Abstract
Natural killer T (NKT) cells play a pivotal role as a bridge between the innate and the adaptive immune response and are instrumental in the regulation of homeostasis. In this review, we discuss the potential for NKT cells to serve as biodrugs in viral infections and in cancer. NKT cells are being investigated for their use as a prognostic biomarker, an immune adjuvant, and as a form of cellular therapy. Historically, the clinical utility of NKT cells was hampered by their low frequency in the blood, discrepancies in nomenclature, and challenges with ex vivo expansion. However, recent advances in the field have permitted the development of several NKT cell-based preclinical and clinical strategies. These new developments pave the way for the successful implementation of NKT cell-based approaches for the treatment of human disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
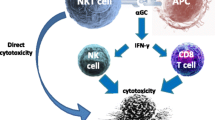
NKT cells can directly mediate lysis of infected and cancer cells, as well as induce other effector cells through their expeditious release of cytokines. |
Adoptive transfer of NKT cells into cancer patients holds promise as NKT cells can target these cells and mediate protection. |
Current immunotherapeutic strategies using chimeric antigen receptors, bispecific T cell engagers, and tumor vaccines are being developed to harness the potential of NKT cells. |
1 Natural Killer T (NKT) Cells
Natural killer T (NKT) cells are an innate-like population of CD1d-restricted T lymphocytes that are characterized by rapid cytokine production following activation [1,2,3,4]. NKT cells express cell surface markers that are characteristic of NK cells (CD56, CD161) and T cells, such as a T-cell receptor (TCR). In addition to their expeditious release of cytokines, after activation NKT cells also upregulate the expression of cell death-inducing molecules, such as perforin, granzymes, and FAS ligand, which allows them to kill cancerous and infected cells [5, 6].
CD1d-restricted NKT cells can be further characterized based on their TCR expression. Type I invariant NKT (iNKT) cells express a specific TCRα chain, Vα14Jα18 in mice and Vα24Jα18 in humans, in combination with specific TCRβ chains (Vβ8.2, 7 or 2 in mice, Vβ11 in humans) [7,8,9,10]. Type I iNKT cells are also noted by their ability to be activated by the glycolipid, α-galactocylceramide (α-GalCer) [11,12,13], presented in the context of CD1d. Type I NKT cells are less frequent in humans than in mice, and make up 0.1–1% of circulating T cells in the blood [14]. In contrast, type II NKT cells express diverse TCRs, are CD1d-restricted, but are unresponsive to α-GalCer [15]. They have been investigated experimentally using type II NKT cell TCR–CD1d-antigen complexes CD1d-tetramers loaded with other lipid antigens, specifically phospholipids, sphingolipids, and glycerolipids [16, 17]. The diversity in the TCR repertoire can make it difficult to thoroughly characterize this population in humans and can lead to some ambiguity when investigating CD1d-specific NKT cells and other NKT-like subpopulations. For example, many human studies investigate CD56+CD3+ NKT-like cells, but this a heterogeneous mixture of T cells that includes mucosal-associated invariant T (MAIT), γδ T cells, activated CD8+ T cells, as well as CD1d-restricted type I and type II NKT cells [18]. Type II NKT cells are thought to be present in higher numbers in humans, compared to type I NKT cells, and gaining a better understanding of their regulation is critical. Fortunately, recent studies from several groups have made significant progress in this area [19, 20].
Similar to classic T-cell subsets, NKT cells develop in the thymus, but they diverge when they reach the double positive stage [21]. In fact, iNKT cell development has been well characterized [22]. Instead of being selected on thymic epithelial cells, they are selected by other double positive thymocytes [23]. This selection event is dependent on engagement between the TCR and CD1d as well as homotypic interactions between the signaling lymphocytic activation molecule (SLAM) family of receptors, which initiate the NKT cell developmental program by upregulating the early growth response 2 (Egr2) and promyelocytic leukemia zinc finger (PLZF) transcription factors [24,25,26,27,28]. iNKT cells can be divided into subsets similar to CD4 T-helper (Th) subsets. NKT1 cells express the transcription factor T-box expressed in T cells (T-bet) and primarily secrete gamma interferon (IFN-γ); NKT2 cells express high levels of GATA binding protein 3 (GATA3) and PLZF and secrete Th2-type cytokines, such as IL-4 and IL-13. NKT17 express intermediate levels of PLZF, are RAR-related orphan nuclear receptor (ROR)γt+ and secrete IL-17 [29,30,31]. Despite effector differentiation occurring during thymic development, significant plasticity in cytokine production has been demonstrated after stimulation [32]. Other NKT cell subsets have been described, such as IL-9 producing NKT cells at mucosal surfaces, B-cell lymphoma 6 (BCL6) expressing NKTFH (follicular helper) cells that produce IL-21, and NKT10 cells, which express the transcription factor Nuclear Factor, Interleukin 3 Regulated (Nfil3/E4BP4), rather than PLZF, and produce IL-10 [33,34,35,36]. Notably, iNKT cell subsets can regulate other lymphocyte subpopulations developing around them [37].
In contrast to classic T-cell subsets, the majority of iNKT cells are tissue resident and do not circulate [38,39,40]. iNKT cells express non-lymphoid tissue homing chemokine receptors such as CCR2, CCR5, and CXCR3. NKT cells have different modes of activation. Specifically, iNKT cells can be activated through antigen-dependent and antigen-independent mechanisms [41, 42]. For example, iNKT cell effector functions can be induced by danger signals (ex. toll like receptor (TLR) signaling) or by cytokines such as IL-12 and IL-18 [43, 44]. In humans, iNKT cells express CD4+, CD8+, or neither (CD4-CD8-), referred to as double negative (DN) [16,17,18,19]; however, in mice iNKT cells express CD4+ or are DN [15] because they express the transcription factor Th-POK (T-helper-inducing POZ/Krüppel-like factor), which blocks CD8 expression [45]. While most of the reports on α-GalCer-reactive NKT describe iNKT cells, α-GalCer-reactive, CD1d-restricted NKT cells that use different TCR α-chains have been identified in mice [46] and humans [47,48,49]. There are numerous populations of NKT-like cells, which can express diverse αβ TCRs, recognize different lipid antigens (5), and express a variety of markers associated with natural killer (NK) cells.
While NKT cells comprise a relatively small population of T cells, their ability to bridge innate and adaptive immune responses establishes them as an important regulatory cell population. In addition to their expeditious release of cytokines, NKT cells can lyse infected or malignant cells [50,51,52,53]. However, NKT cell number and activity are reduced in multiple cancer types and in chronic infections; therefore, understanding factors that regulate their development and effector functions are of significant interest [54,55,56].
2 NKT Cells and Viral Infections
NKT cells are thought to play a key role in controlling viral infections, primarily due to their production of high levels of IFN-γ and the fact that many viruses have evolved mechanisms to downregulate CD1d-mediated antigen presentation to NKT cells [57,58,59,60,61,62,63]. Studies investigating the contribution of NKT cells in antiviral immune responses in humans are limited [64], but in the context of HIV-1, NKT cells have been shown to be reduced following infection [65,66,67,68]. In addition, in chronically infected patients, iNKT cells have been reported to have an exhausted phenotype [69]. Importantly, iNKT cells have been shown to recognize HIV-1-infected DCs, and therefore can play a critical role during the early stages of infection [65].
COVID-19, the disease caused by the novel coronavirus SARS-CoV-2, is one of the most devastating global pandemics in modern history [70, 71]. As of August 2022, the coronavirus disease 2019 (COVID-19) pandemic has resulted in 581.8 million confirmed cases and 6.4 million deaths have been reported globally (World Health Organization). The symptoms from the disease can vary widely, and many studies have focused on immune profiling of COVID-19 patients to identify factors involved in susceptibility to infection and disease pathology [70, 72]. Given the ability of NKT cells to respond to virally infected cells, several studies have examined iNKT and NKT-like cells in COVID-19 patients [18, 73,74,75,76,77]. For example, Liu et al. investigated circulating iNKT (Vα24Jα18+) and NKT-like (CD56+CD3+) cells in 49 COVID-19-convalescent individuals (CI) compared to 27 matched SARS-CoV-2-unexposed individuals (UI) [73]. They observed a significant decrease in the percentage of both iNKT and NKT-like cells in the CI compared to UI cohort months after recovery. In a study that recruited three cohorts of participants from centers across Germany and France, it was found that the frequency of circulating NKT-like cells (CD56+CD3+) served as a predictive biomarker for disease severity in COVID-19 patients [74]. However, as noted by Koay and colleagues, the majority of CD56+CD3+ are not iNKT cells [18]. Moreover, when Koay et al. examined circulating NKT cells from hospitalized patients using α-GalCer-loaded tetramers, no significant differences in iNKTs were observed between COVID-19 patients that were indicative of disease severity. Taken together, these studies suggest that infection with SAR-CoV-2 can lead to a reduction in circulating NKT-like cells and that these cells may serve as a prognostic or predictive biomarker of disease. In contrast, additional mechanistic studies are needed to determine if classic iNKT cells respond to SARS-CoV-2 infected cells and if the virus utilizes specific mechanisms to subvert CD1d-mediated antigen presentation. It would be intriguing to investigate the effectiveness of the adoptive transfer of NKT cells into virally infected patients, particularly as several studies have demonstrated that patients with mutations in immune-related genes or primary immune deficiency diseases that result in NKT cell deficiency can also have increased susceptibility to viral infections [78,79,80].
3 NKT Cells and Adoptive Immunotherapy
One immunotherapeutic strategy that has transformed the treatment of B-cell malignancies is chimeric antigen receptors (CARs). CARs are synthetic receptors engineered to contain a single-chain variable fragment (scFv) that permits specific extracellular antigen recognition and binding and a CD3ζ domain, the intracellular domain through which the TCR signals [81]. Traditionally, T cells are transduced or transfected with the CAR and then infused into patients for cancer immunotherapy. CARs consisting of only the extracellular scFv and intracellular CD3ζ are known as first-generation CARs. However, these CARs still need endogenous co-stimulation for T-cell activation against the tumor. The addition of either one or two co-stimulatory endodomains to CD3ζ, known as second- and third-generation CARs, respectively, improves proliferation, in vivo persistence, and antitumor efficacy. Fourth-generation CARs have also been engineered to include a transgene that encodes for a cytokine to promote activation of the cell attached to the CAR and further improve antitumor efficacy [82].
CAR-T cells are very effective for the treatment of B cell malignancies; however, success in solid tumors has been limited by the immunosuppressive tumor microenvironment and due to challenges in the identification of suitable targets. Given that iNKT cells are CD1d-restricted, they have the ability to target different tumor types. In neuroblastoma, the GD2 ganglioside has been shown to be an effective target. Therefore, Heczey et al. generated and expanded ex vivo CAR.GD2-NKT cells [83], based on the GD2 antibody clone 14.G2a. CAR.GD2-NKT cells are cytotoxic against GD2-positive neuroblasts and against CD1d-positive cells, indicating the dual-specific cytotoxicity of CAR.GD2-NKT cells. The inclusion of co-stimulatory endodomains, CD28 (G28z) or 4-1BB (GBBz) or both (G28BBz), resulted in improved survival of the CAR-NKT cells. To examine the impact in vivo, CAR.GD2 NKT cells were adoptively transferred into a metastatic neuroblastoma xenograft model, and it was found that the inclusion of these co-stimulatory domains resulted in improved survival. In addition, the frequency of tumor-infiltrating CAR.GD2 NKT cells was greater than that of CAR.GD2 T cells demonstrating the ability of CAR.GD2 NKT cells to localize to tumor sites [83]. A concern of CAR-T cell immunotherapy has been the induction of graft versus host disease (GVHD). In a hu-NSG mice model, it was found that CAR.GD2 NKT cells did not induce GVHD, indicating the allogeneic potential of CAR-NKT cells compared to CAR-T cells [83] (Fig. 1).
Advantages of chimeric antigen receptor (CAR)-invariant NKTs (iNKTs). CAR-iNKT cells can recognize tumor cells through both their standard iNKT T-cell receptor (TCR) and the specific anti-tumor antigen CAR, leading to targeted cytotoxicity. The intracellular portion of the CAR consists of the CD3ζ domain for TCR signaling. The CAR can also be modified to include a co-stimulatory endodomain and a cytokine transgene to increase production of pro-inflammatory cytokines and enhance overall antitumor efficacy. CAR-iNKT cells hold great promise for immunotherapy as they overcome various obstacles that hinder the efficacy of other commonly used immunotherapies. CAR-iNKT cells can persist in vitro and in vivo to minimize tumor recurrence. Unlike traditional CAR-T cells composed of classic CD4 and CD8 T cells, CAR-iNKT cells do not induce an allogenic response against healthy cells and therefore prevent the induction of graft-versus-host disease (GVHD). CAR-iNKT cells can also infiltrate tumor sites and localize to tumor sites, maximizing their antitumor potential
While CAR.GD2 NKT cells were shown to increase survival in mice, recurrence of tumor emphasized the need to enhance in vivo persistence of these transduced NKT cells. In another set of studies focused on neuroblastoma, GD2.CAR NKT cells were engineered to co-express IL-15 with either CD28 or 4-1BB co-stimulatory endodomain, further denoted by GD2.28z.15 and GD2.BBz.15, respectively, to evaluate in vivo persistence of CAR-NKTs [84]. However, through functional testing it was shown that CAR-NKT cells expressing 4-1BB undergo activation-induced cell death leading to reduced CAR-NKT cell numbers during ex vivo expansion. The co-expression of IL-15 with the CD28 endodomain promoted survival and in vitro functional fitness of GD2.CAR NKT cells through increased cellular expansion and greater control over tumor cells. The GD2.CAR NKT cells with and without IL-15 co-expression were adoptively transferred into NSG mice. The GD2.28z.15 construct allowed for enhanced in vivo expansion and persistence of NKT cells without significant cytotoxicity [84]. A close examination of the neuroblastoma nodules in the liver, spleen, bone marrow, and lungs of NSG mice, revealed that GD2.28z.15 NKT cells were present at high numbers indicating that these CAR-NKT cells are capable of effectively infiltrating and persisting in tumor tissues [84] (Fig. 1).
Based on these promising in vitro and in vivo results [83, 84], GD2.CAR-NKT cells are currently being evaluated in a clinical trial for children with relapsed or resistant neuroblastoma (NCT03294954) [85]. The interim results demonstrated efficacy of autologous CAR-NKT cells, specifically GD2.CAR-NKT cells with co-expression of IL-15, to effectively and safely expand and traffic to tumor sites in patients with refractory neuroblastoma. In the past, low numbers of circulating NKT cells have been a major of concern; however, these studies demonstrate that CAR-NKTs can be successfully expanded ex vivo on a clinical scale to treat patients (Fig. 1).
More than half of patients with B-cell lymphomas that are treated with anti-CD19 CAR (CAR19)-T cell immunotherapy relapse, indicating the need to develop more effective immunotherapeutic strategies [86, 87]. Due to the effector functions of iNKT cells and the expression of CD1d on these cells, the generation of a CAR19-iNKT cell holds promise for a greater anti-tumor effect in B-cell malignancies [88, 89]. CAR19-iNKT cells exposed to α-GalCer, a potent iNKT cell agonist, resulted in increased cytotoxicity against CD1d+ and CD1d+CD19+ targets, but not CD1d-CD19- and CD1d-CD19+ targets [88]. The interaction of CD1d on target cells with the CAR19-iNKTcells is important due to the dual targeting of CD1d and CD19. Compared to CAR19-T cells, CAR19-iNKT cells had greater proliferation and expansion in B lineage malignancies. When CAR19-iNKT and CAR19-T cells were infused into tumor-engrafted NSG mice with CD1d+CD19+ B cell malignancy, the CAR19-iNKT cells had improved overall and tumor-free survival, indicating the enhanced in vivo anti-tumor activity of CAR19-iNKT cells compared to CAR19-T cells. Therefore, the use of iNKT cells in a CAR-based immunotherapy could be effective in cancers that express CD1d. In a study investigating lymphoma in the brain, it was found that the majority of the mice treated with CAR19-iNKT cells were able to decrease brain tumor burden below a detectable threshold, indicating the ability of CAR19-iNKT cells to control and eliminate brain metastases. Even in mice that relapsed, the CAR19-iNKT cells were able to persist and lead to a second remission [87]. Due to the encouraging results from Rotolo et al. [87] and others, a clinical trial (NCT03774654) has been initiated for relapsed or refractory B-cell malignancies investigating the efficacy of allogeneic CAR-NKT cells by utilizing CD19 specific CAR-NKT cells that co-express CD28 and IL-15.
Moreover, iNKT cells have been shown to induce CD8 T-cell cross-priming, which leads to long-term CD8 T-cell responses. Recent studies by Simonetta and colleagues demonstrate that allogenic CAR-iNKT cells can induce host CD8 T-cell cross-priming in a B-cell lymphoma mouse model [90]. In BALB/c BATF3-/- mice, which are defective in CD8 T-cell cross-priming, the antitumor effect was decreased compared to wildtype controls. The authors found that the co-administration of allogenic CAR-iNKT cells and autologous CD8 T cells significantly enhanced tumor control and prolonged survival, compared to treatment with either cell type alone [90]. These data suggest that the effectiveness of CAR-iNKT cells is enhanced by the presence of CD8 T-cell cross priming. Allogenic CAR-iNKT primed CD8 T cells were transferred into lethally irradiated BALB/c mice and resulted in prolonged survival compared to mice receiving unprimed CD8 T cells. These results suggegst a key role for allogenic CAR-iNKT treatment in promoting long-term CD8 T-cell anti-tumor responses. Overall, these studies show that CAR-iNKT cells can induce CD8 T-cell cross-priming and enhances their antitumor efficacy, as well as highlights the potential of CAR-iNKT cell therapy as an off-the-shelf immunotherapy.
Overexpression of chondroitin sulfate proteoglycan-4 (CSPG4), also known as high molecular-weight-melanoma-associated antigen (HMW-MAA), is associated with the progression of many types of cancer such as melanoma, breast cancer, squamous cell carcinoma, mesothelioma, neuroblastoma, and sarcoma [91]. Simon et al. [92] developed a method to generate CSPG4-CAR NKT cells. Specifically, DNA-based constructs or transient RNA-based constructs can be used to enable T cells to express CARs. In this study the authors assessed the effectiveness of transduction using RNA-based constructs to standard DNA-based transduction, because of the advantages provided by RNA, such as the lack of chromosomal integration and genetic alteration, and potential for decreased side effects. CSPG4-CAR NKTs were able to eliminate human melanoma cells in vitro by producing a large amount of pro-inflammatory cytokines. Cytotoxicity levels were similar between these mRNA-based CAR NKT cells and traditionally transfected CAR-T cells when tested against a melanoma cell line A375M [92]. The results from this study show that CAR-NKT cells can be a safe and effective platform, similar to CAR-T cells for immunotherapy.
CAR-iNKTs have also been tested in multiple myeloma (MM) by using MM-associated antigen CD38 and B-cell maturation antigen (BCMA) to direct the iNKTs to the tumor cells [93]. The BCMA-CAR iNKT cells were designed based on BCMA-CAR T cells and are currently being tested (clinical trial:NCT02658929) [94]. Previous work optimized a CD38B1-CAR that targets cells expressing high levels of CD38, thereby only targeting MM cells and not normal healthy cells [95]. BCMA-CAR iNKTs were able to mediate cytotoxicity against the MM cell line UM9 [93]. UM9 cells only express intermediate levels of CD38, thus treatment with CD38-CAR iNKTs resulted in ~60% cell lysis. When tested against MM1.s, a CD1d positive cell line that expresses high levels of BCMA and CD38, both BCMA-CAR iNKTs and CD38-CAR iNKTs completely eliminated the tumor cells. Importantly, both BCMA-CAR iNKTs and CD38-CAR iNKTs were able to lyse primary MM cells, even those with little or no CD1d expression. Upon stimulation with α-GalCer, both CD38-CAR and BCMA-CAR iNKTs were able to expand ex vivo and maintain their antitumor efficacy [93]. Another recruiting clinical trial is evaluating the use of CAR-iNKT cells co-expressing CD19 and IL-15 for targeting of B-cell tumors. This study aims to determine the safety, efficacy, and feasibility of this allogenic iNKT cell therapy (clinical trial number NCT04814004; clinicaltrials.gov). Please see Table I for a summary of strategies targeting NKT cells for cancer immunotherapy.
4 Antibody-Based Therapies for Invariant NKTs (iNKTs)
The implementation of immune checkpoint inhibitors (ICIs) has completely transformed the treatment of cancer [96]. The US Food and Drug Administration (FDA) approved the first ICI, ipilimumab, a mAb that targets cytotoxic T-lymphocyte-associated antigen (CTLA)-4 in 2011, and mAbs targeting programmed death (PD)-1 and PD-L1 subsequently received FDA approvals [97]. PD-1 (CD279), a co-inhibitory molecule, is a member of the CD28 family [98], along with its ligands PD-L1 and PD-L2. In a study investigating the role of the PD-1 pathway on α-GalCer-induced iNKT cell anergy in mice, it was found that of the use of PD-1/PD-L mAbs simultaneously with α-GalCer treatment blocked the induction of iNKT cell anergy. In addition, inhibiting PD-1/PD-L interactions led to an increase in α-GalCer-treatment-induced anti-tumor responses. PD1 appears to play a critical role in α-GalCer-induced iNKT cell anergy because it was significantly abrogated in PD1-deficient animals [99]. Another study investigating the role of PD-1/PD-L in human iNKT cells found that activation with α-GalCer resulted in PD-1 upregulation, whereas PD-L1 blockade enhanced iNKT cell effector functions, as indicated by Th1 cytokine production and cytotoxicity [100].
5 NKT Cell Activation Using Soluble CD1d Proteins
In addition to PD-L1, tumors cells can express many different inhibitory factors that suppress iNKT cell activation. In order to overcome these suppressive factors and α-GalCer-activation-induced anergy, several studies have investigated the utility of recombinant soluble CD1d proteins loaded with α-GalCer [101,102,103]. It was found that α-GalCer/sCD1d can be repeatedly injected in mice without inducing iNKT exhaustion and lead to sustained iNKT and NK cell activation, as well as DC maturation. Furthermore, the authors found that treatment of HER2+B16 melanoma tumor-bearing mice with a fusion protein containing α-GalCer/sCD1d and an HER2-specific scFv antibody fragment resulted in a significant reduction in tumor burden [101]. Specifically, it was found that liver iNKT cells from α-GalCer/sCD1d-anti-HER2-treated mice remained responsive after repeated injections. Mechanistically, when the authors examined mice injected with either α-GalCer/sCD1d-anti-HER2 or with α-GalCer/sCD1d protein, it was found that treatment with the HER2-targeted α-GalCer/sCD1d protein was able to redirect iNKT, NK, and T cells to the tumor site [101]. Another group investigated the function of a bispecific fusion protein composed of human CD1d joined to a scFv fragment specific for CD19, in order to target NKT cells to B-cell malignancies. It was found that following the loading of αGC, the CD1d-CD19 fusion protein was able to activate iNKT cell effector function both in vitro and in vivo [102].
In contrast to scFv, which are antibody fragments produced by fusing one variable region of the heavy chain (VH) and one variable region of the light chain (VL), bi-specific T-cell engagers (BiTEs) are composed of two scFvs connected by a short peptide linker [104]. BiTEs typically target one CD3 molecule and one tumor antigen, such as Blinatumomab, which targets CD3 and CD19 [104]. Importantly, it has been shown that BiTEs can induce potent iNKT cell responses that can enhance tumor cell death (Fig. 2). It was shown that when PBMC from healthy donors were cultured with a CD3xPD-L1 BiTE in the presence or absence of PD-L1+ human melanoma C8161 cells, the BiTE induced high levels of IFN-y, due in part to the activation of NKT cells [105]. Notably, in this study NKT cells were classified as CD3+CD56+, thus this population is NKT-like [105]. Lameris and colleagues developed CD1d-specific single-domain antibodies (VHH), that can elicit potent iNKT cell activation in the absence of an exogenous antigen like α-GalCer by its intrinsic ability to interact with CD1d and the type I NKT TCR [106]. Treatment with this platform greatly enhanced type I NKT cell-mediated antitumor activity in both in vitro and in vivo models [106]. Based on this technology, a bispecific fusion protein composed of two VHH domain antibodies linked via a short, five amino acid glycine-serine linker, called LAVA-051, has been developed. LAVA-051 activates Vγ9Vδ2 T cells and type I NKT cells and induces killing of CD1d-expressing tumor cells, and is currently being tested in the clinic (clinical trial NCT04887259).
Bi-specific T-cell engagers (BiTEs) involve the fusion of the single chain fragment variables of two monoclonal antibodies to bind both a T cell and a tumor cell with the goal of redirecting T cells to the tumor cells. Due to the invariant T-cell receptor (TCR) of natural killer T (NKT) cells, BiTEs are capable of also binding to the CD3 chain of NKT cells crosslinking them to antigen-specific tumor cells and allowing for direct NKT cell-mediated killing. Similarly, fusion of the scFV region of a HER2 antibody to a soluble CD1d loaded with αGalCer activates NKT cells to target and directly kill HER2 positive tumor cells
6 Additional Strategies Used to Manipulate iNKTs
Oncolytic viruses are being investigated as an approach to enhance antitumor immune responses, due to their ability to selectively infect and kill tumor cells. Gebremeskel and colleagues investigated the effectiveness of two different viruses, vesicular stomatitis virus (VSV) and reovirus, in combination with α-GalCer-loaded DCs, in immunocompetent mouse models of breast and ovarian cancer [107]. The combination of either oncolytic VSV or reovirus with NKT cell immunotherapy resulted in an increase in survival of ID8 ovarian cancer tumor-bearing mice. In contrast, only treatment with VSV in combination with NKT cell immunotherapy led to a decrease in metastasis and an increase in survival in the 4T1 breast cancer model [107]. A recent study from this group investigated the utility of VSV expressing IL-15 in combination with anti-PD-1 mAb and NKT cell-based immunotherapy for the treatment of pancreatic cancer [108]. It was found that while tumors relapsed over time in both subcutaneous and orthotopic Panc02 tumor models, combination of VSV-IL-15 and NKT cell activation correlated with immune cell infiltration, decreased pancreatic tumor burden, and increased survival, which was further enhanced by PD-1 blockade [108].
As highlighted above, there are many strategies utilized by tumors to evade or suppress NKT cell-mediated antitumor immune responses and several groups are developing strategies to restore NKT cell effector functions (Fig. 3). One subset of immunosuppressive cells are myeloid-derived suppressor cells (MDSCs), which have been implicated in fostering an immunosuppressive tumor environment through secretion of cytokines such as TGF-β and IL-10, which supports the development of regulatory T cells (Tregs). Ko and colleagues sought to investigate whether MDSCs loaded with α -GalCer and tumor-specific peptide could serve as antigen-presenting cells and induce antigen-specific immune responses [109]. It was found that the inclusion of an NKT cell agonist significantly enhanced anti-tumor immunity. Moreover, in a study employing a B16F10 melanoma model, it was found that injection of α-GalCer resulted in an increased number of tumor-infiltrating, IFN-γ-producing NKT cells in the tumor, and favored iNOS+F4/80+CD11b+ macrophages (M1) over the CD206+F4/80+CD11b+ macrophages (M2) in the spleen and tumor, and a concomitant reduction in tumor burden [110]. Importantly, it was found that depletion of F4/80+ macrophages completely abrogated the α-GalCer-induced reduction in tumor growth [110], which further suggests a role for targeting monocytes and macrophages in iNKT cell-based immunotherapeutic strategies. In fact, there have been several clinical studies investigating the efficacy of NKT-cell based immunotherapy (see Table 1). In an open-label, single-arm, phase II clinical trial (UMIN000007321) in patients with advanced or recurrent non-small-cell lung cancer (NSCLC) refractory to first-line chemotherapy, blood-derived α-GalCer-pulsed antigen presenting cells (APCs) were intravenously administered to 35 patients [111]. The mean estimated survival time (MST) estimated for all 35 patients was 21.9 months (95% 14.8–26.0), with one patient showing partial response. The administration of α-GalCer-pulsed APCs significantly increased the number of NK cells, IFN-γ-producing cells, and effector CD8+T cells, but did not cause any severe adverse events [111]. The results from the trial warrant further randomized trials.
In addition to promoting direct natural killer T (NKT)-cell mediating killing of tumor cells, target treatments and immunotherapies have been developed to enhance the ability of NKT cells to indirectly eliminate tumor cells. Activated NKT cells produce cytokines, such as IFN-γ, TNF-α, and GM-CSF, that can help promote the activation of NK cells, CD8+ T cells, and in combination with CD40/CD40L interactions lead to the maturation of dendritic cells (DCs), further enhancing anti-tumor immune responses. Oncolytic viruses can increase antigen presentation. α-GalCer-loaded DCs increase the activation of invariant NKT (iNKT), NK, and T cells. CD1d-antibody fusion proteins increase the cytotoxicity of iNKT, NK, and T cells against tumor cells
7 Discussion
It is time to finally harness the potential of iNKT cells and develop strategies to facilitate their use in the clinic. Recent clinical studies have demonstrated that they can be used in CAR-based strategies, can enhance graft versus leukemia (GvL) responses, and serve as a prognostic or predictive biomarker in many disease contexts. In fact, elegant preclinical studies from Dr. Yang’s group have investigated the in vivo efficacy of hematopoietic stem cell-engineered iNKT (HSC-iNKT) cell-based therapy for the treatment of melanoma and multiple myeloma [112, 113]. However, due to challenges inherent to the field such as the nomenclature (iNKT vs. NKT-like), low circulating frequency in human blood, and relatively limited number of investigators focused on therapeutic strategies targeting unconventional lymphocytes, their implementation into clinical practice has been slow. Given the recent promising results using CAR-iNKTs, bispecific platforms and monocyte-based approaches, these nonconventional lymphocyte subpopulations are important therapeutic targets for the treatment of cancer and infectious diseases.
References
Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int Immunol. 1995;7(7):1157–61.
Fowlkes BJ, Kruisbeek AM, Ton-That H, Weston MA, Coligan JE, Schwartz RH, et al. A novel population of T-cell receptor ab-bearing thymocytes which predominantly expresses a single Vb gene family. Nature. 1987;329(6136):251–4.
Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11(3):197–206. https://doi.org/10.1038/ni.1841.
Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11(2):131–42. https://doi.org/10.1038/nri2904.
Ingram Z, Madan S, Merchant J, Carter Z, Gordon Z, Carey G, et al. Targeting natural killer T cells in solid malignancies. Cells. 2021. https://doi.org/10.3390/cells10061329.
Shissler SC, Lee MS, Webb TJ. Mixed signals: co-stimulation in invariant natural killer T cell-mediated cancer immunotherapy. Front Immunol. 2017;8:1447. https://doi.org/10.3389/fimmu.2017.01447.
Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of Va14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–9.
Koseki H, Imai K, Nakayama F, Sado T, Moriwaki K, Taniguchi M. Homogenous junctional sequence of the V14+ T-cell antigen receptor alpha chain expanded in unprimed mice. Proc Natl Acad Sci U S A. 1990;87(14):5248–52. https://doi.org/10.1073/pnas.87.14.5248.
Lantz O, Bendelac A. An invariant T cell receptor a chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180(3):1097–106.
Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24-JαQ/Vb11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med. 1994;180:1171–6.
Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, et al. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J Immunol. 1998;161:3271–81.
Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, et al. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38(12):2176–87. https://doi.org/10.1021/jm00012a018.
Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12(12):845–57. https://doi.org/10.1038/nri3328.
Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16(11):1114–23. https://doi.org/10.1038/ni.3298.
Dasgupta S, Kumar V. Type II NKT cells: a distinct CD1d-restricted immune regulatory NKT cell subset. Immunogenetics. 2016;68(8):665–76. https://doi.org/10.1007/s00251-016-0930-1.
Nair S, Boddupalli CS, Verma R, Liu J, Yang R, Pastores GM, et al. Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation. Blood. 2015;125(8):1256–71. https://doi.org/10.1182/blood-2014-09-600270.
Patel O, Pellicci DG, Gras S, Sandoval-Romero ML, Uldrich AP, Mallevaey T, et al. Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor. Nat Immunol. 2012;13(9):857–63. https://doi.org/10.1038/ni.2372.
Koay H-F, Gherardin NA, Nguyen THO, Zhang W, Habel JR, Seneviratna R, et al. Are NKT cells a useful predictor of COVID-19 severity? Immunity. 2022;55(2):185–7. https://doi.org/10.1016/j.immuni.2022.01.005.
Singh AK, Tripathi P, Cardell SL. Type II NKt cells: an elusive population with immunoregulatory properties. Front Immunol. 2018;9:1969. https://doi.org/10.3389/fimmu.2018.01969.
Yang Zhou J, Werner JM, Glehr G, Geissler EK, Hutchinson JA, Kronenberg K. Identification and isolation of type II NKT cell subsets in human blood and liver. Front Immunol. 2022;13: 898473. https://doi.org/10.3389/fimmu.2022.898473.
Pellicci DG, Koay H-F, Berzins SP. Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge. Nat Rev Immunol. 2020;20(12):756–70. https://doi.org/10.1038/s41577-020-0345-y.
Shissler SC, Webb TJ. The ins and outs of type I iNKT cell development. Mol Immunol. 2019;105:116–30. https://doi.org/10.1016/j.molimm.2018.09.023.
Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164(5):2412–8.
Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–6.
Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27(5):751–62. https://doi.org/10.1016/j.immuni.2007.08.020.
Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, et al. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10(3):306–13. https://doi.org/10.1038/ni.1696.
Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9(9):1055–64. https://doi.org/10.1038/ni.1641.
Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. https://doi.org/10.1016/j.immuni.2008.07.011.
Lee YJ, Starrett GJ, Lee ST, Yang R, Henzler CM, Jameson SC, et al. Lineage-specific effector signatures of invariant NKT cells are shared amongst gammadelta T, innate lymphoid, and Th cells. J Immunol. 2016;197(4):1460–70. https://doi.org/10.4049/jimmunol.1600643.
Engel I, Seumois G, Chavez L, Samaniego-Castruita D, White B, Chawla A, et al. Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat Immunol. 2016;17(6):728–39. https://doi.org/10.1038/ni.3437.
Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25(2):161–7. https://doi.org/10.1016/j.coi.2013.01.003.
Cameron G, Godfrey DI. Differential surface phenotype and context-dependent reactivity of functionally diverse NKT cells. Immunol Cell Biol. 2018. https://doi.org/10.1111/imcb.12034.
Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124(9):3725–40. https://doi.org/10.1172/JCI72308.
Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011;12(5):450–9. https://doi.org/10.1038/ni.2020.
Tonti E, Fedeli M, Napolitano A, Iannacone M, von Andrian UH, Guidotti LG, et al. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J Immunol. 2012;188(7):3217–22. https://doi.org/10.4049/jimmunol.1103501.
Monteiro M, Agua-Doce A, Almeida CF, Fonseca-Pereira D, Veiga-Fernandes H, Graca L. IL-9 expression by invariant NKT cells is not imprinted during thymic development. J Immunol. 2015;195(7):3463–71. https://doi.org/10.4049/jimmunol.1403170.
Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14(11):1146–54. https://doi.org/10.1038/ni.2731.
Fan X, Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell. 2016;164(6):1198–211. https://doi.org/10.1016/j.cell.2016.02.048.
Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018;18(9):559–74. https://doi.org/10.1038/s41577-018-0034-2.
Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1–ICAM-1 interactions. J Exp Med. 2011;208(6):1179–88. https://doi.org/10.1084/jem.20102630.
Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101–17. https://doi.org/10.1038/nri3369.
Brutkiewicz RR. CD1d ligands: the good, the bad, and the ugly. J Immunol. 2006;177(2):769–75.
Georgiev H, Ravens I, Benarafa C, Förster R, Bernhardt G. Distinct gene expression patterns correlate with developmental and functional traits of iNKT subsets. Nat Commun. 2016;7(1):13116. https://doi.org/10.1038/ncomms13116.
Lee YJ, Starrett GJ, Lee ST, Yang R, Henzler CM, Jameson SC, et al. Lineage-specific effector signatures of invariant NKT cells are shared amongst γδ T, innate lymphoid, and Th cells. J Immunol. 2016;197(4):1460–70. https://doi.org/10.4049/jimmunol.1600643.
Engel I, Hammond K, Sullivan BA, He X, Taniuchi I, Kappes D, et al. Co-receptor choice by Vα14i NKT cells is driven by Th-POK expression rather than avoidance of CD8-mediated negative selection. J Exp Med. 2010;207(5):1015–29. https://doi.org/10.1084/jem.20090557.
Uldrich AP, Patel O, Cameron G, Pellicci DG, Day EB, Sullivan LC, et al. A semi-invariant Vα10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen–recognition properties. Nat Immunol. 2011;12(7):616–23. https://doi.org/10.1038/ni.2051.
Brigl M, van den Elzen P, Chen X, Meyers JH, Wu D, Wong C-H, et al. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J Immunol. 2006;176(6):3625–34. https://doi.org/10.4049/jimmunol.176.6.3625.
Gadola SD, Dulphy N, Salio M, Cerundolo V. Vα24-JαQ-independent, CD1d-restricted recognition of α-galactosylceramide by human CD4<sup>+</sup> and CD8αβ<sup>+</sup> T lymphocytes. J Immunol. 2002;168(11):5514–20. https://doi.org/10.4049/jimmunol.168.11.5514.
López-Sagaseta J, Kung JE, Savage PB, Gumperz J, Adams EJ. The molecular basis for recognition of CD1d/α-galactosylceramide by a human non-Vα24 T cell receptor. PLoS Biol. 2012;10(10): e1001412. https://doi.org/10.1371/journal.pbio.1001412.
Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198(7):1069–76. https://doi.org/10.1084/jem.20030630.
Arase H, Arase N, Kobayashi Y, Nishimura Y, Yonehara S, Onoe K. Cytotoxicity of fresh NK1.1+ T cell receptor α/β+ thymocytes against a CD4+8+ thymocyte population associated with intact Fas antigen expression on the target. J Exp Med. 1994;180(2):423–32.
Niemeyer M, Darmoise A, Mollenkopf HJ, Hahnke K, Hurwitz R, Besra GS, et al. Natural killer T-cell characterization through gene expression profiling: an account of versatility bridging T helper type 1 (Th1), Th2 and Th17 immune responses. Immunology. 2008;123(1):45–56. https://doi.org/10.1111/j.1365-2567.2007.02701.x.
Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–6.
Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, et al. Antitumor cytotoxicity mediated by ligand-activated human Vα24 NKT cells. Cancer Res. 1999;59(20):5102–5.
Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122(4):617–22.
Muhammad Ali Tahir S, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J Immunol. 2001;167(7):4046–50.
Littwitz-Salomon E, Schimmer S, Dittmer U. Natural killer T cells contribute to the control of acute retroviral infection. Retrovirology. 2017;14(1):5. https://doi.org/10.1186/s12977-017-0327-8.
Renukaradhya GJ, Webb TJ, Khan MA, Lin YL, Du W, Gervay-Hague J, et al. Virus-induced inhibition of CD1d1-mediated antigen presentation: reciprocal regulation by p38 and ERK. J Immunol. 2005;175(7):4301–8.
Cho S, Knox KS, Kohli LM, He JJ, Exley MA, Wilson SB, et al. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology. 2005;337(2):242–52. https://doi.org/10.1016/j.virol.2005.04.020.
Hage CA, Kohli LL, Cho S, Brutkiewicz RR, Twigg HL 3rd, Knox KS. Human immunodeficiency virus gp120 downregulates CD1d cell surface expression. Immunol Lett. 2005;98(1):131–5. https://doi.org/10.1016/j.imlet.2004.10.025.
Yuan W, Dasgupta A, Cresswell P. Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat Immunol. 2006;7(8):835–42. https://doi.org/10.1038/ni1364.
Raftery MJ, Hitzler M, Winau F, Giese T, Plachter B, Kaufmann SH, et al. Inhibition of CD1 antigen presentation by human cytomegalovirus. J Virol. 2008;82(9):4308–19. https://doi.org/10.1128/jvi.01447-07.
Chen N, McCarthy C, Drakesmith H, Li D, Cerundolo V, McMichael AJ, et al. HIV-1 down-regulates the expression of CD1d via Nef. Eur J Immunol. 2006;36(2):278–86. https://doi.org/10.1002/eji.200535487.
Juno JA, Keynan Y, Fowke KR. Invariant NKT cells: regulation and function during viral infection. PLoS Pathog. 2012;8(8): e1002838. https://doi.org/10.1371/journal.ppat.1002838.
Paquin-Proulx D, Gibbs A, Bächle SM, Checa A, Introini A, Leeansyah E, et al. Innate invariant NKT cell recognition of HIV-1–infected dendritic cells is an early detection mechanism targeted by viral immune evasion. J Immunol. 2016;197(5):1843–51. https://doi.org/10.4049/jimmunol.1600556.
Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002;195(7):869–79.
Van der Vliet HJ, Von Blomberg BME, Hazenberg MD, Nishi N, Otto SA, Van Benthem BH, et al. Selective decrease in circulating Vα24+ Vβ11+ NKT cells during HIV type 1 infection. J Immunol. 2002;168(3):1490–5.
Sandberg JK, Fast NM, Palacios EH, Fennelly G, Dobroszycki J, Palumbo P, et al. Selective loss of innate CD4+ Vα24 natural killer T cells in human immunodeficiency virus infection. J Virol. 2002;76(15):7528–34.
Moll M, Kuylenstierna C, Gonzalez VD, Andersson SK, Bosnjak L, Sönnerborg A, et al. Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur J Immunol. 2009;39(3):902–11. https://doi.org/10.1002/eji.200838780.
Ziegler CGK, Miao VN, Owings AH, Navia AW, Tang Y, Bromley JD, et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell. 2021;184(18):4713-33.e22. https://doi.org/10.1016/j.cell.2021.07.023.
Berlin DA, Gulick RM, Martinez FJ. Severe covid-19. N Engl J Med. 2020;383(25):2451–60. https://doi.org/10.1056/NEJMcp2009575.
Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–66. https://doi.org/10.1056/NEJMcp2009249.
Liu J, Yang X, Wang H, Li Z, Deng H, Liu J, et al. Analysis of the long-term impact on cellular immunity in COVID-19-recovered individuals reveals a profound NKT cell impairment. MBio. 2021. https://doi.org/10.1128/mBio.00085-21.
Kreutmair S, Unger S, Núñez NG, Ingelfinger F, Alberti C, De Feo D, et al. Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia. Immunity. 2021;54(7):1578-93.e5. https://doi.org/10.1016/j.immuni.2021.05.002.
Zingaropoli MA, Perri V, Pasculli P, Cogliati Dezza F, Nijhawan P, Savelloni G, et al. Major reduction of NKT cells in patients with severe COVID-19 pneumonia. Clin immunol (Orlando, Fla). 2021;222:108630. https://doi.org/10.1016/j.clim.2020.108630.
Zhang J-Y, Wang X-M, Xing X, Xu Z, Zhang C, Song J-W, et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat Immunol. 2020;21(9):1107–18. https://doi.org/10.1038/s41590-020-0762-x.
Mazzoni A, Salvati L, Maggi L, Capone M, Vanni A, Spinicci M, et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Investig. 2020;130(9):4694–703. https://doi.org/10.1172/JCI138554.
Khan MA, Khan A. Role of NKT cells during viral infection and the development of NKT cell-based nanovaccines. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9090949.
Rigaud S, Fondanèche M-C, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444(7115):110–4. https://doi.org/10.1038/nature05257.
Locci M, Draghici E, Marangoni F, Bosticardo M, Catucci M, Aiuti A, et al. The Wiskott-Aldrich syndrome protein is required for iNKT cell maturation and function. J Exp Med. 2009;206(4):735–42. https://doi.org/10.1084/jem.20081773.
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73. https://doi.org/10.1056/NEJMra1706169.
Cortés-Selva D, Dasgupta B, Singh S, Grewal IS. Innate and innate-like cells: the future of chimeric antigen receptor (CAR) cell therapy. Trends Pharmacol Sci. 2021;42(1):45–59. https://doi.org/10.1016/j.tips.2020.11.004.
Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124(18):2824–33. https://doi.org/10.1182/blood-2013-11-541235.
Xu X, Huang W, Heczey A, Liu D, Guo L, Wood M, et al. NKT cells coexpressing a GD2-specific chimeric antigen receptor and IL15 show enhanced in vivo persistence and antitumor activity against neuroblastoma. Clin cancer Res. 2019;25(23):7126–38. https://doi.org/10.1158/1078-0432.Ccr-19-0421.
Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26(11):1686–90. https://doi.org/10.1038/s41591-020-1074-2.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44. https://doi.org/10.1056/NEJMoa1707447.
Rotolo A, Caputo VS, Holubova M, Baxan N, Dubois O, Chaudhry MS, et al. Enhanced anti-lymphoma activity of CAR19-iNKT cells underpinned by dual CD19 and CD1d targeting. Cancer Cell. 2018;34(4):596-610.e11. https://doi.org/10.1016/j.ccell.2018.08.017.
Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest. 2016;126(6):2341–55. https://doi.org/10.1172/jci83476.
Ngai H, Tian G, Courtney AN, Ravari SB, Guo L, Liu B, et al. IL-21 selectively protects CD62L(+) NKT cells and enhances their effector functions for adoptive immunotherapy. J Immunol. 2018;201(7):2141–53. https://doi.org/10.4049/jimmunol.1800429.
Simonetta F, Hirai T, Lohmeyer JK, Maas-Bauer K, Alvarez M, Wenokur A, et al. Allogeneic chimeric antigen receptor-invariant natural killer T cells exert both direct and indirect antitumor effects through host CD8 T cell cross-priming. Biol Blood Marrow Transplant. 2020;26(3, Supplement):S42. https://doi.org/10.1016/j.bbmt.2019.12.109.
Ilieva KM, Cheung A, Mele S, Chiaruttini G, Crescioli S, Griffin M, et al. Chondroitin sulfate proteoglycan 4 and its potential as an antibody immunotherapy target across different tumor types. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2017.01911.
Simon B, Wiesinger M, März J, Wistuba-Hamprecht K, Weide B, Schuler-Thurner B, et al. The generation of CAR-transfected natural killer T cells for the immunotherapy of melanoma. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19082365.
Poels R, Drent E, Lameris R, Katsarou A, Themeli M, van der Vliet HJ, et al. Preclinical evaluation of invariant natural killer T cells modified with CD38 or BCMA chimeric antigen receptors for multiple myeloma. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22031096.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–37. https://doi.org/10.1056/NEJMoa1817226.
Drent E, Themeli M, Poels R, de Jong-Korlaar R, Yuan H, de Bruijn J, et al. A rational strategy for reducing on-target off-tumor effects of CD38-chimeric antigen receptors by affinity optimization. Mol Ther. 2017;25(8):1946–58. https://doi.org/10.1016/j.ymthe.2017.04.024.
Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–57. https://doi.org/10.1158/2159-8290.Cd-20-1680.
Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):738. https://doi.org/10.3390/cancers12030738.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. https://doi.org/10.1084/jem.192.7.1027.
Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immuno (Baltimore, Md: 1950). 2009;182(5):2816–26. https://doi.org/10.4049/jimmunol.0803648.
Kamata T, Suzuki A, Mise N, Ihara F, Takami M, Makita Y, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol immunother CII. 2016;65(12):1477–89. https://doi.org/10.1007/s00262-016-1901-y.
Corgnac S, Perret R, Derré L, Zhang L, Stirnemann K, Zauderer M, et al. CD1d-antibody fusion proteins target iNKT cells to the tumor and trigger long-term therapeutic responses. Cancer Immunol Immunother. 2013;62(4):747–60. https://doi.org/10.1007/s00262-012-1381-7.
Das R, Guan P, Wiener SJ, Patel NP, Gohl TG, Evans E, et al. Enhancing the antitumor functions of invariant natural killer T cells using a soluble CD1d-CD19 fusion protein. Blood Adv. 2019;3(5):813–24. https://doi.org/10.1182/bloodadvances.2018028886.
Zhang L, Donda A. Redirecting iNKT cell antitumor immunity with α-GalCer/CD1d-scFv fusion proteins. Methods Mol Biol. 2021;2388:175–80. https://doi.org/10.1007/978-1-0716-1775-5_16.
Tian Z, Liu M, Zhang Y, Wang X. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J Hematol Oncol. 2021;14(1):75. https://doi.org/10.1186/s13045-021-01084-4.
Horn LA, Ciavattone NG, Atkinson R, Woldergerima N, Wolf J, Clements VK, et al. CD3xPDL1 bi-specific T cell engager (BiTE) simultaneously activates T cells and NKT cells, kills PDL1(+) tumor cells, and extends the survival of tumor-bearing humanized mice. Oncotarget. 2017;8(35):57964–80. https://doi.org/10.18632/oncotarget.19865.
Lameris R, Shahine A, Pellicci DG, Uldrich AP, Gras S, Le Nours J, et al. A single-domain bispecific antibody targeting CD1d and the NKT T-cell receptor induces a potent antitumor response. Nature Cancer. 2020;1(11):1054–65. https://doi.org/10.1038/s43018-020-00111-6.
Gebremeskel S, Nelson A, Walker B, Oliphant T, Lobert L, Mahoney D, et al. Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-002096.
Nelson A, Gebremeskel S, Lichty BD, Johnston B. Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression. J Immunother Cancer. 2022. https://doi.org/10.1136/jitc-2021-003923.
Ko H-J, Lee J-M, Kim Y-J, Kim Y-S, Lee K-A, Kang C-Y. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182(4):1818–28. https://doi.org/10.4049/jimmunol.0802430.
Paul S, Chhatar S, Mishra A, Lal G. Natural killer T cell activation increases iNOS<sup>+</sup>CD206<sup>-</sup> M1 macrophage and controls the growth of solid tumor. J Immunother Cancer. 2019;7(1):208. https://doi.org/10.1186/s40425-019-0697-7.
Toyoda T, Kamata T, Tanaka K, Ihara F, Takami M, Suzuki H, et al. Phase II study of α-galactosylceramide-pulsed antigen-presenting cells in patients with advanced or recurrent non-small cell lung cancer. J Immunother Cancer. 2020;8(1): e000316. https://doi.org/10.1136/jitc-2019-000316.
Zhu Y, Smith DJ, Zhou Y, Li Y-R, Yu J, Lee D, et al. Development of hematopoietic stem cell-engineered invariant natural killer T cell therapy for cancer. Cell Stem Cell. 2019;25(4):542-57.e9. https://doi.org/10.1016/j.stem.2019.08.004.
Li YR, Zhou Y, Kim YJ, Zhu Y, Ma F, Yu J, et al. Development of allogeneic HSC-engineered iNKT cells for off-the-shelf cancer immunotherapy. Cell Rep Med. 2021;2(11): 100449. https://doi.org/10.1016/j.xcrm.2021.100449.
Gopisetty A, Chen Y, Nguyen Q, Chiriva-Internati M. Abstract 1535: Allogenic CAR iNKT as a cell therapy platform targeting tumor antigen Isomesothelin. Cancer Res. 2021;81(13_Supplement):1535. https://doi.org/10.1158/1538-7445.Am2021-1535.
Stirnemann K, Romero JF, Baldi L, Robert B, Cesson V, Besra GS, et al. Sustained activation and tumor targeting of NKT cells using a CD1d-anti-HER2-scFv fusion protein induce antitumor effects in mice. J Clin Invest. 2008;118(3):994–1005. https://doi.org/10.1172/jci33249.
Aoyama S, Nakagawa R, Nemoto S, Perez-Villarroel P, Mulé JJ, Mailloux AW. Checkpoint blockade accelerates a novel switch from an NKT-driven TNFα response toward a T cell driven IFN-γ response within the tumor microenvironment. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-002269.
Kharkwal SS, Johndrow CT, Veerapen N, Kharkwal H, Saavedra-Avila NA, Carreño LJ, et al. Serial stimulation of invariant natural killer T cells with covalently stabilized bispecific t-cell engagers generates antitumor immunity while avoiding anergy. Can Res. 2021;81(7):1788–801. https://doi.org/10.1158/0008-5472.Can-20-2219.
Acknowledgements
There are many studies examining NKT cell-based therapies, thus only recent reviews and closely related articles have been cited. We apologize to those whose work may have been omitted due to space limitations. The figures were created using templates on BioRender.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This article was supported by funds through the National Cancer Institute—Cancer Center Support Grant (CCSG)—P30CA134274, NIH NIGMS R25GM113262, and funds through the Maryland Department of Health’s Cigarette Restitution Fund Program—CH-649-CRF.
Conflicts of interest/Competing interests
T.J.W. is the CEO of WebbCures, LLC, co-founded IMMUNE3D, Screen Therapeutics, and is on the scientific advisory board for Immunaccel Labs. The other authors, C.K., S.S., and E.B.A. declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
CK, SS, EAB, and TJW wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kratzmeier, C., Singh, S., Asiedu, E.B. et al. Current Developments in the Preclinical and Clinical use of Natural Killer T cells. BioDrugs 37, 57–71 (2023). https://doi.org/10.1007/s40259-022-00572-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00572-4