Abstract
Engineered red blood cells (RBCs) appear to be a promising method for therapeutic drug and protein delivery. With a number of agents in clinical trials (e.g., dexamethasone 21-phosphate in ataxia telangiectasia, asparaginase in pancreatic cancer/acute lymphoblastic leukemia, thymidine phosphorylase in mitochondrial neurogastrointestinal encephalomyopathy, RTX-134 in phenylketonuria, etc.), this leading article summarizes the ongoing efforts in developing these agents, focuses on the clinical progress, and provides a brief background into engineered RBCs and the different ways in which they can be exploited for therapeutic/diagnostic purposes. References to available data on safety, efficacy, and tolerability are reported. Due to the continuous progress in this field, the information is updated as of January 2020 from databases, websites, and press releases of the involved companies and information that is in the public domain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Engineered RBCs have now reached clinical development with some products already in phase III. |
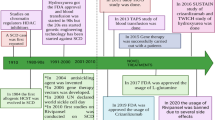
Leading companies in phase III include EryDel SpA and Erytech Pharma; others have received FDA and/or EMA clearance for patient recruitment (Rubius Therapeutics and St George’s, University of London with Orphan Technologies). |
Orphan drug designation has been obtained by the FDA and EMA for several drug products; several regulatory paths to approval have been defined with the main regulatory agencies. |
1 Engineered Erythrocytes
Red blood cells (RBCs) are the most numerous cells present in the blood. In a human being, 4–5 million RBCs are present in each microliter of blood. By a simple estimate of 5 L of blood in a 70 kg adult, a total of approximately 20–25 × 1012 RBCs can be calculated. In transfusion medicine, it is common practice to collect blood from healthy donors to be transfused into compatible patients in need. This operation, which usually requires 400–450 mL of blood, can be safely repeated many times every year, as our hematopoietic system has the ability to replace the removed RBCs with new erythrocytes with an output of 2.5 million cells per second. Of interest, when mature human RBCs enter into circulation, they can survive for more than 110–120 days and are removed from circulation only when they are senescent. Thus, the removal of the RBCs from the blood is a very sophisticated process capable of distinguishing, among all circulating RBCs, only those that have reached their programmed life span. Human RBCs possess additional advantageous characteristics. Due to an extreme differentiation process, they have lost the nucleus and other organelles and are thus considered a nonviable container of about 90 fl with a biconcave shape and a diameter of 7–8 µm. This apparently simple cell is able to squeeze through human capillaries (which are < 2 µm in diameter) without being damaged and to travel throughout the circulatory system in about 20 s. Because of these special properties, years ago several investigators hypothesized that erythrocytes could be used not only to replace blood lost from trauma or for other reasons, but could represent an invaluable cell source easily available for the delivery of therapeutic and contrasting agents in the circulatory system.
Human erythrocytes have been suggested as carriers and bioreactors since 1973 [1]. The basis for these potential applications derived from the seminal work of Hoffman [2, 3], who demonstrated that pores opened on the erythrocyte membrane in hypotonic conditions can be resealed, restoring the main properties of the native cell membrane. Since then, many groups have documented the feasibility of this approach in different animal species [4] for the encapsulation of therapeutic proteins, antigens, contrasting agents, small chemical entities [5, 6] as well as for the delivery of agents coupled to the external surface of the RBC membrane [7,8,9]. Thus, in addition to osmotic-based procedures, RBC engineering can be obtained by multiple strategies, as reviewed in [10, 11]. It is worth noting that RBCs can serve both as a delivery system for the release of drugs in circulation and to keep the therapeutic agent inside the cell while the metabolite to be degraded crosses the RBC membrane. Both approaches are already in clinical studies today.
2 Clinical Progress
The clinical developments reported in this section have received approval by international regulatory agencies; many have also received the designation of ‘orphan drug’, and the most advanced are based on solid preclinical and/or phase I/II clinical investigations. Table 1 summarizes the therapeutic goals and the drugs discussed in this paper.
2.1 Red Blood Cells (RBCs) for the Delivery of Dexamethasone
Dexamethasone is a glucocorticoid analog without mineralocorticoid activity and with the highest potency with respect to hydrocortisone. Because of its chemistry, it can easily cross cell membranes (including the RBC membrane) by simple diffusion. As a consequence, it is not retained within the RBCs upon encapsulation. This problem was solved by Magnani et al. [12] by encapsulating the corticosteroid derivative dexamethasone 21-phosphate (Dexa 21-P). Specific equipment to perform the encapsulation procedure was also developed [13]. The idea of using a non-diffusible prodrug of dexamethasone using autologous erythrocytes was also very beneficial in terms of pharmacokinetics. In fact, Dexa 21-P is slowly converted to the diffusible dexamethasone by RBC resident enzymes and detected in circulation for > 1 month in human volunteers receiving a single infusion of Dexa 21-P-loaded RBCs [14]. In the same study sponsored by EryDel SpA, it was also shown that the processed RBCs have normal in vivo survival and are fully compliant with the rules for FDA-defined transfusion products. Mambrini et al. [13] were also able to document that the human RBCs can be efficiently loaded with a large range of Dexa 21-P concentrations while maintaining the RBCs’ fundamental properties. Dexa 21-P-loaded RBCs have been used in several investigator-initiated clinical studies in chronic obstructive pulmonary disorder (COPD) patients [15], cystic fibrosis [16], steroid-dependent irritable bowel disease (IBD) [17], Crohn’s disease [18, 19], and ulcerative colitis [20, 21], documenting safety and clinical effect in hundreds of patients. Most recently, EryDel SpA started a registration program in ataxia telangiectasia patients. The treatment received orphan drug designation by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The phase II trial enrolled 22 patients receiving a monthly administration of autologous RBCs loaded ex vivo with Dexa 21-P for 6 months. It was very successful, reaching both primary and secondary endpoints [22], namely improvement of the neurological conditions measured by a validated International Cooperative Ataxia Rating Scale (ICARS) and adaptive behavior measured by the Vineland Adaptive Behavior Scale (VABS), and an acceptable safety profile. Based on these results, EryDel started a phase III registration study. This is an international, multi-center, 1-year, randomized, prospective, double-blind, placebo-controlled phase III study designed to assess the effect of two non-overlapping dose ranges of Dexa 21-P, administered by IV infusion once per month, on neurological symptoms of patients with ataxia telangiectasia. This study is being conducted in 22 centers located in the US, Europe, Israel, Asia, Africa, and Australia (for more details, visit http://www.attest-trial.com). A total of 180 patients will be enrolled equally into three groups of two Dexa 21-P doses and a placebo. Patients completing this study will be allowed to continue treatment in an open-label extension study.
2.2 RBCs for the Delivery of Asparaginase
The idea of using RBCs for the delivery of asparaginase was proposed by Ihler et al. [1], but without any experimental evidence for its feasibility. Preclinical investigations in baboons were reported in 1976 [23], and in monkeys in 1983 [24]. In the years 1990–1996, Ropars and colleagues [25,26,27,28,29] proved the feasibility and provided evidence that asparaginase-loaded homologous RBCs are safe, well tolerated, and effective in reducing plasma asparagine in humans. Results from a phase I/II clinical trial of asparaginase-loaded RBCs (named GRASPA®, property of Erytech Pharma) in the treatment of refractory or relapsed lymphoblastic leukemia were published in 2011 [30]. In 2015, GRASPA was evaluated for the treatment of acute lymphoblastic leukemia in elderly patients in a phase II study [31]. The dose of 100 IU/kg was found to show the best safety and efficacy profile for these elderly patients. However, median overall survival was disappointing in this treatment regimen, being 15.8 and 9.7 months in the 100 and 150 IU/kg cohorts, respectively. Asparaginase-loaded RBCs were also evaluated in a phase I study in patients with pancreatic adenocarcinoma [32] with a reasonable safety profile. Results of a phase IIb study with erythrocyte-containing asparaginase (now named eryaspase) in combination with chemotherapy in second-line treatment of advanced pancreatic cancer were recently published [33]. The study, funded by Erytech Pharma, was conducted using the ERYCAPS® technology. For the entire population, median overall survival in the eryaspase arm was 6.0 months versus 4.4 months in the control arm receiving chemotherapy. Median progression-free survival was 2.0 months in the eryaspase arm and 1.6 months in the control arm. Overall, the incidence of adverse events was similar in the two arms. An open-label, randomized phase III study of eryaspase in combination with chemotherapy compared with chemotherapy alone as second-line treatment in patient with pancreatic cancer is ongoing and recruiting (NCT03665441). The company Erytech Pharma has also planned a phase II trial in triple negative breast cancer (NCT03674242) and in other solid tumors, and a phase II trial in lymphoblastic leukemia.
2.3 RBCs for the Delivery of Thymidine Phosphorylase
Accumulation of thymidine and deoxyuridine in human tissues and body fluids, including blood, is a consequence of a complete or partial absence of the enzyme thymidine phosphorylase and results in a fatal autosomal recessive disorder best described as mitochondrial neurogastrointestinal encephalomyopathy or MNGIE (Online Mendelian inheritance in Man #603041, Genome Database accession #9835128). The biochemical consequences of a high concentration of deoxyribonucleosides is an increased concentration of the corresponding triphosphates within mitochondria, leading to multiple deletions, point mutations, and depletion in mitochondrial DNA. Moran et al. [34] reported the first treatment of a MNGIE patient with autologous erythrocyte-encapsulated recombinant thymidine phosphorylase from Escherichia coli. A detailed description of the clinical and biochemical improvements with this treatment was reported by Bax et al. [35], confirming a successful enzyme replacement therapy for the treatment of MNGIE. These conclusions were confirmed in three patients receiving increasing doses of the encapsulated enzyme [36], and a full phase II, multi-center, multiple dose, uncontrolled, open-label trial will investigate the application of erythrocyte-encapsulated thymidine phosphorylase (EE-TP) as an enzyme replacement therapy for MNGIE. Three EE-TP dose levels are planned with patients eventually receiving the dose level that achieves metabolic correction. The study has a 3-month run-in period followed by a 24-month treatment phase [37, 38]. The treatment has received the designation of orphan drug by both the EMA and FDA. At the date of writing (January 2020), this study is not recruiting yet.
2.4 RBCs for the Delivery of Phenylalanine Ammonia Lyase
Phenylketonuria (PKU, OMIM 261600) is an inborn error of metabolism caused by inherited mutations in the enzyme phenylalanine hydroxylase converting phenylalanine (Phe) into tyrosine. This deficiency leads to systemic accumulation of Phe, leading to intellectual disabilities and neurocognitive impairments even in early and continuously treated patients on a Phe-restricted diet [39, 40]. An enzyme replacement therapy based on pegvaliase administration (enzyme produced by BioMarin Pharmaceutical Inc.) has recently been approved by the FDA (May 2018) and EMA (April 2019) for adults with Phe ≥ 600 µM. The administered enzyme is a pegylated recombinant Anabaema variabilis phenylalanine ammonia lyase (PAL) to be administered daily. About 60% of treated patients in a phase III clinical trial achieved blood Phe ≤ 360 µM, but adverse reactions and anaphylaxis were associated with this therapy [41]. To overcome these limitations, a RBC-mediated delivery of PAL was investigated in a validated murine model of PKU [42, 43]. These experiments provided evidence that pegylation is not necessary when the recombinant enzyme is administered encapsulated within autologous RBCs. Furthermore, the selected dose was equally effective at all time points investigated, proving that anti-drug antibodies do not inactivate the recombinant PAL once encapsulated into autologous RBCs. Recently, Rubius announced that their first product to be developed would be based on the delivery of PAL expressed into RBCs (RTX-134) [49]. It is worth noting that Rubius’ technology, as described on the company website, is based on CD34+ hematopoietic precursor cells collected by apheresis from a healthy O-negative donor and purified. These precursor cell populations are genetically engineered with a lentiviral vector, or gene cassette, to express PAL within the cells. The cells are then exposed to media in a bioreactor to promote further expansion and differentiation until the nucleus is ejected, resulting in mature reticulocytes to be administered to patients in need. The company announced FDA clearance of the Investigational New Drug application in March 2019 [50]. The primary objective of this study is to evaluate the safety and tolerability of RTX-134 following intravenous administration of a single dose. RTX-134 consists of allogeneic human red cells expressing AvPAL (Anabaena variabilis phenylalanine ammonia lyase). The trial is designed to determine a preliminary dose and inform a dosing schedule that is deemed safe, tolerable, and potentially effective. Four dose levels are planned and additional dose levels may be explored. Following administration, subjects will be monitored until 28 days after last detection of RTX-134. Detection of RTX-134 will be evaluated using multiple pharmacokinetic and pharmacodynamic assessments including measurement of trans-cinnamic acid (tCA). The number of participants was originally planned to be 12 and the study was due to start in September 2019. However, currently, based on company disclosure, only the first patient has been treated, thus delays are expected in the reading of the clinical dataFootnote 1.
3 Preclinical Pipeline
The two companies with the most advanced clinical programs (EryDel and Erytech) have a robust pipeline with leading products and developments in additional conditions and new therapeutics. Rubius has also announced a strong pipeline with some preclinical data published. Of interest, due to space limitation, we will address readers to several reviews that have summarized the potential clinical developments of several different candidates already tested in suitable animal models [44,45,46,47,48]. The envisaged applications cover many different areas including delivery of small chemical entities, therapeutic proteins, enzymes, immune modulators, and contrasting agents for diagnostic applications.
4 Concluding Remarks
The technology based on the use of RBCs for the delivery of therapeutic and/or diagnostic agents has strongly evolved from a research field to advanced clinical development programs. Some companies are leading the clinical applications with products currently in phase III trials and robust pipelines. The modalities selected by each company for the production of the modified RBCs are different. While Rubius’ technology is mainly based on the genetic engineering of precursor erythroid cells to be modified by viral vectors, expanded ex vivo and differentiated in cell bioreactors before infusion in patients in need, Erytech Pharma is producing the modified RBCs in dedicated cell facilities starting from blood donations obtained by immune-compatible donors. In contrast, EryDel processes autologous blood at the bedside of the patient. In other words, the patient provides a small amount of blood (i.e., about 50 mL in most studies), which is processed in a dedicated automated system (comprising a drug and medical devices) by a patented technology and re-infused into the original donor within 1.5–2 h from the time of blood collection. Thus, each patient receives their own blood once it is processed by the encapsulation of drugs or other therapeutic agents. This approach is repeated monthly and does not require a centralized cell factory and distribution logistics.
The regulatory authorities in the US and Europe have been involved in several steps of EryDel process development. In addition, clearance of all Investigational New Drug Applications (see Table 2) has greatly contributed to defining the main regulatory requirements in terms of safety and efficacy for the benefit of patients in need. EryDel has shown that transformed RBCs have a 24-h survival and a half-life in circulation that conforms to the FDA criteria for the approval of blood transfusion products [14]. Erytech, based on asparaginase activity [30], has shown that the entrapped asparaginase is detectable in circulation for about 1 month. Procedure-related adverse events of RBC-mediated delivery have not been observed in hundreds of administrations in adults and children. In conclusion, the enormous efforts that a large number of researchers have made in advancing our knowledge of the biology, physiology, immunology, and biochemistry of RBCs is finally contributing to the development of new therapeutics for unmet medical needs, frequently in the area of rare diseases. This great effort is now in the last phase of clinical development for a few products, and this is also thanks to a number of investors and patient associations that have joined their efforts to push this fascinating technology forward to new therapeutic breakthroughs.
Notes
On March 12, 2020 Rubius Therapeutics announced in a press release that “the first patient was dosed in the Phase 1b PKU clinical trial of RTX-134 in January 2020. While there were no reported adverse events and RTX-134 administration was well tolerated, the results from the first patient were uninterpretable possibly due, in part, to the low dose of cells administered and the sensitivity of the flow cytometry assay used to detect circulating cells. As a result of the deprioritization, the current Phase 1b clinical trial in PKU will be discontinued."
References
Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci USA. 1973;70:2663–6.
Hoffman JF. Physiological characteristics of human red blood cell ghosts. J Gen Physiol. 1958;42:9–28.
Hoffman JF. On red blood cells, hemolysis and resealed ghosts. Adv Exp Med Biol. 1992;326:1–15.
DeLoach JR. Hypotonic dialysis encapsulation in erythrocytes of mammalian species. Bibl Haematol. 1985;51:1–6.
Pierige F, Bigini N, Rossi L, Magnani M. Reengineering red blood cells for cellular therapeutics and diagnostics. WIREs Nanomed Nanobiotechnol. 2017. https://doi.org/10.1002/wnan.1454.
Villa CH, Anselmo AC, Mitragotri S, Muzykantov V. Red blood cells: supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev. 2016;106:88–103.
Rossi L, Fraternale A, Bianchi M, Magnani M. Red blood cell membrane processing for biomedical applications. Front Physiol. 2019. https://doi.org/10.3389/fphys.2019.01070.
Ji W, Smith PN, Koepsel RR, Andersen JD, Baker SL, Zhang L, Carmali S, Myerson JW, Muzykantov S, Russell AJ. Erythrocytes as carriers of immunoglobulin-based therapeutics. Acta Biomater. 2020;101:422–35. https://doi.org/10.1016/j.actbio.2019.10.027.
Villa CH, Pan DC, Johnston IH, Greineder CF, Walsh LR, Hood ED, Cines DB, Poncz M, Siegel DL, Muzykantov VR. Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv. 2018;2(3):165–76. https://doi.org/10.1182/bloodadvances.2017011734.
Han X, Wang C, Liu Z. Red blood cells as smart delivery systems. Bioconjug Chem. 2018;29(4):852–60. https://doi.org/10.1021/acs.bioconjchem.7b00758.
Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P, Hekierski H, Chatterjee S, Tao JQ, Parhiz H, Bhamidipati K, Uhler TG, Hood ED, Kiseleva RY, Shuvaev VS, Shuvaeva T, Khoshnejad M, Johnston I, Gregory JV, Lahann J, Wang T, Cantu E, Armstead WM, Mitragotri S, Muzykantov V. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun. 2018;9(1):2684. https://doi.org/10.1038/s41467-018-05079-7.
Magnani M, Rossi L, D’ascenzo M, Panzani I, Bigi L, Zanella A. Erythrocyte engineering for drug delivery and targeting. Biotechnol Appl Biochem. 1998;28(1):1–6.
Mambrini G, Mandolini M, Rossi L, Pierigè F, Capogrossi G, Salvati P, Serafini S, Benatti L, Magnani M. Ex vivo encapsulation of dexamethasone sodium phosphate into human autologous erythrocytes using fully automated biomedical equipment. Int J Pharm. 2017;517(1–2):175–84.
Coker SA, Szczepiorkowski ZM, Siegel AH, Ferrari A, Mambrini G, Anand R, Hartman RD, Benatti L, Dumont LJ. A study of the pharmacokinetic properties and the in vivo kinetics of erythrocytes loaded with dexamethasone sodium phosphate in healthy volunteers. Transfus Med Rev. 2018;32(2):102–10.
Rossi L, Serafini S, Cenerini L, Picardi F, Bigi L, Panzani I, Magnani M. Erythrocyte-mediated delivery of dexamethasone in patients with chronic obstructive pulmonary disease. Biotechnol Appl Biochem. 2001;33(2):85–9.
Rossi L, Castro M, D’Orio F, Damonte G, Serafini S, Bigi L, Panzani I, Novelli G, Dallapiccola B, Panunzi S, Di Carlo P, Bella S, Magnani M. Low doses of dexamethasone constantly delivered by autologous erythrocytes slow the progression of lung disease in cystic fibrosis patients. Blood Cells Mol Dis. 2004;33(1):57–63.
Annese V, Latiano A, Rossi L, Lombardi G, Dallapiccola B, Serafini S, Damonte G, Andriulli A, Magnani M. Erythrocytes-mediated delivery of dexamethasone in steroid-dependent IBD patients—a pilot uncontrolled study. Am J Gastroenterol. 2005;100(6):1370–5.
Castro M, Knafelz D, Rossi L, Ambrosini MI, Papadatou B, Mambrini G, Magnani M. Periodic treatment with autologous erythrocytes loaded with dexamethasone 21-phosphate for fistulizing pediatric Crohn’s disease: case report. J Pediatr Gastroenterol Nutr. 2006;42(3):313–5.
Castro M, Rossi L, Papadatou B, Bracci F, Knafelz D, Ambrosini MI, Calce A, Serafini S, Isacchi G, D’Orio F, Mambrini G, Magnani M. Long-term treatment with autologous red blood cells loaded with dexamethasone 21-phosphate in pediatric patients affected by steroid-dependent Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44(4):423–6.
Bossa F, Latiano A, Rossi L, Magnani M, Palmieri O, Dallapiccola B, Serafini S, Damonte G, De Santo E, Andriulli A, Annese V. Erythrocyte-mediated delivery of dexamethasone in patients with mild-to-moderate ulcerative colitis, refractory to mesalamine: a randomized, controlled study. Am J Gastroenterol. 2008;103(10):2509–16.
Bossa F, Annese V, Valvano MR, Latiano A, Martino G, Rossi L, Magnani M, Palmieri O, Serafini S, Damonte G, De Santo E, Andriulli A. Erythrocytes-mediated delivery of dexamethasone 21-phosphate in steroid-dependent ulcerative colitis: a randomized, double-blind Sham-controlled study. Inflamm Bowel Dis. 2013;19(9):1872–9.
Chessa L, Leuzzi V, Plebani A, Soresina A, Micheli R, D’Agnano D, Venturi T, Molinaro A, Fazzi E, Marini M, Ferremi Leali P, Quinti I, Cavaliere FM, Girelli G, Pietrogrande MC, Finocchi A, Tabolli S, Abeni D, Magnani M. Intra-erythrocyte infusion of dexamethasone reduces neurological symptoms in ataxia teleangiectasia patients: results of a phase 2 trial. Orphanet J Rare Dis. 2014. https://doi.org/10.1186/1750-1172-9-5.
Updike SJ, Wakamiya RT, Lightfoot EN Jr. Asparaginase entrapped in red blood cells: action and survival. Science. 1976;193(4254):681–3.
Updike SJ, Wakamiya RT. Infusion of red blood cell-loaded asparaginase in monkey. Immunologic, metabolic, and toxicologic consequences. J Lab Clin Med. 1983;101(5):679–91.
Kravtzoff R, Colombat PH, Desbois I, Linassier C, Muh JP, Philip T, Blay JY, Gardenbas M, Poumier-Gaschard P, Lamagnere JP, Chassaigne M, Ropars C. Tolerance evaluation of l-asparaginase loaded in red blood cells. Eur J Clin Pharmacol. 1996;51(3–4):221–5.
Kravtzoff R, Desbois I, Lamagnere JP, Muh JP, Valat C, Chassaigne M, Colombat P, Ropars C. Improved pharmacodynamics of L-asparaginase loaded in human red blood cells. Eur J Clin Pharmacol. 1996;49(6):465–70.
Garín MI, Kravtzoff R, Chestier N, Sanz S, Pinilla M, Luque J, Ropars C. Density gradient separation of l-asparaginase-loaded human erythrocytes. Biochem Mol Biol Int. 1994;33(4):807–14.
Ktavtzoff R, Desbois I, Doinel C, Colombat P, Lamagnere JP, Chassaigne M, Ropars C. Immunological response to l-asparaginase loaded into red blood cells. Adv Exp Med Biol. 1992;326:175–82.
Kravtzoff R, Ropars C, Laguerre M, Muh JP, Chassaigne M. Erythrocytes as carriers for l-asparaginase. Methodological and mouse in vivo studies. J Pharm Pharmacol. 1990;42(7):473–6.
Domenech C, Thomas X, Chabaud S, Baruchel A, Gueyffier F, Mazingue F, Auvrignon A, Corm S, Dombret H, Chevallier P, Galambrun C, Huguet F, Legrand F, Mechinaud F, Vey N, Philip I, Liens D, Godfrin Y, Rigal D, Bertrand Y. l-Asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial. Br J Haematol. 2011;153(1):58–65.
Hunault-Berger M, Leguay T, Huguet F, Leprêtre S, Deconinck E, Ojeda-Uribe M, Bonmati C, Escoffre-Barbe M, Bories P, Himberlin C, Chevallier P, Rousselot P, Reman O, Boulland ML, Lissandre S, Turlure P, Bouscary D, Sanhes L, Legrand O, Lafage-Pochitaloff M, Béné MC, Liens D, Godfrin Y, Ifrah N, Dombret H, Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). A phase 2 study of l-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: the GRASPALL/GRAALL-SA2-2008 study. Am J Hematol. 2015;90(9):811–8.
Bachet JB, Gay F, Maréchal R, Galais MP, Adenis A, MsC DS, Cros J, Demetter P, Svrcek M, Bardier-Dupas A, Emile JF, Hammel P, Ebenezer C, Berlier W, Godfrin Y, André T. Asparagine synthetase expression and phase I study with l-asparaginase encapsulated in red blood cells in patients with pancreatic adenocarcinoma. Pancreas. 2015;44(7):1141–7.
Hammel P, Fabienne P, Mineur L, Metges JP, Andre T, De La Fouchardiere C, Louvet C, El Hajbi F, Faroux R, Guimbaud R, Tougeron D, Bouche O, Lecomte T, Rebischung C, Tournigand C, Cros J, Kay R, Hamm A, Gupta A, Bachet JB, El Hariry I. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: an open-label, randomized Phase IIb trial. Eur J Cancer. 2019;124:91–101.
Moran NF, Bain MD, Muqit MM, Bax BE. Carrier erythrocyte entrapped thymidine phosphorylase therapy for MNGIE. Neurology. 2008;71(9):686–8.
Bax BE, Bain MD, Scarpelli M, Filosto M, Tonin P, Moran N. Clinical and biochemical improvements in a patient with MNGIE following enzyme replacement. Neurology. 2013;81(14):1269–71.
Levene M, Bain MD, Moran NF, Nirmalananthan N, Poulton J, Scarpelli M, Filosto M, Mandel H, MacKinnon AD, Fairbanks L, Pacitti D, Bax BE. Safety and efficacy of erythrocyte encapsulated thymidine phosphorylase in mitochondrial neurogastrointestinal encephalomyopathy. J Clin Med. 2019. https://doi.org/10.3390/jcm8040457.
Bax BE, Levene M, Bain MD, Fairbanks LD, Filosto M, Kalkan Uçar S, Klopstock T, Kornblum C, Mandel H, Rahman S, Roubertie A, Scarpelli M, Sedgwick PM, Baru M, Sellos-Moura M, Price J, Horn P, Nirmalananthan N. Erythrocyte encapsulated thymidine phosphorylase for the treatment of patients with mitochondrial neurogastrointestinal encephalomyopathy: study protocol for a multi-centre, multiple dose, open label trial. J Clin Med. 2019. https://doi.org/10.3390/jcm8081096.
Pacitti D, Levene M, Garone C, Nirmalananthan N, Bax BE. Mitochondrial neurogastrointestinal encephalomyopathy: into the fourth decade, what we have learned so far. Front Genet. 2018. https://doi.org/10.3389/fgene.2018.00669.
Bilder DA, Noel JK, Baker ER, Irish W, Chen Y, Merilainen MJ, Prasad S, Winslow BJ. Systematic review and meta-analysis of neuropsychiatric symptoms and executive functioning in adults with phenylketonuria. Dev Neuropsychol. 2016;41(4):245–60.
Leuzzi V, Chiarotti F, Nardecchia F, van Vliet D, van Spronsen FJ. Predictability and inconsistencies of cognitive outcome in patients with phenylketonuria and personalised therapy: the challenge for the future guidelines. J Med Genet. 2019. https://doi.org/10.1136/jmedgenet-2019-106278.
Gupta S, Lau K, Harding CO, Shepherd G, Boyer R, Atkinson JP, Knight V, Olbertz J, Larimore K, Gu Z, Li M, Rosen O, Zoog SJ, Weng HH, Schweighardt B. Association of immune response with efficacy and safety outcomes in adults with phenylketonuria administered pegvaliase in phase 3 clinical trials. EBioMedicine. 2018;37:366–73.
Rossi L, Pierigè F, Carducci C, Gabucci C, Pascucci T, Canonico B, Bell SM, Fitzpatrick PA, Leuzzi V, Magnani M. Erythrocyte-mediated delivery of phenylalanine ammonia lyase for the treatment of phenylketonuria in BTBR-Pah(enu2) mice. J Control Release. 2014;194:37–44.
Pascucci T, Rossi L, Colamartino M, Gabucci C, Carducci C, Valzania A, Sasso V, Bigini N, Pierigè F, Viscomi MT, Ventura R, Cabib S, Magnani M, Puglisi-Allegra S, Leuzzi V. A new therapy prevents intellectual disability in mouse with phenylketonuria. Mol Genet Metab. 2018;124(1):39–49.
Pierigè F, Bigini N, Rossi L, Magnani M. Reengineering red blood cells for cellular therapeutics and diagnostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017. https://doi.org/10.1002/wnan.1454.
Rossi L, Pierigè F, Antonelli A, Bigini N, Gabucci C, Peiretti E, Magnani M. Engineering erythrocytes for the modulation of drugs’ and contrasting agents’ pharmacokinetics and biodistribution. Adv Drug Deliv Rev. 2016;106(Pt A):73–87.
Leuzzi V, Rossi L, Gabucci C, Nardecchia F, Magnani M. Erythrocyte-mediated delivery of recombinant enzymes. J Inherit Metab Dis. 2016;39(4):519–30.
Sprandel U. Erythrocytes as carrier for therapeutic enzymes—an approach towards enzyme therapy of inborn errors of metabolism. Bibl Haematol. 1985;51:7–14.
Magnani M, Serafini S, Fraternale A, Antonelli A, Biagiotti S, Pierigè F, Sfara C, Rossi L. Red blood cell-based delivery of drugs and nanomaterials for therapeutic and diagnostic applications. In: Nalwa HS, editor. Encyclopedia of nanoscience and nanotechnology. New York: American Scientific Publishers; 2011. p. 309–54.
A new era in cellular medicine. Rubius Therapeutics, Inc. 2019. https://ir.rubiustx.com/static-files/d5ef2cfc-3c34-4950-ac6f-b03ca9ff11e4. Accessed 3 Mar 2020.
Rubius Therapeutics Announces FDA Clearance of Investigational New Drug Application for First-Ever Red Cell Therapeutic, RTX-134, for Treatment of Phenylketonuria (press release). Rubius Therapeutics, Inc., 11 Mar 2019. https://www.globenewswire.com/news-release/2019/03/11/1751139/0/en/Rubius-Therapeutics-Announces-FDA-Clearance-of-Investigational-New-Drug-Application-for-First-Ever-Red-Cell-Therapeutic-RTX-134-for-Treatment-of-Phenylketonuria.html. Accessed 3 Mar 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was partially supported by Horizon 2020 IEDAT Grant n. 667946.
Conflicts of interest
Mauro Magnani and Luigia Rossi hold shares in EryDel SpA, a company with interests in the technology of RBC-based drug delivery. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rossi, L., Pierigè, F., Aliano, M.P. et al. Ongoing Developments and Clinical Progress in Drug-Loaded Red Blood Cell Technologies. BioDrugs 34, 265–272 (2020). https://doi.org/10.1007/s40259-020-00415-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-020-00415-0