Abstract
Background
Health interventions contribute to the production of greenhouse gas emissions. Thus, reducing carbon footprint is essential in supporting the UK National Health Service (NHS) pathway to net zero. This study explores the approach in which carbon footprint can be included when applying Health Technology Assessment (HTA) modelling using obesity intervention in the United Kingdom (UK) as a case study.
Methods
Using decision analytic modelling, we conducted an HTA incorporating the impacts of obesity-related treatment decisions on UK carbon emissions. A cohort Markov model was used to track the emissions of the UK population after receiving one of two obesity treatments: semaglutide and bariatric surgery.
Results
This study introduced two new carbon measurement tools that may be useful for future policymaking, incremental carbon footprint effectiveness ratio (ICFER) and incremental carbon footprint cost ratio (ICFCR), which made it possible to assess the emission impacts of proposed health policies. Using the obesity intervention case study, we found that both treatments have an incremental cost-effectiveness ratio (ICER) of < £20,000 per quality-adjusted life-years (QALYs) gained. This is below the UK threshold, indicating that these are cost-effective treatments for obesity, but could increase the NHS carbon footprint. However, it could reduce the overall UK societal carbon footprint by reducing the number of people with obesity. The ICFCR shows a reduction of 1.13–4.51 kgCO2e (kilogram of carbon dioxide equivalent) for every pound spent on obesity treatment.
Conclusion
This study illustrates a case study for estimating the effect of health policies on carbon emissions and provides a quantitative measure for obesity-related treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
To mitigate the impact of climate change and to help the government meet their net zero targets, policymakers should consider the environmental costs and effects of health policies and interventions. |
Currently, there is no standardised way of incorporating the environmental impact of healthcare despite the vital role of the health sector in reducing carbon emissions. |
This study proposed a novel approach to quantifying the population's societal carbon footprint into Health Technology Assessment (HTA) which could help balance future health decisions against environmental costs. |
1 Introduction
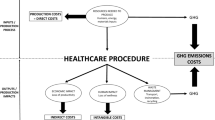
In the UK, the 2019 carbon footprint of the National Health Service (NHS) was 25 MtCO2e (million tonnes of carbon dioxide equivalent), which is around 6% of the UK's total footprint [1, 2]. Thus, the healthcare sector can potentially play a vital role in the mitigation of anthropogenic climate change, by leveraging its influence to drive carbon emission reductions [2]. However, quantifications of the environmental impacts such as carbon emissions on healthcare decision making are generally scarce [3]. This study contributes to the small amount of existing research that integrates carbon emission reduction into the Health Technology Assessment (HTA), a widely established method for health economic evaluations and health-related policy guidance. The literature on incorporating environmental elements and carbon footprint calculation into HTA modelling is growing with the increasing interest in environmental impact reduction [4]. For example, the use of processed-based life cycle assessment (LCA) in HTA has been proposed to internalise environmental externalities [3, 5]. This can be incorporated using various approaches, including decision modification, applying carbon footprint as a criterion in the multi-decision criteria analysis (MCDA), monetisation, or incorporating additional cost in the analysis [3].
This research offers a novel methodological approach to including carbon footprint analysis in HTA modelling and helps shape our understanding of the environmental impact associated with health care. To achieve this, obesity intervention was chosen as a case study, because obesity has a significant impact on the environment. A recent study estimated the yearly global impact of obesity at 1.6% of all emissions, equivalent to 700 MtCO2e, breaking it down into specific societal categories, such as food consumption and transport [6]. This impact comes on top of the increased requirement for obesity treatment in healthcare, which has its own carbon footprint. Therefore, changes in obesity levels will directly affect NHS carbon emissions and the pathway to the NHS net-zero target date of 2045 [7].
The UK NHS estimates that more than one in four adults are obese, with a body mass index (BMI) >30 kg/m2 [8,9,10]. The impact of this can be considered in terms of cost and physical and mental health. A government-commissioned report in 2007 estimated a £7 billion yearly cost of obesity to the NHS, rising to £12.5 billion by 2050 [11]. These cost rises are driven by the higher prevalence of several illnesses (co-morbidities) among people with obesity compared with people with a healthy weight. Each of these requires NHS treatment. The NHS recorded over one million hospital admissions in the UK with a primary or secondary diagnosis of obesity in 2019/2020 [12]. Historically, only bariatric surgery led to large BMI reductions. More conservative interventions, such as drug treatments, showed poor long-term results, with initial weight loss often regained over several years [13]. However, a recent trial highlighted the potential for the drug semaglutide to provide significant weight loss [14], well beyond that achieved by the current approved drug orlistat [15]. Before approving new treatments like semaglutide, NICE (National Institute for Health and Care Excellence) reviews the drug trial results and performs an effectiveness analysis as a part of a health technology assessment (HTA) [16,17,18]. HTAs compare the health effects and cost effectiveness of different treatment scenarios. However, some suggest NICE should also consider healthcare’s environmental impacts [16], especially given the negative impact of climate change on health [19, 20].
This study aims to show that carbon emissions can be integrated into HTA modelling using obesity intervention as a case study. This study has three research objectives: firstly, to perform an HTA to model the difference in cost effectiveness and carbon footprint in the different treatment scenarios of bariatric surgery and semaglutide; secondly, to investigate how reducing obesity affects the prevalence of BMI-related diseases in the UK, and affects NHS spending and carbon emissions; and lastly, to model the effect of lowering BMI on UK societal carbon emissions.
2 Methods
2.1 Markov Model Overview
This study conducted an HTA using a three-part process.
In Part 1, the 2019 UK population was categorised by BMI [10]. Treatment scenarios, either bariatric surgery or semaglutide, were then applied, changing the BMI composition. Each person treated had a one-off cost and one-off carbon footprint.
In Part 2, the BMI-distributed populations from Part 1 were put through a cohort Markov model (Fig. 1) containing six health states: healthy, dead and four morbidity states—cancer, coronary heart disease (CHD), diabetes and stroke. The cancer state is a combination of bowel/colorectal and breast cancer, both of which have been shown to correlate with BMI [11, 13]. Treatment in each morbidity state incurs an NHS cost and related carbon emissions.
Part 3 then combined the population from the treatment scenarios with the outcomes of the cohort Markov model to give the key results from an NHS perspective, comprising an incremental cost-effectiveness ratio (ICER)—the change in cumulative costs from the baseline no-treatment scenario divided by QALYs gained from baseline; an incremental carbon footprint effectiveness ratio (ICFER)—the change in cumulative carbon footprint from baseline over QALYs gained from baseline; and an incremental carbon footprint cost ratio (ICFCR) that refers to the change in cumulative carbon footprint from baseline over the change in cumulative costs from baseline. Finally, taking a wider environmental perspective, societal emissions related to obesity are then aggregated to give societal results. Societal emissions here lean more towards an environmental perspective and do not include indirect costs such as productivity loss for simplification purposes, especially related to obesity treatment.
The time horizon of this study is 50 years, with each year equalling one cycle. This was validated by comparing the model deterministically for 1, 10 and 50 cycles (see Appendix 1in the electronic supplementary material [ESM]). The population enters the model in the healthy state for their BMI category and at the end of each cycle, the population moves proportionally into the different states based on their relevant transition probabilities, as can be seen in Table 1 (see Appendix 2.5 in the ESM). The model assumes that once a member of the population has chronic morbidity, they retain it until death, cannot recover, and cannot develop more than one co-morbidity until death. It is documented that multi-morbidities are more often present in people with obesity and are more complex than dealing with each co-morbidity alone. Multi-morbidities were not included in the study for simplicity [21, 22].
2.2 Scenario Populations
The 2019 UK population of 66,796,800 had a baseline BMI composition as described in Table 2 [10, 37]. Based on the NICE guidelines, all people with obesity are eligible for semaglutide and all Obese III people are eligible for bariatric surgery. The model assumes everybody who gets treatment has already completed the prerequisites of diet and exercise [38]. The treatments assume that everybody achieves the average drop in BMI instantaneously.
2.2.1 Bariatric Surgery Specifications
There are three main types of surgery classed as bariatric surgery: gastric bypass, gastric banding, and sleeve gastrectomy. Gastric bypass was chosen for this study because it gives the largest effect, is a best-case scenario, and is the most common procedure performed in the UK [39]. The effect of surgical intervention on BMI is a 32% drop in a patient’s initial BMI [40]. The cost of bariatric surgery performed on the NHS has not been published but price ranges are published for private bariatric surgery [41]. The average cost used in this study, £10,510, is calculated using the mid-point of the ranges, and their occurrence ratios [39]. The carbon footprint of surgery was taken as the average carbon emissions for a UK hospital surgery, 173 kilograms of carbon dioxide equivalent (kgCO2e)/case [42].
2.2.2 Semaglutide Specifications
The effect of pharmaceutical intervention with semaglutide is an average BMI drop of 5.54 kg/m2 [14]. The annual cost of a 2.4-mg dose of semaglutide is £1,926, using the treatment program published by MIMS (Monthly Index of Medical Specialities) [43]. The carbon footprint of 252.7 kgCO2e/person/year for one year of semaglutide was estimated from information from the drug manufacturer, such as their global sales and carbon footprint and their current drug costs [44,45,46,47]. For the calculation details, see Appendix 1 in the ESM. Semaglutide trial participants received treatment for 68 weeks, so the yearly cost and carbon footprint were multiplied by 1.31 [14].
2.2.3 Scenario Decisions
Both the 100% semaglutide and 100% bariatric surgery scenarios represent the largest conceptual impact of each treatment across its eligible population. Expecting all eligible patients to use an offered treatment is unrealistic and would exceed operational capacity for bariatric surgery, so other scenarios were modelled. The 2.5% semaglutide scenario approximates the 2018 UK uptake of the current weight loss drug, orlistat, which was 389,000 prescriptions or 2.08% of the eligible population [48]. Under the scenario, all these people switch over to semaglutide with minimal additional prescriptions. The 2.5% bariatric surgery scenario represents a desired UK uptake target [49]. The most recent UK statistic is that only 0.27% of the eligible population undergoes bariatric surgery [39]. The 2.5% target represents a 10-fold increase but this is feasible, as seen elsewhere in Europe (e.g., Sweden [49]). The number of Obese III people in the UK is currently rising by 60,000 people a year [49]. Therefore, 2.5% of bariatric surgery scenarios is below the number required to keep the Obese III level constant. Therefore, an intermediate scenario was devised with a 10% surgery rate. Semaglutide achieves a larger weight loss than the current drug orlistat [14, 15]. Therefore, it could be anticipated that uptake will increase. A 10% uptake has been chosen to mirror the bariatric surgery scenario and enable easier comparisons. Bariatric surgery has a larger effect on BMI, such that none of the population remains in Obese class III for the 100% bariatric surgery scenario [Table 2]. However, semaglutide is given to a much wider proportion of the population. For the same percentage of treatment uptake, 8.5 times more people are eligible to receive treatment.
2.3 Utility, Carbon Footprint and Cost Specifications
Utility and cost data for the co-morbidities were obtained from Public Health England’s 2015 data, see Table 1 [22]. Utility values were used to calculate quality-adjusted life years (QALYs). The costs and utility of each co-morbidity were assumed to be constant in relation to BMI. The source calculates the average healthcare costs of ten co-morbidities in 2015 and a baseline cost of anybody not having them (i.e., total healthcare costs divided by the total population). This baseline cost as used in this study is the average healthcare cost of a healthy person not having any of the four study diseases (£1123). For the co-morbidities, the cost listed includes baseline costs, therefore £1123 was removed to represent just the health spending on those four diseases [22]. The carbon footprint of treatment of co-morbidities was calculated by multiplying costs by the NHS carbon intensity, 0.156 kgCO2e/£ [2]. Discount rates for costs and QALYs were applied to allow near-term impacts more importance at the standard rate applied to all economic models considered by NICE of 3.5% [50]. For carbon footprint, no discount rate was used as all carbon emissions are valued equally, “hence maintaining intergenerational equity” [3]. All costs for co-morbidities, semaglutide, and bariatric surgery have been adjusted to 2023 prices [60].
2.4 Uncertainty Analysis
There are two sensitivity analyses performed: a one-way deterministic sensitivity analysis was performed where key input parameters from Part 1 were varied one at a time to a high and a low value to monitor the sensitivity of the key outputs from Part 3. A probabilistic sensitivity analysis using a 1000-run Monte Carlo simulation for Part 2 was also constructed using the distribution of events or the error present in the scientific literature [51]. The distribution types and parameters are detailed in Appendix 2.5 (see ESM). Costs, QALYs and carbon footprint were calculated for each run and averages were only taken on model outputs. The parameters were randomised at the start only and then kept constant for each run of 50 cycles.
2.5 Part 3: Outputs
The Markov model (Part 2) outputs the following values for each BMI category: (i) the number of QALYs spent outside the death state; (ii) costs accrued for each year spent in each state; (iii) carbon footprint accrued for each year spent in each state. Part 3 of the model combines the proportions of the population from each of the scenarios and the overall one-off treatment cost and carbon footprint (CF) for each scenario from Part 1 and the outputs for each BMI from Part 2, to provide the cumulative costs and cumulative carbon footprint (CCF), calculated using Equation (1).
The scenarios are then compared with the baseline no-treatment scenario to provide an ICER, ICFER and ICFCR.
Finally, the obesity-related emissions associated with transport and food consumption are estimated [6]. These are accrued for every life year of an Obese I, Obese II and Obese III person for each scenario, assuming the impact was equal in each category. This value for the societal carbon footprint of obesity is aggregated to the CCF from each scenario to give a cumulative societal carbon footprint. This is then converted into a society framing with a new ‘societal ICFCR’ result.
3 Results
3.1 Model Validation
The model was validated against the NHS budget, the previous cost of obesity measurements and the current carbon footprint of the NHS. To perform the validation, the baseline model was run deterministically with no treatment applied and compared over different timeframes of 1, 10 and 50 cycles. 50 years was chosen to allow assessment over an adult lifespan and also include the NHS Net-Zero target date, see Appendix 1 in the ESM [7]. Fig. 2 shows the proportion of people who are healthy or are still alive by BMI category during the 50 cycles when the model is run deterministically. Healthy-weight people live longer with fewer co-morbidities.
3.2 Scenario Results
Table 3 shows all scenarios have higher cumulative QALYs and cumulative costs than the baseline population undergoing no treatment. The 100% semaglutide scenario accrues over 20 million more QALYs than any other scenario. The magnitude of costs and QALYs increased linearly with percentage of the eligible population given the treatment. All the ICERs per patient are identical for each treatment and are presented together in results tables. All scenarios have a higher CCF relative to the baseline. The ICFER and ICFCR are also shown in Table 3. The aggregation of the CCF with the societal carbon footprint of food consumption and transport results in a net reduction in the overall carbon footprint in this study. Carbon reduction from the baseline scenario and the combined societal ICFCR are shown in Table 4. The 100% semaglutide scenario accumulates at least 200 MtCO2e less societal carbon emissions than the other scenarios. As there is no error associated with the societal emissions as standard (one is tested in the one-way sensitivity analysis), the error is carried over from Table 3.
3.2.1 Uncertainty Analysis
The results from the 1000-run probabilistic Monte Carlo simulation are shown in Fig. 3. Overall, the variation is small, increasing confidence in the results. Costs are correlated with the carbon footprint, R2 = 0.38 for both semaglutide and bariatric surgery.
ICER, ICFCR and ICFER graphs for all scenarios. The line represents the £20,000 NICE ICER threshold [18]. The distance below the line represents the magnitude of the cost effectiveness of each scenario. All results are shown on a log scale. CF carbon footprint, ICER cost-effectiveness ratio (£/QALYs gained), ICFCR incremental carbon footprint cost ratio (kgCO2e/£ spent), ICFER incremental carbon footprint effectiveness ratio (kgCO2e per QALYs gained), kgCO2e kilograms of carbon dioxide equivalent, MtCO2e million tonnes of carbon dioxide equivalent, NHS National Health Service, QALYs quality-adjusted life-years
Table 5 shows the results from the one-way sensitivity analysis. As it was run deterministically, results vary slightly from the probabilistic results above. Semaglutide treatment length must increase to 2 years for the ICER to be higher than for bariatric surgery. Only if semaglutide cost increases 5-fold, does the ICER become higher than for bariatric surgery. If bariatric surgery cost is reduced 10-fold, the ICER becomes lower than for semaglutide, however, the societal ICFCR still shows a smaller relative emissions reduction. At the low carbon footprint values for treatment, the ICFER and ICFCR remain positive. At the high carbon footprint values for treatment, the societal ICFCR remains negative. Only when societal obesity-related emissions are more than 10 times smaller does the societal ICFCR become 0. None of the analyses of the sensitivity of the societal ICFCR shows that bariatric surgery is preferred over semaglutide.
4 Discussion
This study develops two new exploratory measurements to incorporate the environmental impact of health treatment decisions, namely the incremental carbon footprint effectiveness ratio (ICFER) and the incremental carbon footprint cost ratio (ICFCR). If developed, these tools could match the ICER in improving understanding of the emissions impact of policies. The ICFCR highlights whether the health intervention carries a carbon reduction or carbon increase, as well as the magnitude of that change per pound spent. Positive ICFCR shows that the carbon footprint of the NHS rises per pound spent on these treatments.
The main aim of the study is to first take a healthcare perspective and model the cost effectiveness and carbon effectiveness of two treatments for obesity over a 50-year period: bariatric surgery, given to people with a BMI >40 kg/m2, and semaglutide, given to people with a BMI >30 kg/m2. Both treatments have increased costs compared with the no-treatment scenario but also result in increased QALYs gained. ICERs for both treatments are below the UK £20,000 ICER threshold, confirming that both can be considered cost effective. Secondly, the model adopts a wider environmental perspective and investigates how these obesity treatments may impact the societal carbon footprint of the UK. The one-way sensitivity analysis shows the model is robust and not sensitive to the input parameters. Both treatments are always cost effective and always increase the carbon footprint of healthcare.
More importantly, the study does not merely look at the average effect on those treated. It averages the effect of both treatments across the entire population. This averaging across the population could go beyond HTAs and into public policymaking, where costs to the population are considered alongside costs to the individual. The results show that while an obesity reduction increases the NHS carbon footprint, it could reduce the wider UK societal carbon footprint, due to a reduction in food and transport emissions. From the one-way sensitivity analysis, unless these reductions are essentially zero, the societal ICFCR is negative and therefore, carbon is reduced per pound spent. The reduction is costly at £359–£1,030/tCO2e when compared with the £33–£65/tCO2e estimate for the required cost of carbon to reduce carbon emissions to meet the Paris Agreement [52]. Due to the limited research on incorporating carbon emissions into HTA processes, no specific thresholds have been established to assess the value of a health intervention in relation to its opportunity cost. However, this £33–£65/tCO2e could be a potential threshold for the ICFCR going forward and would potentially assist policy decisions to achieve cost-effective carbon savings.
The ICFER measurement explores balancing future health decisions against carbon footprint. This study highlights the simpler scenario where both the carbon footprint and QALYs increase, perhaps requiring a threshold like that used with ICERs to ensure the NHS meets its future carbon budget as well as its cost budget. When in future the effects of climate change are more keenly apparent and the need for decarbonisation is more urgent, it also has a more controversial scenario where both the carbon footprint and QALYs decrease, suggesting some trade-off of health for carbon reductions and raising the question of whether that would ever be a consideration for policymakers. This aligns with similar discussions around health reductions in ICERs where it is argued that this is currently never considered [53].
This study shows an improvement in obesity prevalence does not translate into a direct cost or carbon footprint improvement for the NHS, as people stay healthier for longer and survive longer with co-morbidities, but there are other interventions where this may not be the case. NICE could implement an internal carbon price during HTAs, monetising additional carbon emissions from a scenario and thereby increasing the cost of the least environmentally friendly option. By doing this, an intervention that was borderline on its original HTA may become desirable. By considering an environmental perspective, the study shows that an obesity reduction due to the NHS treatment decision reduces the UK's societal carbon footprint, helping meet the UK net-zero legal obligation [54]. This could place decisions about these interventions/policies alongside some of the other public health initiatives pursued by the Department of Health as an improvement in public health and a reduction in societal carbon footprint, rather than the current reduction in public service costs [55]. For carbon footprint, as with healthcare costs, prevention may be better than cure. Other studies project increasing costs as obesity prevalence increases and recommend obesity prevention measures as a high priority [11, 56]. Further investment in primary and community care would allow more obese patients to be seen and treated before they reach the most carbon-intensive portion of the NHS, which is acute care [2].
The government is widening perspectives to influence the social and environmental performance of companies and their supply chains, such as the Modern Slavery Act and due diligence laws [57, 58]. This could be expanded to include drug licensing decisions and HTAs in future. Carbon footprinting is still nascent and the lack of high-quality data requires a lot of top-down calculations allocating emissions based on spending [2, 3]. The more top-down calculations, the higher the correlation between spending and carbon footprint, as shown in the results of this study. More bottom-up emissions measurements of specific processes or treatments can improve this study and decouple carbon emissions from costs [16]. Consequently, emissions for the treatment of co-morbidities in this study are calculated using pounds spent, and the carbon footprint of semaglutide is based on the allocation of sales-related emissions. The basic assumption is that more expensive treatment is more carbon intensive.
This study has several strengths. Firstly, this study has explored the way we can internalise environmental externalities such as carbon footprint into health economic modelling. We have contributed to the growing literature in this area of research by providing one of the first studies to explore methodological approaches to carbon footprint integration into traditional HTA modelling. Secondly, this study focuses on the incorporation of carbon footprint calculation into HTA beyond monetising. This would help gain a better understanding of carbon footprint calculation in its original form (instead of assuming monetary form), resulting in different metrics that could help policymakers understand not just the health perspective, but also the environmental perspective. The methods presented in the paper could also provide a standardised metric for carbon footprint calculation that can be replicated by other countries if emissions data can be obtained and made available.
Inevitably, as an exploratory approach, this study has limitations. Because of limited data on the UK prevalence of every disease according to standard BMI categories, we derived diabetes mellitus mortality data and stroke morbidity data from other countries [26, 35]. Different studies of the same morbidity show variation in the exact risk ratios between BMI categories, but the risk curve shape and the magnitude of the ratio are similar, so this error is assumed to be low. Additionally, the model is static and not age-dependent which limits the applicable studies and model representativeness. BMI morbidity and mortality risks which change with age could be included in future research. Furthermore, the static model assumes the weight is lost immediately during obesity treatment and not regained. Both treatment types, surgical and pharmaceutical, have demonstrated weight regain after treatment. Bariatric surgery patients on average regain 6% of weight lost after 10 years [59]. Existing drug treatments only maintain weight loss while the drug is taken [15]. The same may apply to semaglutide. If further courses of semaglutide are required beyond 68 weeks, then the associated carbon footprint will increase, although the one-way sensitivity analysis shows increasing to 2-year prescriptions is not significant for the final result.
In common with other cost-effectiveness analysis studies, this current study also has limitations in its capacity to model reality, particularly in terms of incorporating multimorbidity. People with obesity have an increased risk of multimorbidity compared with those of normal weight [21]. In the model, people with diabetes do not develop complications because it does not permit multimorbidity. In reality, they are more likely to have multimorbidity, such as strokes and CHD [30]. The study therefore underestimates the number of diabetes-related deaths and people remain in that state for an unrealistic number of cycles. People with multimorbidities often have a higher cost than the combined individual comorbidities. Therefore, allowing multimorbidities may make the studied obesity interventions more impactful [22]. Moreover, the model does not consider the severity level of cancers, and their remission rates, which may result in an overestimation of cancer prevalence. Cancer patients in remission incur fewer costs than those with active disease, but may be more likely to re-develop cancer than the general population. However, this was not simulated in the cohort model.
Another limitation would be in terms of the replication of the study in another global context. Although useful, it might be challenging to adopt and replicate this study in another context, especially as the carbon footprint data associated with health interventions is not always available in other countries, especially in the Global South. Moreover, internationally standardised quantification of health-related carbon footprint modelling is scarce, thus models often rely on assumptions that might be biased.
Future research making the model dynamic, to include trends of age and obesity over time, would give a wider scope. Carbon emissions analysis could include the average yearly UK carbon footprint per person. This would better represent the effect of longer lives on the UK carbon footprint. Finally, a qualitative and quantitative study into the inclusion and consequent impact of an internal carbon price on an HTA would be a worthwhile undertaking. Carbon pricing is already used across several fields and is likely to become the norm.
5 Conclusion
This study demonstrates the potential of health technology assessments to include the impacts of obesity-related treatment decisions on UK carbon emissions. It found both semaglutide and bariatric surgery are cost-effective treatments for obesity but increase the carbon footprint of UK healthcare. However, they both stand to reduce the societal carbon footprint of the UK, such that the overall carbon footprint impact is negative. The study found that semaglutide is more effective than bariatric surgery at achieving this overall impact, largely because more people are eligible to receive treatment. Finally, this study has introduced two new carbon measurement tools that may be useful for future research and policymaking.
References
DEFRA. UK’s carbon footprint [Internet]. GOV.UK. 2021 [cited 2021 Aug 8]. https://www.gov.uk/government/statistics/uks-carbon-footprint.
Tennison I, Roschnik S, Ashby B, Boyd R, Hamilton I, Oreszczyn T, et al. Health care’s response to climate change: a carbon footprint assessment of the NHS in England. Lancet Planet Health. 2021;5(2):e84-92.
McAlister S, Morton RL, Barratt A. Incorporating carbon into health care: adding carbon emissions to health technology assessments. Lancet Planet Health. 2022;6(12):e993–9.
Marsh K, Ganz M, Nørtoft E, Lund N, Graff-Zivin J. Incorporating environmental outcomes into a health economic model. Int J Technol Assess Health Care. 2016;32(6):400–6.
Thiel CL, Cassels-Brown A, Goel H, Stancliffe R, Steinbach I, Thomas P, et al. Utilizing off-the-shelf LCA methods to develop a ‘triple bottom line’ auditing tool for global cataract surgical services. Resour Conserv Recycl. 2020;1(158): 104805.
Magkos F, Tetens I, Bügel SG, Felby C, Schacht SR, Hill JO, et al. The environmental foodprint of obesity. Obesity. 2020;28(1):73–9.
NHS. Delivering a ‘Net Zero’ National Health Service [Internet]. NHS; 2020 [cited 2021 Aug 8]. https://www.england.nhs.uk/greenernhs/publication/delivering-a-net-zero-national-health-service/.
CDC. All About Adult BMI [Internet]. Centers for Disease Control and Prevention. 2021 [cited 2021 Aug 31]. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html.
NHS. Obesity [Internet]. nhs.uk. 2019 [cited 2021 Sep 7]. https://www.nhs.uk/conditions/obesity/.
NHS, ONS, Moody A. Health Survey for England 2019 [Internet]. NHS Digital. 2020 [cited 2021 Jun 16]. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019.
McPherson K, Marsh T, Brown M. Tackling obesities: future choices—modelling future trends in obesity and the impact on health. London: Government Office for Science, UK Government; 2007.
NHS. Statistics on Obesity, Physical Activity and Diet, England 2021 [Internet]. NHS Digital. 2021 [cited 2021 Aug 15]. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/england-2021.
Panca M, Viner RM, White B, Pandya T, Melo H, Adamo M, et al. Cost-effectiveness of bariatric surgery in adolescents with severe obesity in the UK. Clinical Obesity. 2018;8(2):105–13.
Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002.
Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237–48.
Marsh K, Ganz ML, Hsu J, Strandberg-Larsen M, Gonzalez RP, Lund N. Expanding health technology assessments to include effects on the environment. Value Health. 2016;19(2):249–54.
McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold. Pharmacoeconomics. 2008;26(9):733–44.
NICE. Assessing cost effectiveness - The guidelines manual [Internet]. NICE; 2012 [cited 2021 Aug 31]. https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness.
Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Belesova K, Boykoff M, et al. The 2019 report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. The Lancet. 2019;394(10211):1836–78.
Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Beagley J, Belesova K, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. The Lancet. 2020;397(10269):129–70.
Delpino FM, dos Santos Rodrigues AP, Petarli GB, Machado KP, Flores TR, Batista SR, et al. Overweight, obesity and risk of multimorbidity: a systematic review and meta-analysis of longitudinal studies. Obes Rev. 2023;24(6): e13562.
Public Health England. The health and social care costs of a selection of health conditions and multi-morbidities [Internet]. Public Health England; 2020 [cited 2021 Jun 23] p. 62. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/898189/The_health_and_socialcare_costs_of_a_selection_of_health_conditions_and_multi-morbidities.pdf.
Cancer Research UK. Cancer statistic by cancer type [Internet]. [cited 2021 Jun 23]. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type.
Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. The Lancet. 2014;384(9945):755–65.
SSNAP. SSNAP Annual Portfolio for April 2019-March 2020 admissions and discharges - Clinical Audit Report [Internet]. Kings College London; 2020 [cited 2021 Jun 23]. https://www.strokeaudit.org/results/Clinical-audit/National-Results.aspx.
Kurth T, Gaziano JM, Berger K, Kase CS, Rexrode KM, Cook NR, et al. Body mass index and the risk of stroke in men. Arch Intern Med. 2002;162(22):2557.
BHF. Heart & Circulatory Disease Statistics 2021 [Internet]. 2021 [cited 2021 Jun 23]. https://www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2019.
Canoy D, Cairns BJ, Balkwill A, Wright FL, Green J, Reeves G, et al. Body mass index and incident coronary heart disease in women: a population-based prospective study. BMC Med. 2013;11(1):87.
Whicher CA, O’Neill S, Holt RIG. Diabetes in the UK: 2019. Diabet Med. 2020;37(2):242–7.
Gray N, Picone G, Sloan F, Yashkin A. The relationship between BMI and onset of diabetes mellitus and its complications. South Med J. 2015;108(1):29–36.
ONS. Deaths registered in England and Wales: 2019 [Internet]. 2020 [cited 2021 Jun 23]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2019.
Angelantonio ED, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet. 2016;388(10046):776–86.
Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, et al. Association between obesity and mortality after acute first-ever stroke: the obesity–stroke paradox. Stroke. 2011;42(1):30–6.
Davies AR, Smeeth L, Grundy EMD. Contribution of changes in incidence and mortality to trends in the prevalence of coronary heart disease in the UK: 1996–2005. Eur Heart J. 2007;28(17):2142–7.
Logue J, Walker JJ, Leese G, Lindsay R, McKnight J, Morris A, et al. Association between BMI measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes Care. 2013;36(4):887–93.
Zaccardi F, Dhalwani NN, Papamargaritis D, Webb DR, Murphy GJ, Davies MJ, et al. Nonlinear association of BMI with all-cause and cardiovascular mortality in type 2 diabetes mellitus: a systematic review and meta-analysis of 414,587 participants in prospective studies. Diabetologia. 2017;60(2):240–8.
ONS. Population estimates for the UK, England and Wales, Scotland and Northern Ireland - mid-2019 [Internet]. 2020 [cited 2021 Aug 25]. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2019estimates.
NICE. Obesity: identification, assessment and management [Internet]. NICE; 2014 [cited 2021 Feb 28] p. 37. https://www.nice.org.uk/guidance/cg189/resources/obesity-identification-assessment-and-management-pdf-35109821097925.
Small P, Mahawar K, Walton P, Kinsman R. Third Registry Report [Internet]. The National Bariatric Surgery Registry of The British Obesity and Metabolic Surgery Society; 2020 [cited 2021 Aug 21]. (The UK National Bariatric Surgery Registry). http://new.e-dendrite.com/sites/default/files/2021-01/NBSR%202020.pdf.
Bromberger B, Porrett P, Choudhury R, Dumon K, Murayama KM. Weight Loss interventions for morbidly obese patients with compensated cirrhosis: a Markov decision analysis model. J Gastrointest Surg. 2014;18(2):321–7.
NHS. Weight loss surgery - Availability [Internet]. nhs.uk. 2017 [cited 2021 Aug 21]. https://www.nhs.uk/conditions/weight-loss-surgery/who-can-have-it/.
MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1(9):e381–8.
Wegovy | MIMS online [Internet]. [cited 2023 Sep 11]. https://www.mims.co.uk/drugs/nutrition/obesity/wegovy.
Novo Care. Explaining list price for Saxenda [Internet]. 2021 [cited 2021 Aug 21]. https://www.novocare.com/saxenda/let-us-help/explaining-list-price.html.
Novo Nordisk. Wegovy approved in US - Investor Conference Call [Internet]. 2021 [cited 2021 Jun 24]. https://investor.novonordisk.com/wegovy-tm-approved-in-the-us/?page=1.
Novo Nordisk. ESG Portal [Internet]. 2021 [cited 2021 Aug 21]. https://www.novonordisk.com/sustainable-business/esg-portal.html.
Novo Nordisk. Novo Nordisk Quarterly Financial Report Q1 2019 [Internet]. 2019 May [cited 2021 Aug 21]. https://www.novonordisk.com/content/dam/nncorp/global/en/investors/irmaterial/quarterly_financial_reports/2019/20190503_Financial%20statement%20Q1%202019.pdf.
NHS. Statistics on Obesity, Physical Activity and Diet, England, 2020 Part 3: Adult overweight and obesity [Internet]. NHS Digital. 2020 [cited 2021 Mar 1]. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/england-2020/part-3-adult-obesity-copy.
Welbourn R, le Roux CW, Owen-Smith A, Wordsworth S, Blazeby JM. Why the NHS should do more bariatric surgery; how much should we do? BMJ. 2016;11(353): i1472.
NICE. Guide to the methods of technology appraisal 2013. Glossary-Discounting [Internet]. 2013 [cited 2021 Sep 4]. https://www.nice.org.uk/process/pmg9/chapter/glossary#discounting-2.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006. (Handbooks in Health Economic Evaluation).
Stern N, Stiglitz J. Report on High-Level Commission on Carbon Prices [Internet]. Carbon Pricing Leadership Coalition; 2017 [cited 2021 Sep 10]. https://static1.squarespace.com/static/54ff9c5ce4b0a53decccfb4c/t/59b7f2409f8dce5316811916/1505227332748/CarbonPricing_FullReport.pdf.
Klok RM, Postma MJ. Four quadrants of the cost-effectiveness plane: some considerations on the south-west quadrant. Expert Rev Pharmacoecon Outcomes Res. 2004;4(6):599–601.
UK CCC. Net Zero Technical report [Internet]. UK Committee For Climate Change; 2019 [cited 2021 Aug 31]. https://www.theccc.org.uk/publication/net-zero-technical-report/.
Public Health England. PHE Strategy 2020 to 2025 [Internet]. GOV.UK. 2019 [cited 2023 May 26]. https://www.gov.uk/government/publications/phe-strategy-2020-to-2025.
Cancer Research UK, Hunt D, Knuchel-Takano A, Bhimjiyani A, UK Health Forum. Tipping the Scales: why preventing obesity makes economic sense [Internet]. CRUK; 2016 [cited 2021 Aug 15] p. 35. http://www.healthlumen.com/wp-content/uploads/2020/04/Tipping-The-Scales-CRUK-Full-Report-FINAL.pdf.
LeBaron G, Rühmkorf A. Steering CSR through home state regulation: a comparison of the impact of the UK bribery act and modern slavery act on global supply chain governance. Global Pol. 2017;8:15–28.
DEFRA. Government sets out world-leading new measures to protect rainforests [Internet]. GOV.UK. 2020 [cited 2021 Jul 9]. https://www.gov.uk/government/news/government-sets-out-world-leading-new-measures-to-protect-rainforests.
Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
CCEMG-EPPI-Centre Cost Converter v.1.4 [Internet]. [cited 2023 Sep 11]. https://eppi.ioe.ac.uk/costconversion/default.aspx.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of Funding
The publication of this work was supported by King's College London School of Social Science and Public Policy (SSPP) Subvention Fund.
Potential Conflicts of Interest
The authors report no conflicts of interest that are directly relevant to the content of this article.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code Availability
The custom codes for the model are available in the supplementary information files.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Author Contributions
This manuscript was written and edited by MK, ZS and NZ. The HTA model was developed by MK with supervision from ZS and NZ. Critical revision of the paper was done by MK, ZS and NZ. All authors have contributed significantly to the work and have approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kindred, M., Shabrina, Z. & Zakiyah, N. Exploratory Approach to Incorporating Carbon Footprint in Health Technology Assessment (HTA) Modelling: Cost-Effectiveness Analysis of Health Interventions in the United Kingdom. Appl Health Econ Health Policy 22, 49–60 (2024). https://doi.org/10.1007/s40258-023-00839-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00839-z