Abstract
Background
Improved multiple sclerosis (MS) diagnosis and increased availability of intravenous disease-modifying treatments can lead to overburdening of infusion centres. This study was focused on developing a decision-support tool to help infusion centres plan their operations.
Methods
A discrete event simulation model (‘ENTIMOS’) was developed using Simul8 software in collaboration with clinical experts. Model inputs included treatment-specific clinical parameters, resources such as infusion chairs and nursing staff, and costs, while model outputs included patient throughput, waiting time, queue size, resource utilisation, and costs. The model was parameterised using characteristics of the Charing Cross Hospital Infusion Centre in London, UK, where 12 infusion chairs were deployed for 170 non-MS and 860 MS patients as of March 2021. The number of MS patients was projected to increase by seven new patients per week.
Results
The model-estimated waiting time for an infusion is, on average, 8 days beyond clinical recommendation in the first year of simulation. Without corrective action, the delay in receiving due treatment is anticipated to reach 30 days on average at 30 months from the start of simulation. Such system compromise can be prevented either by adding one infusion chair annually or switching 7% of existing patients or 24% of new patients to alternative MS treatments not requiring infusion.
Conclusion
ENTIMOS is a flexible model of patient flow and care delivery in infusion centres serving MS patients. It allows users to simulate specific local settings and therefore identify measures that are necessary to avoid clinically significant treatment delay resulting in suboptimal care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The operations of infusion centres that serve multiple conditions, including multiple sclerosis, can be simulated through a discrete event simulation (DES) model with reasonable apparent accuracy. |
A DES model that maximises the efficiency of an infusion suite can help inform decision making about healthcare resource allocation. |
1 Introduction
Multiple sclerosis (MS) is a chronic autoimmune disorder of the central nervous system, affecting an estimated 2.2 million people worldwide [1, 2]. The prevalence of MS is steadily rising as diseased patients live longer. In the UK alone, the prevalence of MS increased by 29% between 1990 and 2016, reaching 106,454 patients, with 130 new cases diagnosed each week [3, 4]. Although MS is currently incurable, several disease-modifying therapies (DMTs) exist for relapsing forms of MS (RMS) [5].
Currently approved MS DMTs include immunomodulatory and anti-inflammatory drugs [6] administered orally as subcutaneous injectables or as intravenous infusions over multiple cycles [7]. DMTs can be categorised depending on their degree of efficacy in preventing relapses; however, identifying DMTs for treatment is based on medical or financing guidelines and the patient’s perception of risks [8]. Currently, the treatments recognised to have the highest impact on relapse activity include alemtuzumab, natalizumab, and ocrelizumab. All these treatments are administered as infusions in specialist infusion centres under clinical supervision, as per their summary of product characteristics (SmPC).
Infusion centres serve patients with neurological diseases and other conditions (e.g., oncological and rheumatological) with finite resources, putting them at risk of being overburdened. Literature indicates that infusion centres commonly experience long waiting times: the time from treatment decision to administration, and waiting times from patient arrival until start of infusion [9]. This can lead to suboptimal clinical outcomes and patient dissatisfaction [9, 10]. Delays in treatment administration warrant more effective short-term resource utilisation.
The waiting time and queue size in infusion centres are influenced by capacity constraints, such as the number of available infusion chairs and available staff, scheduling techniques, time taken from patient’s arrival to infusion start, last-minute additions or appointment cancellations, and time taken to manage infusion-related adverse events [9, 11]. Other factors that impact waiting time and queue size include patient resistance to treatment decision and insurance-related issues [12,13,14]. Finally, external unexpected circumstances, such as the coronavirus disease 2019 (COVID-19) pandemic, place centres under further strain. This impacts several constraints related to scheduling, chair capacity, implementation of additional COVID-related screening, additional cleaning and disinfection activities between infusions, and extended working hours [15].
Infusion-related capacity constraints are well-studied in other therapeutic areas, including oncology, but are not well-characterised in MS [16, 17]. Adopting appropriate resource modelling techniques may allow for improved capacity constraint management. Thokala et al. defined resource modelling as the quantitative assessment of technology diffusion curves, their related resource requirements, and their capacity constraints [11]. The authors prescribe studies utilising discrete event simulation (DES) modelling techniques, which provide estimates of resource use and availability over time of a given service setting [11, 18]. Given the complexity of real-life clinical settings, it is crucial to develop the most parsimonious model, which would simultaneously allow for simplicity, relevance, and high predictive power.
This paper describes a model called ENTIMOS (meaning ‘genuine’, ‘honest’ in Greek) developed using Simul8®Footnote 1 DES software (Simul8 Corporation) to maximise the efficiency of an infusion suite based on the most appropriate allocation of system resources. In particular, the model simulates the time and resource utilisation associated with three intravenously administered DMTs for MS, viz. ocrelizumab, natalizumab, and alemtuzumab [19,20,21], and patient waiting time associated with their administration. The infusion pathway and input parameters can be adjusted in the model to reflect the situation in a particular infusion centre, and a case study is presented in this study using data from Charing Cross Hospital in London, UK. We discuss the benefits of using this model for patients, healthcare providers and payers, reflecting also on its generalisability, adaptability and limitations.
2 Materials and Methods
2.1 Conceptual Modelling
Qualitative information regarding MS infusion pathways was collected from infusion centres serving MS patients across the US and UK. Centres were identified as part of a parallel prospective study (unpublished) focusing on understanding the details of the infusion process in various countries. In the US, 13 centres were contacted by email, of which six responded, while in the UK, three centres were contacted, of which two responded. A larger number of centres were needed in the US to identify variations in process flows, whereas the two UK centres are nationally representative of large infusion centres according to the clinicians interviewed. Site administrators, nurses and pharmacists at the selected centres were requested to complete three separate questionnaires between April and July 2020. Different aspects investigated for each respondent type are detailed in Table 1. Information from the sites was summarised as a single common model process flow, whose clinical accuracy was validated by a clinical neurologist in the UK.
This process flow was used as the conceptual modelling framework for ENTIMOS. Given the degree of variation across infusion centres, the model combines common elements across the process flows with both conditional statements (i.e., if-else/case-type constructs), and user customisation options. The case study in this paper uses information on site processes provided by Charing Cross Hospital.
The ENTIMOS model was implemented in Simul8, a proprietary DES software [22].
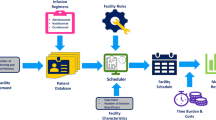
2.2 Process Flow
Figure 1 depicts the process flow of the model, while the model interface and visual settings are presented in Fig. 2 (electronic supplementary material [ESM] 4 includes the study figures). In the model, as MS DMT is prescribed, providing pharmacies must approve treatment reimbursement before scheduling the first infusion appointment. The time to hospital pharmacy approval is assumed to vary between 4 and 20 weeks, as per the hospital pharmacist information. Once the patient’s appointment is scheduled, laboratory tests must be performed to rule out contraindications for treatment (e.g., active hepatitis B virus infection), followed by vital signs checks and patient screening as well as optional oral pretreatment. Users can decide whether these happen ahead of time outside of the clinic (default) versus in a waiting room or in an infusion chair. On the day of the infusion, patients check in to the clinic and receive pretreatment with intravenous anti-inflammatory drugs. Thereafter, MS DMT is administered as per their SmPC or the centre’s standard operating procedures (SOPs). Handling of any infusion-related reactions (IRRs) is determined based on type and severity, and includes stop, slowdown infusion, or slowdown with symptomatic treatment. Following infusion, a patient is monitored over a prescribed period, which varies across DMTs as per the centre’s SOP. Default durations of each of these processes are listed in ESM Table 1. These values were based on responses provided in the qualitative evaluation, by a clinical neurologist, or subsequently from literature, unless otherwise indicated. Patient resistance to treatment and insurance-related issues are currently not considered in this model.
Current user interface. Input settings include Centre settings (chair and bed capacity, treatment pathway, number of patients, scheduling, did not attend rate, number of staff, staffing costs) and Treatment settings (posology, IRR rates, medication and IRR costs, payer approval time). IRR infusion-related reaction, IV intravenous
2.3 Input Parameters
2.3.1 Patient Mix
Model inputs related to the patient mix include the number of MS patients and their distribution across the three most used DMTs in the UK (ocrelizumab, natalizumab, alemtuzumab), as well as the number of non-MS patients.
2.3.2 Treatment-Specific Parameters
Model inputs related to treatment include frequency of administration, infusion duration and frequency of IRRs. To populate the model, information on the posology of each of the MS DMTs was obtained from the respective SmPCs (Table 2). Additionally, information on the minimum and maximum duration of the infusion was calculated from the recommended administration rates and the dose. For non-MS DMTs, a generalised infusion duration for the whole non-MS DMT treatment class was created, based on the duration of infusion for the three most used non-MS infusions in the UK (intravenous immunoglobulin, infliximab, and rituximab) and their respective SmPCs. The treatment-specific assumed frequency of IRRs was based on the SmPC, literature (including results of clinical trials for the DMTs) or on a clinical insight, if there were no data available. These assumptions are detailed in ESM Table 2. First-infusion IRR rate is assumed to be higher than succeeding infusions, except for natalizumab.
The timing of infusion appointment was scheduled using triangular distribution, while the period between appointments was fixed as per the drug posology. The model is executed on a first-come, first-served scheduling; however, prioritisation was applied for alemtuzumab patients who needed 5 consecutive days of treatment, i.e., if they started the treatment, they would be scheduled with priority the next day.
For each non-MS patient, the expected number of visits per year were sampled and time to next appointment was estimated by assuming that the time between these treatment visits remained constant.
2.3.3 Healthcare Resources and Costs
Resources included in the model are the number of infusion chairs operating during a defined number of hours per day in the infusion centre, and nursing staff. Infusion chairs are modelled as a constraint in the model. Daily centre opening times can be user-specified.
Staff resource parameters include the number of staff nurses available and the time they spend on pretreatment, infusion, IRR handling, recovery and monitoring, and the percentage of time spent on activities not directly relating to patient care. Rather than using staff nurses as a resource constraint, a total pool of nurse hours (e.g., per week) spent on patient care was calculated. This was deemed reasonable based on clinical expert advice and to ensure balance between model usability/simplicity and complexity/realism. Following depletion of the total pool of staff nurse hours available based on the number of nurses employed by the centre, it was assumed that all remaining activity was taken over by agency nurses charging a different rate from staff nurses. Time spent by pharmacists and neurologists was omitted in the model owing to the largely limited amount of time they spend in the infusion process, as compared with nurses. Other than labour, costs in the model include medication (DMT, premedication, medication used for IRR resolution) and supply costs [23]. Cost inputs are listed in ESM Table 3. The costs were according to the 2019/2020 National Tariff, which included both providers’ and National Health Services’ costs.
2.3.4 Scheduling Considerations
In case of patients who did not attend their scheduled appointment, it is assumed that the infusion chair is blocked for the duration of the originally scheduled appointment and cannot be used by another patient. This was deemed reasonable by the clinical expert as it is not feasible to schedule another patient at short notice.
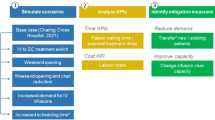
2.4 Model Outputs and Scenario Simulation
The outputs of ENTIMOS include six key performance indicators (KPIs) that reflect the efficiency of an infusion centre’s operations across different domains: patient throughput, patient waiting time, patient queue size, staff utilisation, chair utilisation, costs (Table 3).
2.4.1 Waiting Time and System Compromise
System compromise is defined as patients facing an average delay of ≥ 30 days before receiving their next due infusion (as per approved use in SmPC) due to increasing waiting times for appointments in an infusion centre. It was selected as the focal metric due to its clinical relevance and potentially direct detrimental impact on MS progression [9, 10]. A centre can still operate under system compromise; however, the prolonged treatment delays put patients at risk. Waiting time indicates the extent to which a centre can avoid severe implications of missed or delayed patient treatment. This metric also helps centres understand how to mitigate treatment delays.
2.4.2 Corrective Action to Reach System Equilibrium
System equilibrium is defined as a situation where the average waiting time for the next due infusion does not exceed 30 days, evaluated monthly. Annual marginal corrective actions are defined as those that are required to prevent system compromise, resulting in a system equilibrium over the entire simulation horizon. The annual cycle for corrective actions reflects yearly planning cycles at Charing Cross Hospital. Besides these automatically evaluated corrective actions, the model provides flexibility to define various scenarios that can be evaluated on a granular, monthly level through manual model runs.
The model allows for simulation of effects of three types of corrective action to reduce waiting times: (1) adding new infusion chairs, and (2) switching out new patients or (3) existing patients, by referring them to other clinics or offering them an alternative treatment not requiring infusion. New patients switched out are assumed not to enter the infusion suite. Existing patients who are switched out are removed from the system gradually over the duration of the entire year, proportionately to their distribution at day 1 (i.e., of each 100 patients who were shifted out, 64 were ocrelizumab patients and 36 were natalizumab patients). Since alemtuzumab was not used at Charing Cross Hospital at the time of the study, it is not included here.
The marginal annual corrective action is automatically determined by the model through a binary search algorithm for patients switching, and stepwise increase for infusion chairs by an increment of one chair. User interface for manual testing of different corrective actions and automated search of marginal corrective actions is presented in Fig. 3.
2.4.3 Simulation Horizon
The current version of the model supports simulations up to 5 years. In this analysis, a 3-year horizon was chosen to reflect a typical planning horizon at an infusion centre. To build a realistic queue of patients being already in care at time 0, base-case simulations were run over a 24-week warm-up period before collecting the results. Twenty-four weeks was selected as the minimum warm-up period time, allowing all patients to show up in the system at least once before results are collected and removing seasonality effect (all patients with the same treatment being scheduled on the same day). Different durations of the warm-up period were tested, and warm-up periods longer than 24 weeks prolonged the computation time without changing the results substantially.
2.5 Model Verification and Validation
The verification and validation of ENTIMOS was performed in multiple steps. The process flow used by the model was validated for clinical accuracy and level of detail by two experts (Dr. Richard Nicholas and Dr. Praveen Thokala) via videoconferencing. To reduce the possibility of bias, the model code was verified by Dr. Thokala, an independent health economist who did not participate in its programming. Definitions of system compromise and model outputs were then validated by Dr. Nicholas, a clinical expert involved in the process.
3 Results
For demonstration, the model was parameterised with input parameters collected at the Charing Cross Hospital in March 2021. The Simul8 trial calculator recommended a trial of five simulation runs to obtain a point estimate and 95% confidence interval for the result metrics (Table 4). At that point of time, the infusion unit had 12 infusion chairs and operated 12 h a day, 5 days a week. The infusion appointments were booked starting at 8:00 am, and the longest possible time for a given infusion was blocked to prevent unnecessary in-clinic waiting for patients. Additionally, only three natalizumab slots could be booked/chair/day. The did not attend (DNA) rate was 2%. The infusion centre employed 10 staff nurses and served 860 MS patients (552 ocrelizumab, 308 natalizumab), as well as 170 non-MS patients. The process flow for the administration of non-MS treatments was sufficiently similar to that of MS DMTs and was handled in the model using the same pathway. Based on the advice from a clinical expert (also co-author of this study), the future demand increase was assumed to be seven new MS patients per week, out of whom six (87%) were expected to receive ocrelizumab and one (13%) was expected to receive natalizumab. The number of non-MS patients was assumed to be constant.
3.1 Waiting Time and System Compromise
In a base-case scenario with the input parameters described above, the total number of MS patients in care was estimated to be 1952 (860 existing and 1092 new) and the cumulative number of intravenous administrations was estimated to be 18,677 over a 3-year simulation horizon (Table 4). Average monthly waiting times are presented in Fig. 4. The waiting time for an appointment (MS and non-MS) was estimated to be, on average, 8 days beyond clinical recommendation in year 1, 19 days in year 2, and 31 days in year 3 of the simulation (results not presented). The infusion centre ran at capacity from the beginning of the simulation and was predicted to reach system compromise (where delay in receiving due treatment is 30 days or longer) within 30 months.
3.2 Corrective Actions to Prevent System Compromise
Marginal corrective actions identified by the model as able to maintain system equilibrium over 3 years, as well as selected KPIs for these scenarios, are presented in Table 4. One additional infusion chair per year was able to accommodate the anticipated demand without the need to shift any patient out of the centre during the simulation period. Alternatively, 7% of existing patients (total of 363, resulting in 1589 patients in care after 3 years of simulation) or 24% of new patients (total of 262, resulting in 1690 patients in care after 3 years of simulation) could be shifted out of the centre annually by referring them to other clinics or switching them to alternative non-intravenous treatments, maintaining system equilibrium.
Adding one additional chair per year increased the number of intravenous administrations to 22,020 (+18% from the base-case). In the switching scenarios, the number of intravenous administrations remained at the same level as in the base-case (±1%). The reported minimal differences (29 fewer infusions compared with base-case when switching out 7% of existing patients, compared with an additional 107 when switching out 24% of new patients) are due to the proportion of patients expected to receive ocrelizumab, which is higher among new patients (six of seven MS patients = approximately 86%) compared with existing patients (552 of 860 MS patients = approximately 64%). This assumption was based on the treatment undergone by patients at the Charring Cross Hospital during the study. The number of patients in the waiting queue and their breakdown by treatment type is illustrated on Fig. 5, showing that the proportion of ocrelizumab patients in the waiting line is also increasing with time. This is because ocrelizumab requires longer infusions than natalizumab, leading to these patients potentially waiting longer.
Queue size over the simulation period: number of patients waiting for infusion, broken down by treatment, with and without corrective action. a Queue under the base-case scenario without intervention. b–d Queue under scenarios where marginal corrective actions needed to assure system equilibrium were automatically defined by the model. MS multiple sclerosis
While queue size gradually increased over time in all scenarios (Fig. 5), adding one additional chair per year resulted in the shortest patient queue compared with other considered scenarios (Table 4). However, this led to an increase in the number of nurse hours needed by + 16.41%, corresponding to a + 16.39% increase in labour costs compared with the base-case scenario (from £981,155 to £1,142,009). The increase in labour costs is driven by the need to contract additional nurse time from a nurse bank (+ 41.92% in the agency nurse cost compared with the base-case scenario). Conversely, switching patients out of the infusion centre maintained the number of monthly nurse hours needed and associated costs at a similar level compared with the base-case scenario. Complete cost results are included in ESM 4.
Mean monthly chair utilisation was consistently high (> 90% of chair hours) across all scenarios due to the above-mentioned fact that in all scenarios, the infusion centre operated at capacity. The unused 10% of chair-hours capacity indicates that efficiencies can be achieved at the infusion centre, for example through improving the scheduling methodology.
4 Discussion
This study represents the first DES model estimating the impact of healthcare system constraints on the infusion treatment pathway for MS. Simulation of resource constraints serves as an insightful tool in healthcare systems [18, 24,25,26,27,28,29,30], but to our knowledge have not been used in MS. There is a rise in demand for infusions that puts pressure on treatment centres [11, 14]. In this study, we demonstrate how ENTIMOS, a simulation model, could be used to forecast impending changes and optimise resources to benefit infusion centre administrators, and ultimately the patients. By using a study case, the aim of this paper was to showcase both the functionality of our DES model and its adaptability to other settings.
For centre administrators, a simulation model can provide information needed to anticipate and proactively manage resource constraints and anticipate crises. A modelling approach may also assist patients in receiving more timely treatment with potential for better outcomes and improved experience. This is especially relevant for infusion centres where resources are shared with patients from other therapeutic areas (e.g., oncology, rheumatoid arthritis). Infusion centre chair resources were significantly depleted during peaks of the COVID-19 pandemic, causing prolonged waiting times and detrimental impacts on patient outcomes.
This DES model has three limitations. The estimation of staff resources and associated costs over simulated time horizons were not modelled as a constraint. Instead, it was assumed that agency nurses would be able to undertake any excess activity once staff nurse resources were depleted. This was based on the experience at Charing Cross where no infusion appointment was cancelled due to nurse unavailability at the time the model was developed. However, a new version of the model is currently under development with nurse constraint as an optional function to increase applicability of the model to other infusion centres where staff availability might be the factor responsible for prolonged waiting time.
The current model assumptions do not allow the estimation of pharmacist and neurologist time and costs. While these may be relatively less significant compared with nurse costs, they may have some impact on marginal resource changes over a long period. Finally, in this chronic disease setting, all patients are assumed to remain in the system. Although the model contains a functionality to specify the percentage of patients outflowing each year (e.g. due to death), this option was disabled in the current simulation. The rationale behind this decision was the consideration that the impact of patient outflow on treatment demand and patient waiting times would be insignificant over a 3- to 5-year horizon in this chronic disease setting. Therapy discontinuation is not anticipated to occur with sufficient frequency to have a substantive impact on results. Switching patients to treatments not requiring infusions is specifically captured through scenario analysis where users can explore consequences of switching new and existing patients. Switching across different intravenous DMTs is not modelled explicitly, but changes in treatment patterns can be reflected in the model by specifying the mix of newly diagnosed patients in terms of treatments they receive.
The ENTIMOS model was designed to inform strategic decisions for long-term planning and is currently not suitable for short-term (i.e., weekly or monthly) scheduling decisions. To model these scheduling decisions, exact data on the types of patients and the staff skill mix and rotations would be needed [31, 32]. Long-term simulation predictions are not precise estimates because patient numbers may be different to the parameterised inputs. ENTIMOS can forecast the outputs for strategic planning for any specific local setting.
The design choices in the process flow were based on data collected from a sample of eight diverse international sites in the UK and US that are assumed to be representative of all types of intravenous infusion sites. The model has the flexibility to represent different infusion centres by amending the infusion pathways and input parameters based on the variability in pathways and settings observed in these eight sites. Nevertheless, it is possible that unique patient flows of some infusion centres cannot be adequately represented using ENTIMOS. The modelling approach offers flexibility in being able to estimate the potential impact of different scenarios, including unanticipated events such as COVID-19, and other system changes resulting in the process flow being different from the one currently used in this DES model.
5 Conclusions
Utilising a resource planning tool allows long-term capacity planning with an outlook of increased productivity, quality of service, and efficiency. A decision analytics approach using a model such as ENTIMOS could be utilised to support planning decisions at Charing Cross Hospital and other infusion clinics on an annual basis. The model described here can be adapted for use in different settings by customising the process flow and input parameters related to the number and mix of patients, treatments and centre resources. Infusion suites could benefit from tools outlining how to make the most efficient use of resources, especially now when constrained healthcare systems strive to recover from the COVID-19 pandemic. This type of approach puts patients’ interests at the centre of resource allocation decisions and supports clinics by identifying the necessary corrective measures to assure continuity of care.
Notes
Simul8® is a registered trademark of Simul8 Corporation.
References
Gelfand JM. Chapter 12—multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation. In: Goodin DS, editor. Handbook of clinical neurology, vol. 122. Amsterdam: Elsevier; 2014. p. 269–90.
Stenager E. A global perspective on the burden of multiple sclerosis. Lancet Neurol. 2019;18(3):227–8. https://doi.org/10.1016/s1474-4422(18)30498-8.
MS Society. MS in the UK. Available at: https://www.mssociety.org.uk/sites/default/files/2020-08/MS-in-the-UK_2020.pdf.
Wallin MTCW, Nichols E, Bhutta Z, et al. Global, regional, and national burden of motor neuron diseases 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(12):1083–97. https://doi.org/10.1016/s1474-4422(18)30404-6.
Gold R, Wolinsky JS, Amato MP, Comi G. Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord. 2010;3(6):351–67. https://doi.org/10.1177/1756285610385608.
Berger JR. Functional improvement and symptom management in multiple sclerosis: clinical efficacy of current therapies. Am J Manag Care. 2011;17(Suppl 5 Improving):S146–53.
National MS Society. Disease-modifying therapies for MS. New York: National MS Society; 2020.
Holm M. MS treatment strategy: Hitting hard and early, or minimising risks through stepwise escalation? Brainwork; 2018. Available at: https://www.brainwork.md/ms-treatment-strategy-hitting-hard-and-early-or-minimising-risks-through-stepwise-escalation/.
Plourde CL, Varnado WT, Gleaton BJ, Das DG. Reducing infusion clinic wait times using quality improvement. JCO Oncol Pract. 2020;16(8):e807–13. https://doi.org/10.1200/JOP.19.00643.
Simacek KF, Ko JJ, Moreton D, Varga S, Johnson K, Katic BJ. The impact of disease-modifying therapy access barriers on people with multiple sclerosis: mixed-methods study. J Med Internet Res. 2018;20(10): e11168. https://doi.org/10.2196/11168.
Thokala P, Dixon S, Jahn B. Resource modelling: the missing piece of the HTA jigsaw? Pharmacoeconomics. 2015;33(3):193–203. https://doi.org/10.1007/s40273-014-0228-9.
Melao A. Oral DMTs still common 1st therapy for new ms patients but ocrevus having impact, market report says. Multiple Sclerosis News Today; 2018. Available at: https://multiplesclerosisnewstoday.com/2018/07/27/spherix-global-insights-new-ms-patient-audit-reveals-dtm-use-trends/.
Bindu DY. Time from ‘treatment decision’ to ‘actual start of DMT’ in MS – a local clinical survey. Available at: https://multiplesclerosisacademy.org/resources/delegate-projects/projects-medication/time-from-treatment-decision-to-actual-start-of-dmt-in-ms-a-local-clinical-survey/.
Multiple Sclerosis Trust. 20% rise in the estimated number of people living with MS in the UK. Multiple Sclerosis Trust; 2020. Available at: https://mstrust.org.uk/news/20-rise-estimated-number-people-living-ms-uk.
McPherson S. Infusion suites are adapting in response to COVID-19. WeInfuse News; 2020. Available at: https://weinfuse.com/infusion-suites-are-adapting-in-response-to-covid-19/.
De Cock E, Pivot X, Hauser N, Verma S, Kritikou P, Millar D, et al. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer. Cancer Med. 2016;5(3):389–97. https://doi.org/10.1002/cam4.573.
Sugalski JM, Kubal T, Mulkerin DL, Caires RL, Moore PJ, Fiorarancio Fahy R, et al. National comprehensive cancer network infusion efficiency workgroup study: optimizing patient flow in infusion centers. J Oncol Pract. 2019;15(5):e458–66. https://doi.org/10.1200/jop.18.00563.
Salleh S, Thokala P, Brennan A, Hughes R, Dixon S. Discrete event simulation-based resource modelling in health technology assessment. Pharmacoeconomics. 2017;35(10):989–1006. https://doi.org/10.1007/s40273-017-0533-1.
Biogen. Tysabri prescribing information 2020. Available at: https://www.tysabri.com/content/dam/commercial/tysabri/pat/en_us/pdf/tysabri_prescribing_information.pdf.
Genentech. Twice-yearly dosing of OCREVUS. 2020. Available at: https://www.ocrevus.com/hcp/dosing/administration.html.
Genzyme S. How lemtrada is given: sanofi genzyme. 2020. Available at: https://www.lemtrada.com/about/how-lemtrada-is-given.
Concannon K, Elder M, Hindle K, Tremble J, Tse S. Simulation modeling with SIMUL8 by Kieran Concannon, et al. Mississauga: Visual Thinking International; 2007.
Franken MG, Kanters TA, Coenen JL, de Jong P, Koene HR, Lugtenburg PJ, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs. 2018;29(8):791–801. https://doi.org/10.1097/cad.0000000000000648.
Liang B, Turkcan A, Ceyhan ME, Stuart K. Improvement of chemotherapy patient flow and scheduling in an outpatient oncology clinic. Int J Prod Res. 2015;53(24):7177–90. https://doi.org/10.1080/00207543.2014.988891.
Huggins A, Claudio D, Waliullah M. A detailed simulation model of an infusion treatment center. Proc Winter Simul Conf. 2014;2014:1198–209. https://doi.org/10.1109/WSC.2014.7019977.
Baril C, Gascon V, Miller J. Design of experiments and discrete-event simulation to study oncology nurse workload. IISE Trans Healthc Syst Eng. 2020;10(1):74–86. https://doi.org/10.1080/24725579.2019.1680581.
Alvarado MM, Cotton TG, Ntaimo L, Pérez E, Carpentier WR. Modeling and simulation of oncology clinic operations in discrete event system specification. SIMULATION. 2017;94(2):105–21. https://doi.org/10.1177/0037549717708246.
Bernatchou M, Ouzayd F, Bellabdaoui A, Hamdaoui M. Towards a simulation model of an outpatient chemotherapy unit. In: International Colloquium on Logistics and Supply chain Management. 2017. pp. 177–82.
Lamé G, Dixon-Woods M. Using clinical simulation to study how to improve quality and safety in healthcare. BMJ Simul Technol Enhanc Learn. 2020;6(2):87–94. https://doi.org/10.1136/bmjstel-2018-000370.
Simulation for predictive analytics in healthcare: answering “What If?”. SDLC Partners; 2020. Available at: https://sdlcpartners.com/insights/simulation-for-predictive-analytics-in-healthcare-answering-what-if/.
Harper PR, Gamlin HM. Reduced outpatient waiting times with improved appointment scheduling: a simulation modelling approach. OR Spectrum. 2003;25(2):207–22. https://doi.org/10.1007/s00291-003-0122-x.
Topaloglu S. A shift scheduling model for employees with different seniority levels and an application in healthcare. Eur J Oper Res. 2009;198(3):943–57. https://doi.org/10.1016/j.ejor.2008.10.032.
Acknowledgements
Medical writing assistance was provided by IQVIA employees Kiran Chaudhary and Paranjoy Saharia. The funding for medical writing was provided by Novartis Pharma AG (CH-4002 Basel, Switzerland).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Research and material support for this manuscript was funded by Novartis Pharma AG (CH-4002 Basel, Switzerland).
Author contributions
IB, CL, NA, and PD conceptualised the study. KL, PT, RB, CL, NA, PD, ES, and ZA performed research methodologies. KL, PT, RN, PD, ES, ZA, RB, and NA were involved in formal analysis and investigation. ES and ZA prepared the first draft. All authors reviewed and approved the manuscript. Funding was acquired by NA and supervision was provided by RB and NA.
Conflict of interest
Kristyna Lacinova is a full-time employee of Simul8 Corporation Ltd. Praveen Thokala is the director of PT Health Economics Ltd and advised Novartis on this study on a consultancy basis. He has also performed paid consultancy in the past with IQVIA, RTI-HS, Roche, Pfizer, Daiichi Sankyo, Abbvie, Novo Nordisk and Novartis. Richard Nicholas is a paid employee of Imperial College Healthcare NHS Trust, London, UK, and has acted as a paid consultant in the past for Biogen, Roche, Teva, Merck, and Novartis. He has also received research funds from and worked on clinical trials run by Biogen and Novartis. Pamela Dobay, Erik Scalfaro and Zuzanna Angehrn are full-time employees of IQVIA AG, Basel, which received funding from Novartis Pharma AG to design, conduct and interpret the reported analyses. Roisin Brennan is a full-time employee of Novartis Global Services Centre. Ibolya Boer, Carol Lines, and Nicholas Adlard are full-time employees of Novartis Pharma AG.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable since the model did not use patient-level data. Data were sourced from publicly available literature and assumptions based on clinical experience of one of the co-authors.
Code availability
Models were developed using Simul8 proprietary software; extracts from the model codes were available to the reviewers upon request. The feasibility of making the model publicly available is currently being evaluated.
Ethics approval
Not applicable since patient-level data have not been used in this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lacinova, K., Thokala, P., Nicholas, R. et al. ENTIMOS: A Discrete Event Simulation Model for Maximising Efficiency of Infusion Suites in Centres Treating Multiple Sclerosis Patients. Appl Health Econ Health Policy 20, 731–742 (2022). https://doi.org/10.1007/s40258-022-00733-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00733-0