Abstract
Background
The American College of Surgeons Oncology Group Z0011 trial showed that complete axillary lymph node dissection (cALND) did not improve survival benefits in patients with one or two tumour-involved sentinel lymph nodes and undergoing breast conservation. Still, a considerable number of the Z0011-eligible patients continue to be treated with cALND in various countries. Given the potential economic gain from implementation of the Z0011 recommendations, we quantified population-level impacts of omitting cALND among Z0011-eligible patients in clinical practice.
Methods
This 2-year economic analysis adopted both the perspective of patients under statutory insurance and the societal perspective, using data collected prospectively from 179 German breast cancer units between 2008 and 2015. The estimation of cost savings and health gain relied on a single decision tree, which considered three scenarios: clinical practice at the baseline; actual implementation in routine care; and hypothetical full implementation in all eligible patients.
Results
Data for 188,909 patients with primary breast cancer were available, 13,741 (7.3%) of whom met the Z0011 inclusion criteria. The use of cALND decreased from 94.3% in 2010 to 46.9% in 2015, resulting in a gain of 335 quality-adjusted life-years and a saving of EUR50,334,756 for the society. Had cALND been omitted in all eligible patients, the total gain would have been more than double.
Conclusions
The implementation of the Z0011 recommendations resulted in substantial savings and health gain in Germany. Our findings suggest that it is beneficial to introduce additional policy measures to promote further uptake of the Z0011 recommendations in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although the Z0011 trial showed that complete axillary lymph node dissection (cALND) did not improve survival benefits in early breast cancer patients, a considerable number of Z0011-eligible patients continue to be treated with cALND in various countries. |
Our economic evaluation estimated that the implementation of Z0011 recommendations from 2011 to 2015 in Germany resulted in substantial savings (EUR50,334,756) and health gain (335 QALY) for the society. |
Our findings suggest that the introduction of additional policy measures to promote further uptake of the Z0011 recommendations in clinical practice would be beneficial in Germany and in similar contexts. |
1 Background
In breast cancer, complete axillary lymph node dissection (cALND) was recommended for patients with tumour-affected sentinel lymph nodes (SLNs) until the publication of the American College of Surgeons Oncology Group Z0011 trial in 2010. The Z0011 trial randomised 891 women with T1/2 invasive breast cancer and one or two positive SLNs undergoing breast conservation to sentinel lymph node dissection (SLND) with or without cALND. The trial showed that SLND alone resulted in non-inferior 5-year loco-regional control, disease-free survival and overall survival rates comparable to SLND and cALND [1,2,3,4]. Subsequent prospective randomised trials confirmed that cALND can be safely omitted in clinically node-negative patients with micro-metastases in SLNs [5] or when axillary radiation is given after SLND [6,7,8].
Following the publication of the Z0011 trial results, the use of cALND among the patients who meet the Z0011 inclusion criteria (hereafter referred to as Z0011-eligible patients) has declined globally [9,10,11,12,13,14,15]. Since cALND bears high direct medical costs [16] and is associated with substantial secondary morbidities [17, 18], omitting this procedure among eligible patients is expected to save costs and concurrently improve health. The direct medical costs of cALND were estimated at approximately EUR3,331 in a micro-costing study in France [16]. Moreover, both short- and long-term secondary morbidities are more frequent in patients undergoing SLND and cALND than SLND alone [17,18,19,20]. Lymphedema, a chronic morbid condition with arm swelling and restriction of movement occurs in 20% of patients undergoing cALND versus 5.6% of patients undergoing SLND [17], leading to a decline in quality of life [21] and productivity loss among patients [22].
Still, cALND continues to be performed among a considerable proportion of Z0011-eligible patients in Germany and in other high-income countries [12, 13, 15, 23]. For example, two studies reported that the proportion of cALND remained high, at about 44% in Australia between 2011 and 2017 [12], and at 77% in Hong Kong between 2014 and 2019 [23]. Given that breast cancer is the most prevalent cancer worldwide, impacting nearly 7.8 million women at the end of 2020 [24], it is important to understand the magnitude of cost savings and health gain from the implementation of the Z0011 recommendations in clinical routine care. This evidence on population-level impact not only helps health professionals and patients fully appreciate the benefits of omitting cALND but also establishes the evidence on the value of additional policy measures which promote the further uptake of the Z0011 recommendations into clinical practice. We identified only one study which assessed the hypothetical cost savings resulting from implementing the Z0011 recommendations and did it only with reference to a single hospital in the USA [25]. To provide policy traction and promote the further uptake of the Z0011 recommendations into clinical practice, we quantified the population-level impact of omitting cALND among the Z0011-eligible patients. Specifically, our economic evaluation assessed both the actual and the potential cost savings and health gain of implementing the Z0011 recommendations into clinical practice of breast cancer care in Germany. We expect to strengthen the existing evidence base and to enhance the translation of the Z0011 recommendations into routine clinical practice.
2 Method and Data
2.1 Study Design and Data Sources
In our economic evaluation, we estimated the cost savings and health gain attributable to the implementation of the Z0011 recommendations into the routine management of breast cancer patients in Germany. Our retrospective analysis adopted both the perspective of a patient insured under the German statutory insurance (hereafter referred to as statutory insured patient) and a societal perspective. The perspective of statutory insured patients considered the direct medical costs incurred within the health sector (e.g., personnel, equipment, and drugs) paid by either the social health insurance or by the patients during the provision of the surgical procedure and of the treatment for subsequent morbidities. Accordingly, our cost calculation of statutory insured patients included the patient co-payment for in-patient care [26] and patient out-of-pocket payments for prescriptions which were not reimbursed by social health insurance. The assessment from the societal perspective additionally considered non-medical costs incurred outside the health sector (e.g., productivity loss, travel costs, and out-of-pocket payments) owing to the secondary morbidities induced by cALND. We applied a decision-modelling approach [27] and estimated both cost savings and quality of life improvement in a single decision tree model, as described below.
Guided by the current literature that both short- and long-term morbidities associated with SLND and cALND concentrated in the first two years after the surgery [17, 18], our model captured the relevant morbidities and associated costs for two years. We selected 2015, the most recent year of our main dataset, to be the base year of our analysis.
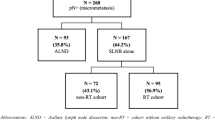
We used data from a nationally representative dataset of 188,909 patients diagnosed with primary breast cancer from 179 breast cancer units, which were prospectively collected between 2008 and 2015 in Germany [13]. Detail on the data quality and validation process has been reported by Hennigs et al [13]. The final dataset used for this analysis consisted of 13,741 patients (7.3% of all breast cancer patients), who had pT1/2cN0M0 invasive breast cancer with one or two tumour-involved SLNs and underwent breast-conserving surgery and adjuvant radiotherapy representing the Z0011 inclusion criteria. Further details on the patient characteristics are reported by Hennigs et al [13].
2.2 Decision Tree Model
Our choice to build a decision tree model was informed by the fact that the overall survival, diseases-free survival and loco-regional control are non-inferior between patients who are treated with SLND alone or SLND and cALND [27]. Thus, only the differences in costs and subsequent morbidities between the two groups should be considered and a decision tree is appropriate to model such differences.
In line with our research questions (estimating both actual and potential benefits of the implementation of the Z0011 recommendations), our decision tree has three arms reflecting three scenarios: clinical practice at baseline (2008–2010) prior to the publication of the Z0011 trial results (baseline scenario) in which almost every patient received cALND; actual practice of cALND from 2011 to 2015 (actual implementation scenario); and hypothetical practice in which cALND would have been omitted in all Z0011-eligible patients (full implementation scenario). Entering each arm of our decision tree are Z0011-eligible patients (with T1/2 tumours and one or two positive SLNs undergoing breast conservation), who then undergo two treatment possibilities: SLND alone or SLND and cALND. The probability of patients entering the SLND alone group or the SLND and cALND group is based on the annual uptake reported in the time-trend analysis by Hennigs et al, which served as the basis for the implementation effect estimates adopted in our decision tree [13]. The decision tree structure was presented in Fig. 1.
Following the axillary procedures, patients enter either one of three mutually exclusive health states: cancer free without any morbidity, cancer free with short-term morbidities, and cancer free with long-term morbidities. The probabilities of patients entering either of these three health states and other epidemiological parameters were informed by existing studies, which were identified via literature searches on different topics, including clinical effectiveness of cALND and SLND, secondary morbidities, costs and cost-effectiveness of these two axillary surgical procedures. Studies were selected with preference being given to systematic reviews based on meta-analysis, randomised controlled trials and observational studies conducted in similar settings. We reported our model epidemiological estimates and their data sources in Table 1.
Given that our decision tree model considered only the associated secondary morbidities on which the current evidence is of adequate quality, our model included only infection and seroma for short-term morbidities, which last for one month [18, 28] and secondary lymphedema as long-term morbidity, which lasts longer than 12 months [17]. The probability of developing infection and seroma following SLND alone (11%) versus SLND and cALND (15%) was informed by both a randomised controlled trial [28] and a systematic review with meta-analysis [18]. Similarly, the probability of lymphedema after SLND alone (5.6%) and versus after SLND and cALND (20%) was informed by an existing systematic review with meta-analysis [17]. Only the proportions of patients who need out-patient treatment (90%) and in-patient treatment (10%) for infection and seroma were informed by direct consultation with two senior clinicians, who have more than ten years of working experience in breast cancer treatment.
2.3 Cost Estimation
We adopted a mixed approach to costing and followed three steps [29]. First, informed by clinical guidelines [30] and the two aforementioned experts, we identified all costs related to the two surgical procedures (SLND and cALND) and the treatment costs (both in-patient and out-patient care) of the resulting morbidities. In the analysed dataset, SLND was performed together with breast conserving surgery (BCS) for nearly all patients (97.35%). Complete axillary lymph node dissection was performed together with BCS and SLND in the same procedure (after frozen section and confirmation of a positive SLN) or as a separate procedure following BCS and SLND. In addition, following either SLND or cALND, patients might be re-operated, with differing re-excision rates following either SLND or cALND.
Second, we measured the actual utilisation of the surgical procedures. Specifically, for the SLND alone group, we calculated the proportion of patients who underwent SLND in the same or separate procedure with BCS. For the SLND and cALND group, we calculated the proportion of patients who underwent cALND in the same or separate procedure with BCS and SLND. We also calculated the re-operation rates for the two groups using the same dataset of 13,741 patients described above. Table 2 reports the average use of SLND and cALND between 2010 and 2015 calculated from the analysed data.
Third, we identified the average cost for each procedure and treatment of associated morbidities from existing data sources and relevant publications. For in-patient care procedures, we first determined the correct diagnosis-related group (DRG) codes and then obtained the yearly unit costs for the corresponding procedures from 2010 to 2015. We retrieved all the annual unit costs of the concerned procedures from the Institute for the Hospital Renumeration System (InEK) data portal [31], an official open data source providing information on the costs of all DRGs in Germany.
For out-patient procedures, we derived the mean cost of a visit for treating wound infections and seroma from the current medical fee schedule [32]. We obtained the estimates on the annual direct and indirect costs of treating lymphedema including productivity loss, travel costs and out-of-pocket payments from an existing micro-costing study in Germany [22]. In this micro-costing study, the authors had used the human capital approach to value the productivity loss due to lymphedema. Table 3 reports the average costs of all procedures and treatments used in our analyses.
Finally, we combined data on utilisation reported in Table 2 with the information on average costs reported in Table 3 to value the costs associated with SLND and cALND.
2.4 Outcome Estimation
We adopted quality-adjusted-life-year (QALY) as health outcome measure. A QALY combines in a single index measure length of life and quality of life [33]. Given that the survival outcomes are almost equivalent, our calculation of QALYs considered only differing morbidities between the two groups. Specifically, QALYs are calculated by multiplying the duration of time spent in a health state by the health-related quality of life weight (HRQoLW) (i.e., utility score) associated with that health state [34]. Health-related quality of life weight of the breast cancer free state (0.87) was obtained from an existing study, which used the EQ-5D-3L questionnaire and elicited quality of life for different breast cancer states from 840 patients in Sweden [35]. We derived the disutility weight due to lymphedema from an existing study in the USA, which applied the Functional Assessment of Cancer Therapy-Breast (FACT-B) instrument to assess the quality-of-life change due to secondary lymphedema among women who were affected by early breast cancer. This study reported that compared to women who did not experience lymphedema, the quality of life of women who experienced lymphedema decreased on average 13.6 (122.7–109.1) points out of a total of 144 possible points using the FACT-B instrument. Accordingly, we estimated the disutility weight of lymphedema to be − 0.094 (− 13.6/144) [21]. Since we found no data, we assumed the disutility weight of infection and seroma to be − 0.03 based on the minimum important difference in utility [36]. We report HRQoLWs used in the model and their data sources in Table 1.
2.5 Statistical Analyses
First, we conducted deterministic analyses, which are the point-estimate analyses using the mean estimates of all model parameters reported in Tables 1, 2 and 3. Specifically, we calculated the average cost incurred and QALY accrued per patient for each of the three scenarios (the baseline, the actual implementation and the full implementation). We then compared both the actual implementation scenario and the full implementation scenario to the baseline scenario by estimating the incremental costs saved and the incremental QALYs gained per patient under each corresponding scenario. The comparison of the actual implementation scenario to the baseline scenario assessed the actual savings and health gain from the observed uptake of the Z0011 recommendations in routine care, while the comparison of the full implementation scenario to the baseline scenario assessed the potential savings and health benefits had the Z0011 recommendations been implemented fully.
We performed the analysis for each year and applied the corresponding yearly estimates on the cALND utilisation rate and the unit cost of the related procedures. Given that the average inflation rate from 2010 and 2015 in Germany was almost zero [37], we did not adjust costs incurred in different years to the base year before we calculated the cumulative economic gain. Also, since our analysis adopted a two-year time horizon, we did not discount costs saved nor health benefits accrued in the second year.
Second, we extrapolated our model findings, applying the proportion of Z0011-eligible patients estimated from the dataset (7.3%) on the national breast cancer cases to estimate the cumulative cost savings and QALYs gained in Germany [38].
Third, in the analysis reflecting the perspective of statutory insured patients, we valued a QALY at EUR19,890, based on the concept of value of statistical life year and the marginal production of health care spending [39]. In the analysis reflecting the society perspective, we valued a QALY at EUR40,000, based on the mean estimate reported in empirical work using the well-being valuation approach [40]. Accordingly, the monetary value of health benefits was estimated by multiplying the number of QALYs gained by the value of a QALY (EUR19,890 for the perspective of statutory insured patients and 40,000 for the societal perspective).
Fourth, given the uncertainty in the estimated value of a QALY, we calculated the cumulative economic gain for both study perspectives for four different QALY values (EUR17,562 and EUR19,890, which were the minimum value and the mean value of a QALY reported in [39], EUR40,000 and EUR60,000, which were the mean value and the maximum value of a QALY reported in [40]). Since applying different values for a QALY inevitably changes the total economic gain for all three scenarios in a similar manner, we only applied the approach for the full implementation scenario.
Finally, we conducted one-way sensitivity analyses (SA) by varying one model parameter at a time to examine the impact of each parameter on the base results [27]. In addition, we examined the joint uncertainty of all model parameters using probabilistic sensitivity analyses (PSA) relying on a Monte Carlo simulation with 5,000 iterations. In each iteration, model parameters were sampled from the assigned mathematical distribution (gamma for cost parameters and beta for epidemiological parameters) within the range specified in Table 2 and the arbitrary range of ± 20% for one model estimate (average cost of an out-patient care visit of infection and seroma) reported in Table 3. We presented the one-way SA results on a Tornado diagram, and the PSA results on a cost-effectiveness scatter plot graph.
2.6 Model Validation and Reporting
Following the guidance on the modelling good research practice [41], we checked the face validity and internal validity of the model by inspecting whether all parameters influenced the model according to the expectations. We reported our analysis following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [42].
3 Results
3.1 Deterministic/Point-Estimate Analysis Results
Tables 4 and 5 report the deterministic analysis results from the perspective of statutory insured patients. Specifically, Table 4 shows costs saved and QALYs gained per patient from 2011 to 2015 under the actual implementation scenario and the full implementation scenario compared with the baseline (clinical practice before Z0011 trial). In 2015, the baseline scenario incurred an average cost of EUR8,130 and accrued 0.8229 QALY per patient, while the corresponding estimates were EUR4,960 and 0.8561 QALY under the full implementation, and EUR6,537 and 0.8396 QALY under the actual implementation. Relative to the baseline scenario, the full implementation scenario would have saved EUR3,170 per patient and the actual implementation scenario saved EUR1,593 per patient in 2015. Concurrently, in comparison with the baseline scenario, the actual implementation and the full implementation gained 0.0167 QALY and 0.0332 QALY per patient, respectively, in 2015 (Table 4). Under the actual implementation, the costs saved and QALYs gained vary by year dependent on the use of cALND.
In Table 5, we report the population-level costs saved and QALYs gained from the perspective of statutory insured patients. Relative to the baseline scenario, the actual implementation scenario resulted in a cost saving of EUR8,224,511 and a gain of 86.20 QALY in 2015 alone, while the corresponding estimates under the full implementation scenario were EUR16,362,307 and 171.37 QALYs. During the entire study period from 2011 to 2015, in relation to the baseline, the actual implementation scenario saved totalled EUR32,029,495 and 335.25 QALYs, equating a total economic gain of EUR38,697,543. The full implementation scenario would have saved a total of EUR82,943,129 and 868.68 QALY, equating a total economic gain of EUR100,221,068.
From the societal perspective, which also includes non-medical costs (e.g., productivity loss, travel costs), our model estimated that the baseline scenario entailed a higher average cost of EUR8,814 and accrued the same QALY (0.8229) per patient as reported for the perspective of statutory insured patients in Table 4. Our model estimated that the total gain for the society from 2011 and 2015 was EUR50,334,756 under the actual implementation scenario; it would have been EUR130,367,237 under the full implementation scenario. Details of the results from the societal perspective are reported in Tables 6 and 7.
As visualised in Fig. 2, applying different values for a QALY changes the total economic gain accordingly. Specifically, when a QALY is valued at the minimum (EUR17,562), the gain under the full implementation scenario decreases to EUR98,199,048 for statutory insured patients and to 110,876,003 for the society. In contrast, when a QALY is valued to the maximum (EUR60,000), the gain increases to EUR135,063,859 for statutory insured patients and 147,740,814 for the society.
3.2 Sensitivity Analysis (SA) Results
The one-way SA results revealed that duration of lymphedema, cost of cALND performed in the same procedure with BCS and SLND and probability of lymphedema following cALND procedure were the three most influential parameters shaping cost savings. For the health gain, the model parameters related to lymphedema including disutility of having lymphedema, the probability of developing secondary lymphedema following cALND, and this probability following SLND were the three most influential. Details of one-way SA results are presented in the Supplementary file
For PSA results, almost all 5,000 pairs of the incremental costs and effects lay in the southeast quadrant of the cost-effectiveness plane (Figs 3, 4), indicating that both the actual implementation and the full implementation scenarios have lower costs and more QALYs relative to the baseline scenario.
4 Discussion
To our knowledge, our study is the first attempt worldwide to determine both the cost savings and health improvement of omitting cALND among Z0011-eligible patients, using data from a representative and high-quality database in Germany [13]. Our analysis indicated that the declining use of cALND (from 94.3% in 2010 to 46.9% in 2015) saved a total of about EUR38,697,543 (EUR1479 per patient) for the statutory insured patients and about EUR50,334,756 (EUR1,924 per patient) for the society between 2011 and 2015. Such savings were equivalent to approximately 4.5–5.8% of the average healthcare costs attributed to breast cancer care (EUR33,237 per patient) in Germany [43].
Similar patterns in cost reduction and health improvement were observed for other invasive surgical procedures, for example, the watch-and-wait approach in the management of rectal cancer [44, 45] or minimally invasive versus open surgery techniques in the treatment of degenerative lumbar spinal conditions [46]. Similarly, a recent cost‐effectiveness study demonstrated that observation was superior to sentinel lymph node biopsy for postmenopausal breast cancer women with negative axillary ultrasound [47].
Given the current context of the rampaging COVID-19 pandemic [48] and the widespread consequences of climate change on health [49], policy makers and health care managers are pushed into a serious situation to make rational use of scarce resources. Our study contributes important evidence for evidence-based policy making, which potentially promotes the efficient use of healthcare resources. Specifically, our study showed that omitting cALND among Z0011-eligible patients produced both substantial cost savings and health gain. This finding is novel and important to promote the further uptake of the Z0011 recommendations into routine clinical care. Having the full knowledge of all relevant benefits and harms of cALND for Z0011-eligible patients may facilitate the surgeons and patients to make rational decisions on their treatment options and opt for not doing cALND as recommended by the Z0011 trial. Furthermore, our estimates on the benefits of implementing the Z0011 recommendations into clinical practice establish powerful evidence on the population-level impact of translating the results of practice-changing trials like Z0011 into clinical practice, thus highlighting the importance of conducting and providing grants for such trials.
In addition, we found that the hypothetical full implementation scenario produced a much larger gain (more than two-fold) compared to the actual implementation scenario. This finding is of particular importance and suggests that it is beneficial to introduce additional policy measures to promote the further uptake of the Z0011 recommendations in routine care such as the Choosing Wisely Campaign to de-implement the low-value breast cancer surgery in the USA [50].
Our findings should be appraised against several methodological considerations. First, our model has not included all the possible morbidities following cALND. Since we only focused on the morbidities for which existing data are of adequate quality (e.g., wound infections, seroma and secondary lymphedema), we might underestimate the true gain of omitting cALND by ignoring other secondary morbidities such as restricted motion and paraesthesia [18]. Second, our cost assessment is based on the costs of statutory insured patients, who account for 87.7% of the population in Germany [51]. Nonetheless, since costs for patients insured privately may be higher than those set in the public system, we need to acknowledge that real gains could be even higher than the gains detected in our study. Third, we derived the HRQoLW for the cancer-free state and the disutility weight of lymphedema from existing studies conducted in other countries [21, 35]. Therefore, these weights might not reflect the true utilities for the German population. Still, since these weights were applied equally for both groups (cALND and SLND), we expect the uncertainty of these weights not to affect our core results on the incremental gain of one intervention compared to the other. Lastly, our economic analysis specifically illustrated the impact of the Z0011 trial results on the costs and population health in Germany. Although we analysed national data, we assume on the one hand that the results are principally transferable to other developed countries with similar health care systems. On the other hand, the exact numbers cannot be directly applied to other settings, given that our model inputs are based on specific data from Germany.
5 Conclusion and Recommendation
The actual implementation of the Z0011 recommendations into routine practice resulted in substantial cost savings and concurrently improved patient health at the population level in Germany. Our findings suggest that the introduction of additional policy measures to promote the further uptake of the Z0011 recommendations in routine care (e.g., updating treatment guideline or conducting information events to show the full benefits of omitting cALND among Z0011-eligible patients) will produce a much larger gain to both statutory insured patients and the society in Germany and similar context.
References
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–33.
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75.
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318(10):918–26.
Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M, Hunt KK. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264(3):413–20.
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305.
Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJH, Mansel RE, Cataliotti L, Westenberg AH, Klinkenbijl JHG, Orzalesi L, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–10.
Sávolt Á, Péley G, Polgár C, Udvarhelyi N, Rubovszky G, Kovács E, Győrffy B, Kásler M, Mátrai Z. Eight-year follow up result of the OTOASOR trial: the Optimal Treatment Of the Axilla—Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: a randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol (EJSO). 2017;43(4):672–9.
Huang T-W, Kuo KN, Chen K-H, Chen C, Hou W-H, Lee W-H, Chao T-Y, Tsai J-T, Su C-M, Huang M-T, et al. Recommendation for axillary lymph node dissection in women with early breast cancer and sentinel node metastasis: a systematic review and meta-analysis of randomized controlled trials using the GRADE system. Int J Surg. 2016;34:73–80.
Ong CT, Thomas SM, Blitzblau RC, Fayanju OM, Park TS, Plichta JK, Rosenberger LH, Hyslop T, Shelley Hwang E, Greenup RA. Patient age and tumor subtype predict the extent of axillary surgery among breast cancer patients eligible for the American College of Surgeons Oncology Group Trial Z0011. Ann Surg Oncol. 2017;24(12):3559–66.
Morigi C, Peradze N, Galimberti V, Leonardi MC, Radice D, Santomauro GI, Bagnardi V, Intra M, Firpo E, Veronesi P. Feasibility and surgical impact of Z0011 trial criteria in a single-Institution practice. Breast J. 2020;26(7):1330–6.
Malik AA, Urooj N, Shamim R, Salim M, Bano R, Chaudhry Z, Khan HM, Khan AI. Managing the axilla in early breast cancer. Impact of ACOSOG Z0011 trial in changing practices in a low middle income country. Asian Pac J Cancer Prev APJCP. 2017;18(8):2079–82.
Ngui NK, Hitos K, Hughes TMD. Effect of the American College of Surgeons Oncology Group Z0011 trial on axillary management in breast cancer patients in the Australian setting. Breast J. 2019;25(5):853–8.
Hennigs A, Köpke M, Feißt M, Riedel F, Rezai M, Nitz U, Moderow M, Golatta M, Sohn C, Schneeweiss A, et al. Which patients with sentinel node-positive breast cancer after breast conservation still receive completion axillary lymph node dissection in routine clinical practice? Breast Cancer Res Treat. 2019;173(2):429–38.
Wang T, Baskin AS, Dossett LA. Deimplementation of the choosing wisely recommendations for low-value breast cancer surgery: a systematic review. JAMA Surg. 2020;155(8):759–70.
Joyce DP, Lowery AJ, McGrath-Soo LB, Downey E, Kelly L, O’Donoghue GT, Barry M, Hill ADK. Management of the axilla: has Z0011 had an impact? Ir J Med Sci (1971-). 2016;185(1):145–9.
Classe JM, Baffert S, Sigal-Zafrani B, Fall M, Rousseau C, Alran S, Rouanet P, Belichard C, Mignotte H, Ferron G, et al. Cost comparison of axillary sentinel lymph node detection and axillary lymphadenectomy in early breast cancer. A national study based on a prospective multi-institutional series of 985 patients ‘on behalf of the Group of Surgeons from the French Unicancer Federation.’ Ann Oncol. 2012;23(5):1170–7.
DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–15.
Glechner A, Wöckel A, Gartlehner G, Thaler K, Strobelberger M, Griebler U, Kreienberg R. Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: a systematic review and meta-analysis. Eur J Cancer. 2013;49(4):812–25.
Rao R, Euhus D, Mayo HG, Balch C. Axillary node interventions in breast cancer: a systematic review. JAMA. 2013;310(13):1385–94.
Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, Kissin M, Mansel RE. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95(3):279–93.
Beaulac SM, McNair LA, Scott TE, LaMorte WW, Kavanah MT. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. 2002;137(11):1253–7.
Gutknecht M, Herberger K, Klose K, Purwins S, Dietz D, Blome C, Augustin M. Cost-of-illness of patients with lymphoedema. J Eur Acad Dermatol Venereol. 2017;31(11):1930–5.
Man V, Lo MS, Kwong A. The applicability of the ACOSOG Z0011 Criteria to breast cancer patients in Hong Kong. Chinese Clinical Oncology; Vol 10, No 3 (June 2021): Chinese Clinical Oncology 2021.
Breast cancer. https://www.who.int/news-room/fact-sheets/detail/breast-cancer. Accessed 1 June 2020
Camp MS, Greenup RA, Taghian A, Coopey SB, Specht M, Gadd M, Hughes K, Smith BL. Application of ACOSOG Z0011 criteria reduces perioperative costs. Ann Surg Oncol. 2013;20(3):836–41.
Siegel M, Busse R. Can people afford to pay for health care? New evidence on financial protection in Germany. Copenhagen: World Health Organization, Regional Office for Europe; 2018.
Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied methods of cost-effectiveness analysis in health care. Oxford: Oxford University Press; 2011.
Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. JNCI J Natl Cancer Inst. 2006;98(9):599–609.
Mogyorosy Z, Smith P. The main methodological issues in costing health care services: a literature review. York: Centre for Health Economics, University of York; 2005.
Empfehlungen gynäkologische Onkologie Kommission Mamma. https://www.ago-online.de/leitlinien-empfehlungen/leitlinien-empfehlungen/kommission-mamma. Accessed 15 Nov 2019
InEK Datenportal. https://www.g-drg.de/Datenbrowser_und_Begleitforschung/G-DRG-Report-Browser/aG-DRG-Report-Browser_2020. Accessed 15 Jan 2020
EBM online version. https://www.kbv.de/html/2460.php. Accessed 20 Feb 2020
Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006;21(5):402–8.
Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21.
Rautalin M, Färkkilä N, Sintonen H, Saarto T, Taari K, Jahkola T, Roine RP. Health-related quality of life in different states of breast cancer—comparing different instruments. Acta Oncol. 2018;57(5):622–8.
Nichol MB, Epstein JD. Separating gains and losses in health when calculating the minimum important difference for mapped utility measures. Qual Life Res. 2008;17(6):955.
Germany Inflation Rate 1960–2022. https://www.macrotrends.net/countries/DEU/germany/inflation-rate-cpi. Accessed 15 Sept 2020
German Centre for Cancer Registry Data RKI: Database Query with estimates for cancer incidence, prevalence and survival in Germany, based on data of the population based cancer registries. 2021.
Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–35.
Himmler S, Stöckel J, van Exel NJA, Brouwer WBF: The value of health - Empirical issues whenestimating the monetary value of a QALY based on well-being data. In: SOEP papers on multidisciplinary panel data research, no. 1101. Berlin: German Institute for Economic Research (DIW Berlin); 2020.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Mak. 2012;32(5):733–43.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–50.
Kreis K, Plöthner M, Schmidt T, Seufert R, Schreeb K, Jahndel V, Maas S, Kuhlmann A, Zeidler J, Schramm A. Healthcare costs associated with breast cancer in Germany: a claims data analysis. Eur J Health Econ. 2020;21(3):451–64.
Rao C, Sun Myint A, Athanasiou T, Faiz O, Martin AP, Collins B, Smith FM. Avoiding radical surgery in elderly patients with rectal cancer is cost-effective. Dis Colon Rectum. 2017;60(1):30–42.
Miller JA, Wang H, Chang DT, Pollom EL. Cost-effectiveness and quality-adjusted survival of watch and wait after complete response to chemoradiotherapy for rectal cancer. J Natl Cancer Inst. 2020;112(8):792–801.
Vertuani S, Nilsson J, Borgman B, Buseghin G, Leonard C, Assietti R, Quraishi NA. A cost-effectiveness analysis of minimally invasive versus open surgery techniques for lumbar spinal fusion in Italy and the United Kingdom. Value in Health. 2015;18(6):810–6.
McEvoy AM, Poplack S, Nickel K, Olsen MA, Ademuyiwa F, Zoberi I, Odom E, Yu J, Chang S-H, Gillanders WE. Cost-effectiveness analyses demonstrate that observation is superior to sentinel lymph node biopsy for postmenopausal women with HR + breast cancer and negative axillary ultrasound. Breast Cancer Res Treat. 2020;183(2):251–62.
Hiscott J, Alexandridi M, Muscolini M, Tassone E, Palermo E, Soultsioti M, Zevini A. The global impact of the coronavirus pandemic. Cytokine Growth Factor Rev. 2020;53:1–9.
World Health Organization (WHO): Climate change and health: key facts. 2021. https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health. Accessed 20 Jan 2022
Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801–2.
Federal Ministry of Health. The German healthcare system: Strong. Reliable. Proven. Publikationsversand der Bundesregierung, Berlin; 2020.
Acknowledgements
We would like to thank Dr Sascha Baller for the technical review of the DRG codes used in our analysis and Salvador Shabbir for the language editing assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding disclosures
This research received funding from the German Cancer Aid (grant No. 70112082).
Role of funder
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interests
There is no conflict of interest to declare.
Ethics approval
The study was approved by the ethics committee of Heidelberg University Hospital and was conducted in accordance with the Declaration of Helsinki. The study was deemed to be without risk, relying exclusively on anonymised routinely collected data. As such, the ethics committee did not request approval for consent for this designated analysis.
Consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and material
The data and materials related to this publication can be made available upon a written request to the corresponding author.
Code availability (software application or custom code)
The authors have provided the original model with the associated codes to the editor during the peer review process. The original model and the associated code will be provided upon a written request to the corresponding author.
Author contributions
Concept and design: HTN, MDA, JH, AH. Acquisition of data: Nguyen, De Allegri, Heil, AH. Analysis and interpretation of data: HTN, MDA, JH, AH. Drafting of the manuscript: HTN. Critical revision of the paper for important intellectual content: MDA, JH, AH. Statistical analysis: HTN, MDA. Obtaining funding: JH and AH. Administrative, technical, or logistic support: MDA, JH, AH. Supervision: MDA, JH, AH.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nguyen, H.T., De Allegri, M., Heil, J. et al. Population-Level Impact of Omitting Axillary Lymph Node Dissection in Early Breast Cancer Women: Evidence from an Economic Evaluation in Germany. Appl Health Econ Health Policy 21, 275–287 (2023). https://doi.org/10.1007/s40258-022-00771-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00771-8