV13 evaluations have been published in other settings [20, 30, 43–56]. Published cost-effectiveness analyses are available for the USA, England, Germany, Italy, and The Netherlands, with a wide range of immunization strategies and different populations tested. Comparison of results between studies is difficult because of model assumptions, differences in healthcare system organizations, epidemiology, year of cost values, and other country-specific factors, but some of them can be mentioned as illustrative examples. In the USA, PCV13 was dominant (higher effectiveness with lower cost) when the cost effectiveness of the addition of one dose of PCV13 to the previously recommended PPV23 dose in adults with selected immunocompromising conditions was explored [44], and was more cost effective than PPV23 in immunocompromised or older adults [46, 47]. Routine PCV13 at ages 50 and 65 years yielded a $US45,100 per QALY ratio compared with current recommendations in the USA [50]. At a European level, PCV13 was dominant in Italy [45] for immunization of the at-risk population 50–79 years, and in Germany it was dominant for adults (>18 years) [53]. Most recently, cost-utility analyses in The Netherlands [54] and Italy [49] reported that PCV13 vaccination in adults aged >65 years is cost effective. In England [51], however, the yielded ratio of vaccinating people (>2 years old) with high-risk conditions against IPD using PCV13 was above £30,000 (€37,216) per QALY, and some studies suggested that use of PCV13 in the elderly or adult at-risk population could affect healthcare budgets in Germany [55] and the UK [56].
The model structure of the present work has been used previously to estimate the cost effectiveness of PCV13 in other frameworks such as in a study in the USA [43], which concluded that the administration of one dose of PCV13 in adults ≥50 years would result in a great reduction in the overall burden of PD, being a dominant strategy compared with PPV23 [43].
Despite these studies, it is important to note that this is the first evaluation addressing the question of cost effectiveness for conjugate pneumococcal vaccination that is specially focused in COPD adults and applying the vaccine efficacy data from the CAPiTA trial [32]. Therefore, no comparison with other studies in a similar population is feasible.
The present study has some limitations and assumptions to be considered. In the absence of efficacy data for pneumococcal pneumonia in an ambulatory care setting, we assumed similar efficacy as in the inpatient setting. Given that more than half of COPD patients who develop pneumonia are treated in an inpatient setting and the cost of hospitalization is a key cost driver, the impact of such an assumption is likely to be minimal. Some parameters such as the waning of vaccine protection in the long-term and indirect effects are uncertain. However, a conservative estimate for indirect effects was employed for the base-case scenario and sensitivity analyses for waning effect confirmed the robustness of the results. The present model was developed from a third-party payer perspective; thus, it did not include indirect costs that could be useful for a societal analysis. It is difficult to incorporate reliable data derived from PD in terms of indirect costs. However, the inclusion of indirect costs would lead to a lower ICER, as the working-age population (50–64 years) is expected to have less work loss due to pneumococcal pneumonia. Also, one could argue that PCV13 had little gain in terms of QALY measurement (only 0.0024 per patient), but also the incremental cost was negligible (less than €4 and even dominant in older groups). Finally, Spanish evidence on the incidence and case-fatality rate in PD is lacking in this specific group of patients considered. Nevertheless, data from COPD patients in the UK applied in the model would not differ substantially from the Spanish COPD population due to similarities in healthcare organizations and patient management in both countries.
5 Conclusion
Based on reasonable assumptions regarding PCV13 and PPV23 effectiveness as well as the available epidemiological and cost data, the use of one dose of PCV13 for COPD patients aged ≥50 years, instead of CVP with PPV23, is expected to lead to a decline in IPD, inpatient and outpatient NBP cases, and their related deaths. Furthermore, the incremental costs of this vaccination strategy are counterbalanced in part by savings from averted PD cases. The proposed vaccination strategy is a highly cost-effective option compared with current vaccination with PPV23 based on the accepted Spanish cost-effectiveness threshold of €30,000 per QALY over a lifetime horizon from the NHS perspective.
References
Licciardi PV, Toh ZQ, Dunne E, Wong SS, Mulholland EK, Tang M, et al. Protecting against pneumococcal disease: critical interactions between probiotics and the airway microbiome. PLoS Pathog. 2012;8:e1002652.
Krueger P, St Amant O, Loeb M. Predictors of pneumococcal vaccination among older adults with pneumonia: findings from the Community Acquired Pneumonia Impact Study. BMC Geriatr. 2010;10:44.
Van Hoek AJ, Andrews N, Waight PA, Stowe J, Gates P, George R, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65:17–24.
Picazo JJ, González-Romo F, García Rojas A, Peréz-Trallero E, Gil Gregorio P, de la Cámara R, et al. Consensus document on pneumococcal vaccination in adults with risk underlying clinical conditions [in Spanish]. Rev Esp Quimioter. 2013;26:232–52.
Inghammar M, Engström G, Kahlmeter G, Ljungberg B, Löfdahl CG, Egesten A. Invasive pneumococcal disease in patients with an underlying pulmonary disorder. Clin Microbiol Infect. 2013;19:1148–54.
Shea KM, Edelsberg J, Weycker D, Farkouh RA, Strutton DR, Pelton SI. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1:ofu024.
British Thoracic Society. Guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl. III):iii1–55.
Sicras-Mainar A, Ibáñez-Nolla J, Cifuentes I, Guijarro P, Navarro-Artieda R, Aguilar L. Retrospective epidemiological study for the characterization of community-acquired pneumonia and pneumococcal pneumonia in adults in a well-defined area of Badalona (Barcelona, Spain). BMC Infect Dis. 2012;12:283.
Spanish National Statistical Institute. http://www.ine.es. Accessed 1 Apr 2015.
Gil-Prieto R, García-García L, Álvaro-Meca A, Méndez C, García A, Gil de Miguel A. The burden of hospitalisations for community-acquired pneumonia (CAP) and pneumococcal pneumonia in adults in Spain (2003–2007). Vaccine. 2011;29(3):412–6.
Prato R, Tafuri S, Fortunato F, Martinelli D. Why it is still important that countries know the burden of pneumococcal disease. Hum Vaccin. 2010;6:918–21.
Jiang Y, Gauthier A, Annemans L, van der Linden M, Nicolas-Spony L, Bresse X. Cost-effectiveness of vaccinating adults with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in Germany. Expert Rev Pharmacoecon Outcomes Res. 2012;12:645–60.
Ministerio de Sanidad y Consumo. Vacunación en adultos: recomendaciones año 2004. http://www.msssi.gob.es/ciudadanos/proteccionSalud/vacunaciones/docs/recoVacunasAdultos.pdf. Accessed 1 Apr 2015.
Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
Pitsiou GG, Kioumis IP. Pneumococcal vaccination in adults: does it really work? Respir Med. 2011;105:1776–83.
Metersky ML, Dransfield MT, Jackson LA. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine? Chest. 2010;138:486–90.
Walters JA, Smith S, Poole P, Granger RH, Wood-Baker R. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010; 11:CD001390.
Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55:259–64.
Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–20.
Feldman C, Anderson R. Review: current and new generation pneumococcal vaccines. J Infect. 2014;69:309–25.
Core data sheet: PCV13. http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf. Accessed 24 Apr 2015.
Miravitlles M, Soriano JB, García-Río F, Muñoz L, Duran-Tauleria E, Sanchez G, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64:863–8.
Spanish National Health Survey 2011/2012. http://www.msssi.gob.es/estadEstudios/estadisticas/encuestaNacional/encuesta2011.htm. Accessed 27 Apr 2015.
Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41.
Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–63.
Metcalf BJ, Gertz RE Jr, Gladstone RA, Walker H, Sherwood LK, Jackson D, et al. Strain features and distributions in pneumococci from children with invasive disease before and after 13 valent conjugate vaccine implementation in the United States. Clin Microbiol Infect. 2015. doi:10.1016/j.cmi.2015.08.027.
National Institute of Statistics. Hospital Morbidity Survey 2012. http://www.ine.es. Accessed 17 Oct 2014.
Centers for Disease Control and Prevention. National Center for Health Statistics. http://www.cdc.gov/nchs/?. Accessed 16 Oct 2014.
Merino-Sánchez M, Alfageme-Michavila I, Reyes-Núñez N, Lima-Alvarez J. Prognosis in patients with pneumonia and chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2005;41:607–11.
Kuhlmann A, Theidel U, Pletz MW, Graf von der Schulenburg JM. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Health. Econ Rev. 2012;2:4.
Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30:6802–8.
Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25.
Torres A, Rodriguez Créixems M, Grau I, Molinos L, Rajas O, Linares P, et al. Serotypes distribution and clinical features of invasive pneumococcal disease in immunocompromised vs immunocompetent adults in Spain, 2010–2011 (ODIN STUDY). Eur Respir J. 2012;40:P3981.
Menéndez R, Torres A, España PP, Pérez-Trallero E, López Hontangas JL, Marco F, et al. Pneumococcal serotypes causing community-acquired pneumonia (CAP) among hospitalized adults in Spain using a new urinary antigen detection (UAD) test. The CAPA study. ERJ. 2014;44(Suppl 58):P1810. http://erj.ersjournals.com.proxy1.athensams.net/content/44/Suppl_58/P1810.
Sisk JE, Whang W, Butler JC, Sneller VP, Whitney CG. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann Intern Med. 2003;138:960–8.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22:4203–14.
Bennett JE, Sumner W 2nd, Downs SM, Jaffe DM. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154:43–8.
Ministerio de Sanidad, Servicios Sociales e Igualdad. Diagnosis Related Groups. http://www.msssi.gob.es/estadEstudios/estadisticas/cmbd.htm. Accessed 1 Apr 2015.
Consejo General de Colegios Oficiales de Farmacéuticos. https://botplusweb.portalfarma.com/botplus.aspx. Accessed 1 Apr 2015.
Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. BOE de 24 de Mayo 2010;126. http://www.boe.es/boe/dias/2010/05/24/pdfs/BOE-A-2010-8228.pdf. Accessed 1 Apr 2015.
Lopez-Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11:513–20.
Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. What is an efficient health technology in Spain? Gac Sanit. 2002;16:334–43.
Weycker D, Sato R, Strutton D, Edelsberg J, Atwood M, Jackson LA. Public health and economic impact of 13-valent pneumococcal conjugate vaccine in US adults aged ≥50 years. Vaccine. 2012;3(30):5437–44.
Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31:6011–21.
Liguori G, Parlato A, Zamparelli AS, Belfiore P, Gallé F, Di Onofrio V, Società Italiana di Health Horizon Scanning (SIHHS), et al. Adult immunization with 13-valent pneumococcal vaccine in Campania region, South Italy. Hum Vaccin Immunother. 2014;10:492–7.
Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31:3950–6.
Smith KJ, Wateska AR, Nowalk MP, Raymund M, Lee BY, Zimmerman RK. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in U.S. older adults. Am J Prev Med. 2013;44:373–81.
Pradas R, Gil de Miguel A, Álvaro A, Gil-Prieto R, Lorente R, Méndez C, et al. Budget impact analysis of a pneumococcal vaccination programme in the 65-year-old Spanish cohort using a dynamic model. BMC Infect Dis. 2013;13:175.
Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adults pneumococcal vaccination strategies in Italy. Hum Vaccin Immunother. 2013;9:699–706.
Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307:804–12.
Rozenbaum MH, van Hoek AJ, Fleming D, Trotter CL, Miller E, Edmunds WJ. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ. 2012;345:e6879.
Jiang Y, Gauthier A, Annemans L, van der linden M, Nicolas-Spony L, Bresse X. A public health and budget impact analysis of vaccinating at-risk adults and the elderly against pneumococcal diseases in Germany. Expert Rev Pharmacoecon Outcomes Res. 2012;12:631–43.
Kuhlmann A, Theidel U, Pletz MW, von der Schulenburg JM. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Health Econ Rev. 2012;2:4.
Mangen MJ, Rozenbaum MH, Huijts SM, Van Werkhoven CH, Postma DF, Atwood M, et al. Vaccination of older adults in Netherlands using the 13-valent pneumococcal conjugate vaccine: a cost-utility analysis [oral presentation no. O151]. In: European Congress of Clinical Microbiology and Infectious Diseases; 25–28 Apr 2015; Copenhagen.
Jiang Y, Gauthier A, Annemans L, van der Linden M, Nicolas-Spony L, Bresse X. A public health and budget impact analysis of vaccinating at-risk adults and the elderly against pneumococcal diseases in Germany. Expert Rev Pharmacoecon Outcomes Res. 2012;12(5):631–43.
Jiang Y, Gauthier A, Keeping S, Carroll S. A public health and budget impact analysis of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res. 2014;14(6):901–11.
Acknowledgments
We are indebted to Blanca Gros (former employee of Pharmacoeconomics and Outcomes Research Iberia, Madrid, Spain), Pablo Guijarro (former employee of Pfizer S.L.U), Rogier Klok (Pfizer Inc., Collegeville, PA, USA), and Reiko Sato (Pfizer Inc., Collegeville, PA, USA) for their critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Pfizer S.L.U., Alcobendas (Madrid), Spain.
Conflict of interest
N. Lwoff and J. Rejas are employees of Pfizer S.L.U. I. Oyagüez and M. Echave are employees of Pharmacoeconomics and Outcomes Research Iberia (PORIB), Madrid, Spain, a consultant company specialized in economic evaluation of health technologies, which has received financial support for the present model customization and manuscript drafting. F. Antoñanzas, M. Campins, R. Menéndez, and J.M. Rodríguez González-Moro have received an unrestricted grant, as members of an advisory board, to validate the model inputs. All authors had complete access to the data, participated in the analysis and/or interpretation of results, and have approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rodríguez González-Moro, J.M., Menéndez, R., Campins, M. et al. Cost Effectiveness of the 13-Valent Pneumococcal Conjugate Vaccination Program in Chronic Obstructive Pulmonary Disease Patients Aged 50+ Years in Spain. Clin Drug Investig 36, 41–53 (2016). https://doi.org/10.1007/s40261-015-0345-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0345-z