Abstract
Introduction
Administration of intravenous (IV), high-efficacy treatments (HETs) for the treatment of multiple sclerosis (MS) poses a high resourcing and planning burden on infusion centres, resulting in treatment delays that may increase the risk of breakthrough disease activity. Simulation tools can be used to systematically analyse capacity scenarios and identify and better understand constraints, therefore enabling decision-makers to optimise patient care. We have previously applied ENTIMOS, a discrete event simulation model, to assess scenarios of patient flow and care delivery using real-life data inputs from the neurology infusion suite at Charing Cross Hospital London. The model predicted that, given current capacity and projected demand, patients would experience high-risk treatment delays within 30 months.
Objective
This study aimed to address key healthcare challenges for MS patient care management as seen from a neurologist’s perspective. We used ENTIMOS to predict the impact of several distinct and clinically plausible scenarios on patient waiting times at the same MS infusion suite and to quantify mitigation strategies needed to assure continuity of care.
Methods
We used real-world experience of an expert neurologist to identify five clinical scenarios: (1) switching patients to a subcutaneous (SC) MS treatment of the same therapeutic agent, in the same hospital setting; (2) extending opening times to the weekend; (3) reducing the number of infusion chairs (to simulate social distancing measures applied during the coronavirus disease 2019 [COVID-19] pandemic); (4) increasing demand for infusions; and (5) increasing the scheduling approval time. Patient waiting time for next due infusion and time to high-risk treatment delays (≥ 30 days) were the main analysed model outputs. Previously published base case results were used as reference. All hypothetical scenarios were run over a 3-year horizon (with the exception of scenario 1, which was run over a 3- and 5-year horizon). Strategies to mitigate treatment delays were analysed and discussed.
Results
Switching 50% of patients to SC treatment of the same therapeutic agent administered in hospital postponed the predicted high-risk treatment delays to shortly beyond the 3-year simulation timeframe (month 38). Weekend opening reduced waiting times from 20 days to 4 days and prevented treatment delays, however, at elevated labour costs. Reducing the infusion chairs increased waiting time to 53 days on average and 86 days at the end of the simulation, leading to high-risk treatment delays within 6 months. An increased demand for infusions increased waiting time to 26 days on average and 47 days at the end of the simulation, leading to high-risk treatment delays within 22 months. Prolonged scheduling approval time did not reduce the waiting time, nor postpone the high-risk treatment delays.
Conclusion
Decision makers need transparency on capacity constraints to better plan resourcing at infusion suites and MS treatments. Our results showed that various mitigation measures, such as increasing capacity by additional infusion chairs per year and transferring patients to other infusion suites, may help prevent treatment delays. Switching patients from IV to an SC version of the same therapeutic agent reduced the waiting time moderately and postponed high-risk treatment delays. It was insufficient to prevent high-risk treatment delays in the long term.
Plain Language Summary
Patients with multiple sclerosis and other neurological conditions receive therapies that are often given intravenously. Due to increasing demand for intravenous infusions, specialist infusion centres face challenges with scheduling and insufficient personnel numbers, which contributes to the increasing costs of care. Computer-based decision support tools can help hospital administrators predict demand for infusions, plan resources and estimate overall costs. We used a computer-based decision support tool, “ENTIMOS”, to predict demand at a multiple sclerosis infusion suite in London and to simulate possible solutions. The tool predicted that over the next 3 years patients would face increasing waiting time for their treatment and many would experience high-risk treatment delays of 30 days or longer. We tested several different, realistic scenarios where treatment demand was exacerbated and alleviated: we tested what would happen if patients were discharged from the infusion suite (decreasing demand), if the centre opened for 7 days instead of 5 days a week (increasing capacity), if social distancing measures were in place (decreasing capacity), and other scenarios. We found that high-risk treatment delays could be avoided if the centre adds infusion chairs to the suite or switches patients out of the infusion suite (e.g. to a treatment administered at home). The most effective long-term solution would be to have treatment options for multiple sclerosis that could be taken by patients at home. These treatments would be required to have the same benefits and the same or lower risk as the intravenous infusion therapies that are used today. It would help reduce labour costs of healthcare and may enable patients with multiple sclerosis to manage their disease at home.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
This study applied ENTIMOS, a previously published decision support tool, to simulate five scenarios representing plausible hypothetical developments in multiple sclerosis (MS) care. |
The model simulated patient demand for MS infusion services over 3 years and tested the impact of mitigation measures on the key output: high-risk treatment delays of 30 days or longer. |
While increasing centre capacity and weekend opening may help in the short term, switching patients from intravenous to subcutaneous high-efficacy therapy administered outside of infusion sites appears an effective long-term strategy to reduce MS infusion demand without increasing the labour cost of MS care. |
1 Introduction
The outlook for patients with multiple sclerosis (MS) evolved substantially since the first disease-modifying treatment (DMT) was introduced in the 1990s, as more efficacious DMTs that markedly reduce relapse rates and slow disability accumulation were developed. Several currently available DMTs for MS (alemtuzumab, natalizumab and ocrelizumab) are administered as infusions in hospital settings, under specialist care, and are considered to be high-efficacy treatments (HETs) [1,2,3,4], while natalizumab has also been approved for subcutaneous (SC) use [5], and a phase 3 clinical study of the non-inferiority of SC versus intravenous (IV) ocrelizumab is ongoing [6]. Although natalizumab is administered SC, it must still be administered in an infusion chair [5]. The rise in MS prevalence coupled with a range of other drugs administered IV to treat other neurological conditions propels the demand for infusion services [7].
The increased demand for infusion services creates challenges in scheduling and optimal resourcing, leading to prolonged waiting time for treatment [7, 8]. Serving patients requiring IV treatments in a timely manner depends on the availability of resources, such as infusion chairs and qualified staff. Inadequate infusion centre capacity may increase the risk of patients experiencing lesions and/or relapses. In some centres, the lack of infusion capacity can impact other therapy areas that rely on IV administration, such as oncology. The management of resources is crucial as new treatments become reality, requiring enough resources in place to serve all patients. While infusion-related capacity constraints are well studied in areas such as oncology [9, 10], there is a scarcity of detailed demand flow analyses at MS-treating infusion suites [11].
The coronavirus disease 2019 (COVID-19) pandemic has further highlighted the need for rare event and emergency planning [12]. During the pandemic, infusion centres had to reduce the number of chairs to comply with social distancing regulations. This further prolonged waiting times in centres already operating at capacity, thus delaying treatments and impacting health outcomes [12]. The increased demand for infusion services and virtual care during the pandemic, as well as the backlog of patients and treatments post-pandemic, highlighted the threat of potential future lockdowns and similar rare events on capacity [13, 14]. Hence, it is important that infusion suites optimally allocate and plan resources to anticipate and avoid treatment delays, and that patients with MS are shifted towards integrated care for treatment and monitoring outside of infusion suites whenever possible.
Decision support tools using real-world data to simulate patient journey and resource utilisation in infusion suites could help infusion centres with planning resources and decision making. Hospital staff, such as clinicians, nurses and hospital managers, could use it to anticipate demand given the current resources and explore ways to allocate them in the future.
We have previously generated ENTIMOS [11], a peer-reviewed decision support tool, and used it to customise resource planning in the Charing Cross Hospital infusion suite in London, UK. The model estimated that, given the suite capacity as of March 2021 and projected demand, patients can be expected to experience high-risk treatment delays within 30 months from the start of simulation [11].
This study builds on the previously published base case and assesses the impact of five distinct clinical scenarios, which focussed on current or anticipated challenges at infusion suites and offered strategies and mitigations to reduce patient waiting time in the neurology infusion suite at the Charing Cross Hospital in London.
2 Methods
2.1 Study Overview
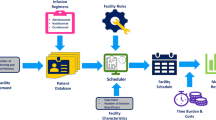
This study was conducted in three steps (Fig. 1). A base case has been established previously for the Charing Cross Hospital infusion suite, London, UK, given their capacity and resources available at the time of data collection in March 2021 [11], and was used as reference.
Study overview: A three-step approach was used to conduct the study, by defining and simulating relevant clinical scenarios, analysing KPIs, and identifying mitigation measures for improving MS care. aThis scenario comprised scheduling approval time and additional measures that would result in infusion scheduling delay (see “Methods” Sect. 2.3.6 for details). bFor example, simulate switch to home-based care with non-IV MS therapy. IV intravenous, MS multiple sclerosis, SC subcutaneous, KPI key performance indicator
Five hypothetical but clinically plausible scenarios were specified by the first author of this article, Consultant Neurologist Prof. Richard Nicholas, given the experience of leading a large tertiary referral neurology unit. These scenarios, which can be viewed as sensitivity analyses to the original publication, listed the key factors expected to influence (both exacerbate and alleviate) the demand for infusions in the coming years. The study was initiated while the COVID-19 pandemic was ongoing; therefore, these scenarios reflected the social distancing measures that were implemented at that time (and that are likely to be implemented when we are confronted with a future pandemic).
The primary focus was to predict the moment in time when patients would experience high-risk treatment delays, and thus be at risk of disease progression and/or exacerbation [7, 8]. This key outcome was defined as average waiting time for the next due infusions of ≥ 30 days (compared with the approved treatment schedule indicated in the Summary of Product Characteristics [SmPC]). Scenarios were also compared using time and cost key performance indicators (KPIs).
Finally, potential mitigation measures focusing on either reducing demand or improving capacity were identified (see Fig. 1).
2.2 The Decision Support Tool
The ENTIMOS discrete event simulation model (described in detail by Lacinova et al. [11]) has been developed as a decision-support tool using information about patient flow, MS infusion care delivery pathways and site processes collected from site administrators, nurses, pharmacists and clinicians at the London Charing Cross Hospital infusion suite, as of March 2021. Data on centre and treatment settings, such as the number of infusion chairs, patients and nurses, and treatments administered, were collected to build a process flow. The main output data are patient waiting time, as well as costs. For a full list of input and output parameters, see Lacinova et al. [11]. The clinical accuracy and relevance of the tool were validated by a consultant neurologist (Prof. Richard Nicholas).
2.3 Clinical Scenarios
We defined a set of clinically plausible scenarios which addressed current or anticipated challenges at infusion suites (scenarios 3 and 4) as well as plausible strategies to mitigate them (scenarios 1, 2 and 5) without increasing the resources such as number of chairs or reducing the patient number. In cases where high-risk treatment delays were nevertheless predicted, we used the model to estimate how many more resources will be needed or how many patients would need to be discharged to maintain adequate, continuous care. If different measures result in reduced patient waiting time, labour costs can be considered as an additional parameter to select the optimal mitigation strategy.
2.3.1 Base Case
The base case scenario used real-world inputs from the London Charing Cross Hospital infusion suite, as of March 2021. Inputs, such as the number of infusion chairs, patients, nurses, treatments used, etc., were used to simulate a realistic expectation of patient number, queue size and waiting times over a simulation time of 3 years (scenario 1.2 was also simulated over 5 years). Charing Cross Hospital infusion suite is a neurology-only suite that has 12 infusion chairs, employs ten staff nurses and treats 860 patients with MS and 170 patients with non-MS conditions; further model inputs and results of the base case have been discussed in full elsewhere [11]. In this study, the base case results were used as a reference (baseline data) for assessing the potential impact of the other five scenarios on MS care.
2.3.2 Scenario 1: IV to SC Treatment Switch
Scenario 1.1 was designed to evaluate the effect of reducing the demand for infusions by switching patients from IV to SC therapy in the hospital setting. In this scenario, 50% of patients receiving a monthly IV MS HET (natalizumab) were switched to SC administration (n = 154) of the same therapeutic agent, with otherwise similar characteristics. Only 50% of patients were chosen for treatment switch because, as revealed in a recent qualitative study to determine the drivers of patient treatment preference, which involved telephone interviews with people living with relapsing MS and healthcare professionals, ~ 40% of patients with negative experiences would choose to avoid treatment that involves injection (either SC or IV) or self-administration [15]. All new natalizumab patients were assumed to be prescribed an SC version of this drug. As this drug requires supervision from the medical personnel during administration, patients were assumed to occupy an infusion chair regardless of the administration route; therefore, this scenario simulated the effect of difference in treatment duration (1–2 h for IV and 0.1–0.7 h for SC treatment) [16]. The pre-treatment for both routes of administration was assumed to be the same. The posology inputs used for the SC version of MS HET are described in Supplementary Table 2 (see the electronic supplementary material). To determine the long-term feasibility of this scenario, a sensitivity analysis of the same settings was simulated over a longer (5-year) horizon (scenario 1.2).
2.3.3 Scenario 2: Weekend Opening
In scenario 2, the infusion service included weekends (i.e. extending the opening times from 5 to 7 days a week) to model centre capacity in line with COVID-19 response measures.
2.3.4 Scenario 3: Weekend Opening and Chair Reduction
To simulate social distancing measures that were required during the COVID-19 pandemic and may be needed in future under assumed epidemic/pandemic conditions, the third scenario reduced the number of infusion chairs by 50% (from 12 to 6) on top of the weekend opening.
2.3.5 Scenario 4: Increased Demand for IV Infusions
The fourth scenario evaluated the impact of a hypothetical IV HET that might be approved and reimbursed in the UK for a chronic, progressive neurodegenerative disease (CPND) that currently lacks an HET. The demand for IV infusions from CPND patients matched the demand from patients with MS (860 existing patients at simulation start and seven new patients per week, on the top of MS patients). It was assumed, based on clinical opinion, that a high proportion (35%) of patients with CPND would experience adverse events that would require them to skip the subsequent infusion [17]. This scenario did not consider the resources needed to conduct imaging prior to infusion and monitor patients recovering from the adverse events of the CPND HET. Supplementary Table 3 describes the posology inputs used to simulate the CPND IV case (see the electronic supplementary material).
2.3.6 Scenario 5: Infusion Scheduling Delays for New Patients with MS
The fifth scenario studied the impact of infusion scheduling delays. In routine clinical practice, infusion delays are sometimes employed as a gatekeeping tactic to shorten the patient queue. The clinics postpone the start of therapy for newly diagnosed patients, allowing for more time to schedule and treat existing patients. The tactical delays can be applied to multiple steps in the continuum of patient care, namely screening of the patient’s blood results, obtaining consent for the infusion, clinical review and multidisciplinary team approval, infusion scheduling, and dose screen and approval by pharmacy. While clinical review and multidisciplinary team approval were deemed outside of control of the infusion suite administration, all other factors listed above were included in scenario 5.
Based on clinical experience from the Charing Cross Hospital in London, this scenario assumed that after 12 months of simulation, the scheduling delay for new patients will have increased from an average 12 weeks (range 4–20 weeks) to 23 weeks (range 20–26 weeks).
2.4 Simulation Time
To reflect a typical planning horizon at an infusion centre, all scenarios were run for a simulation time of 3 years. An additional sensitivity analysis over a period of 5 years was run for scenario 1.2, to interrogate the long-term feasibility of patient switch from IV to SC MS HET when treatment would still be administered within the infusion suite.
2.5 Model Outputs: KPIs
To evaluate the efficiency of the infusion centre, three KPIs were used: waiting time (days), time to high-risk treatment delays (month) and direct costs (labour) (£). Patient waiting time refers to the number of days patients must wait to receive their next due infusion and is calculated as the average on a monthly basis. Time to high-risk treatment delays refers to the point in time when patients face treatment delays that put them at risk of disease progression and/or exacerbation, and is expressed as the month in which the average patient waiting time reaches ≥ 30 days (compared with the approved treatment schedule indicated in the SmPC) [7, 8]. Reaching this point can be considered a “system compromise” [11]. Labour costs, based on studies by Franken et al., North et al., Perry, and Schmier et al. are a direct result of nurse hours spent on infusion administration and management of infusion-related adverse reactions and are expressed as the sum accrued over the entire simulation [18,19,20,21]. Fixed costs for acquiring new chairs and other operational costs (e.g. additional infusion rooms if existing rooms are at capacity) were not considered in the model for simplicity. The entire list of outputs that can be generated by the simulation tool has been described by Lacinova et al. [11].
2.6 Mitigation Measures
Mitigation measures are adjustments in the resources or patient numbers which prevent patient waiting time from reaching a threshold of 30 days, therefore avoiding high-risk treatment delays.
This analysis included three mitigation measures: discharging new patients with MS from the infusion centre, discharging existing patients with MS or adding infusion chairs. Patients discharged from the infusion centre could either be referred to the care of another MS infusion centre or switched to oral or SC treatment options that would allow self-administered and/or home-based care. Discharged patients are the ones for whom there is effectively no capacity in the infusion centre and, therefore, a new care arrangement needs to be found for them. In the technical specifications of the tool (see [11]), discharge is represented through a “switch-out” option that removes patients from the simulation. Quantification of mitigation strategies was only completed for those scenarios where high-risk treatment delays were reached within the simulation time frame.
3 Results
3.1 Base Case
The base case has been described previously [11]. Briefly, Charing Cross Hospital infusion suite was operating at capacity from the beginning of the simulation. High-risk treatment delays were reached within 30 months. The labour costs in the base case were measured, given that this is the most important cost component in helping to differentiate between different courses of action for treatment. These labour costs were forecasted to a total of £981,155 over 3 years. The model predicted that to avoid high-risk treatment delays during the 3-year horizon, one of the following mitigation measures would be needed: adding one infusion chair or by switching 7% of existing patients or 24% of new patients to alternative MS treatments not requiring infusion. [11].
Increasing the chair capacity by one infusion chair annually (i.e. three chairs over 3 years) would require 16.41% extra nurse hours per month to serve these chairs, bringing the total number of nurse hours to 1508 h per month and incurring additional £160,854 in labour costs [11].
These results were used as a reference (baseline data) for assessing the potential impact of the five hypothetical scenarios (Table 1). Mitigations measures needed to keep average waiting time below 30 days that were identified by the model are presented in Table 2.
3.2 Scenario 1: IV to SC Treatment Switch of the Same Therapeutic Agent
The reduced duration of SC MS HET administration allowed for a better patient turnaround, increasing the total number of all MS HET administrations from 18,703 in the base case to 21,171. This is also reflected in the average waiting time that was reduced from 20 days, which was the base case used for comparison in each case, to 13 days, accompanied by slightly increased labour cost compared with the base case (£1,005,734 vs £981,155, respectively; Table 1), considering that in-hospital administration was assumed for both MS HET administration routes, IV and SC. At the end of the simulation, 640 patients were waiting for an appointment to receive their due treatment and the average waiting time in the last month reached 29 days. A sensitivity analysis of the same settings simulated over a longer (5-year) horizon showed that high-risk treatment delays were reached at month 38, which means that high-risk treatment delays were not prevented but only postponed beyond the 3-year horizon in this scenario.
3.3 Scenario 2: Weekend Opening
When extending the opening times at Charing Cross Hospital infusion suite to 7 days a week, it was anticipated that the high-risk treatment delays could be avoided during the 3 years of simulation. In this scenario, the waiting time at the end of simulation was reduced to 11 days and number of patients waiting for their due appointment dropped to 326; however, higher labour costs (£1,373,804) were forecasted compared with the base case scenario (Table 1).
3.4 Scenario 3: COVID-19: Chair Reduction with Weekend Opening
When simulating the COVID-19 pandemic social distancing measures, extending opening hours while the number of infusion chairs was reduced by half (from 12 to 6) was not sufficient to prevent the high-risk treatment delays of ≥ 30 days, which were predicted to occur within 6 months. The waiting time at the end of the simulation increased to 86 days, while labour costs covering infusion administration and managing the infusion-related reactions decreased to £710,625 (below the base case scenario). To receive their treatment on time under assumed pandemic conditions, the majority of existing and new patients (59% and 79%, respectively) would need to be discharged (Table 2).
3.5 Scenario 4: New Demand for CPND HET IV Infusions
The scenario where a hypothetical CPND treatment is approved in the UK assumed doubling the patient burden on the infusion suite. As a result, the model predicted that high-risk treatment delays would occur 8 months earlier than in the base case scenario (month 22), while the waiting time reached 47 days at the end of the simulation. Furthermore, labour costs would increase to over £1 million. To prevent this situation, 31% of new patients and 14% of existing patients would need to be discharged from the infusion suite each year. This infusion, assumed to take 1 h, has often been fitted by the model within the remaining time between the longer MS infusions, thus allowing for high-volume scheduling of CPND patients without affecting the MS care. These results may underestimate the impact of increased demand for infusions if a CPND IV HET infusion would take longer than the hypothesised 1 h.
3.6 Scenario 5: Infusion Scheduling Delays for New Patients with MS
In this scenario, the waiting time for newly diagnosed, first-time patients increased from 103 to 184 days on average (results not shown). Increase in the scheduling approval time resulted in a shorter time until high-risk delays were reached (month 27 vs month 30 in the base case scenario). The number of patients waiting for their next due appointment increased from 713 patients in the base case to 779 patients. Also, the labour costs and necessary mitigation measures remained similar compared with the base case scenario (Table 1). Delaying access to treatment by a couple of weeks to individual, newly diagnosed patients put them at risk without alleviating the burden to centre patients, overall.
Among the three scenarios (scenarios 1, 2 and 5) testing plausible mitigation measures, only weekend opening (scenario 2) resulted in sustainable reduction of waiting times, conclusively preventing high-risk treatment delays. This scenario was associated with an increased labour cost of 40% (£1,372,804 vs £981,155). Switching half of patients receiving an IV MS HET to an SC version of the same therapeutic agent (also administered in hospital) reduced the waiting time moderately and postponed high-risk treatment delays beyond the 3-year horizon, to month 38. Therefore, it was insufficient to prevent high-risk treatment delays in a long term.
In the two scenarios (scenarios 3 and 4) reflecting hypothetical but plausible future clinical developments (future pandemic and approval of a new CPND HET), a significant increase in the waiting time is expected, leading to acceleration of high-risk treatment delays. To prevent high-risk treatment delays, infusion chairs need to be added, which is often difficult due to physical or cost constraints, or a significant proportion of patients need to be discharged from the infusion suite and provided with alternative treatment options that can be administered in a different setting. The model predicted that up to 14% of existing patients need to be discharged each year under hypothetical circumstances (i.e. no capacity in the infusion centre/increased patient burden) [11] and up to 59% in the case of another pandemic requiring social distancing, while new patients cannot be admitted in order to maintain continuity of care for MS patients.
4 Discussion
This study aimed to address key healthcare challenges for MS patient care management as seen from a neurologist’s perspective. With new IV administered treatments being constantly introduced, infusion suites such as the one at Charing Cross Hospital, London, face increasing demand pressures. Several clinical scenarios were simulated to predict outcomes and identify potential mitigation measures for improving MS patient care at the Charing Cross Hospital. The key output was the predicted time to high-risk treatment delays, defined as a delay of ≥ 30 days beyond the due date of next HET infusion due to insufficient centre capacity. This was defined as a key output since the evidence, clinical guidelines and recommendations clearly indicate that patients with MS who do not receive timely treatment are at risk of exacerbation and disease progression [7, 8].
In the previously published base case, we showed that, without any change to the resourcing, capacity and treatment demands at the Charing Cross Hospital, patients would experience high-risk treatment delays within 30 months [11]. In that study, we found that this tipping point could be postponed by switching 7% of existing patients or 24% of new patients to a different MS treatment not requiring infusion (e.g. ofatumumab). This would allow all patients to receive timely infusion appointments without the need to increase resources. Such measures could improve not only the overall management of MS patient care, but also patients’ quality of life, by reducing the time spent at the infusion centre. However, as demonstrated in the current ENTIMOS simulation scenarios, if patients are switched from IV HET to in-hospital administered SC HET of the same therapeutic agent, the high-risk treatment delays would only be postponed by several months and not prevented, as highlighted by the sensitivity analysis.
Alternatively, the centre’s schedule could be extended to weekends (scenario 2), assuming the number of infusion chairs remains unchanged. Such a measure could reduce the patient waiting time dramatically and prevent high-risk treatment delays within 3 years. However, this scenario would have the highest anticipated labour costs, as additional nurse availability would be required. Although efficient at full chair capacity, weekend opening would be insufficient if social distancing measures are required (reduced chair capacity), as was the case during the COVID-19 pandemic (scenario 3). Although the social distancing measures are no longer in place in the UK or indeed the rest of the world, the results of this study highlight that these measures may result in severe reduction in infusion suite capacity. Lockdowns and social distancing measures under assumed epidemic/pandemic conditions should be taken into account when planning emergency response strategies.
The capacity to serve patients with MS is also limited within infusion suites that treat different categories of patients (e.g. neurology, oncology). Therefore, a hypothetical approval of a novel IV HET for another indication, such as treating patients with CPND, would put further strain on the Charing Cross Hospital infusion suite by increasing demand for infusions and exposing patients with MS to high-risk treatment delays much more rapidly (scenario 4).
Increasing the time between diagnosis and treatment initiation (scheduling approval time, scenario 5) for newly diagnosed patients could buy more time for scheduling and treating the existing patients. However, such a measure may not be appropriate at Charing Cross Hospital in London. This would not be a clinically desired solution, as it does not postpone nor prevent the high-risk treatment delays and would put newly diagnosed patients with MS at risk of disease progression by delaying their access to treatment.
Our initial analysis of the base case showed that adding one additional infusion chair per year could help accommodate the current demand for infusions and mitigate high-risk treatment delays [11]. This analysis confirmed that this mitigation measure would also be effective for scenarios 1, 4 and 5. Yet such changes are often not possible due to physical limitations. They incur significant additional costs and would leave the overall patient experience unchanged. Another suggested mitigation measure would be to discharge patients from the infusion centre, for example by transferring them to other treatment centres. However, patient transfers to other centres would address the problem of increased demand only locally and for a limited time. This must be done only when it is possible to simultaneously maintain optimal patient health and provide the highest possible level of specialised care with the potential implications in mind (e.g. risk of poor treatment handover, treating physician losing oversight of patients, potential lack of diagnostics and MS-specific clinical expertise, increased commute hours for patients, additional administrative and organisational efforts).
Instead, a long-term solution could be to switch patients to non-IV alternative therapies administered outside the infusion clinic setting. Integrated care approaches, such as switching patients to oral or SC options and treating them outside the infusion clinics could help reduce hospital visits to only those that are absolutely necessary for diagnosis and monitoring purposes, thus reducing the burden of MS on both the patient and the healthcare system. The results of this study should be considered in the light of the assumptions and limitations previously described by Lacinova et al. [11]. The decision support tool used in this study was designed to estimate the capacity of an infusion suite rather than the entire neurology directorate. Although the tool quantifies the mitigation strategies needed to prevent clinically relevant delays, the feasibility of their implementation lies beyond its scope.
5 Conclusions
This work addresses key healthcare challenges in the planning and management of MS. We analysed clinical scenarios at the Charing Cross Hospital, London, infusion suite using a previously published decision support tool [11] that has been designed to help healthcare professionals and hospital administrators explore how the changes in demand for infusions and resource constraints could potentially affect patient care in their infusion centres. In this article, we built on the previously published base case and explored further how integrating decision support tools into clinical practice could help with finding solutions for better management of MS care, to improve patient access to treatment. At the London Charing Cross Hospital infusion suite, feasible short-term measures for coping with demand for MS care, given the available capacity, could be switching patients from IV HET to in-hospital administered SC HET of the same therapeutic agent where SmPC allows or extending the centre schedule to weekends. To alleviate the burden on infusion suites and accommodate the increasing demand in the long term, an integrated care approach would be needed. New care arrangements need to be created and extensively utilised, to assure continuous delivery of care in infusion centres. Assuming similar safety and efficacy profiles, switching patients from IV HETs to oral or SC HETs administered outside infusion centres holds great potential to simultaneously reduce the use of IV infusion facilities and resources, allow for labour cost savings in MS care and help patients make decisions about their treatment. Infusion suite managers need to monitor the performance and delay and act timely and strategically to avoid compromising patient care.
References
Mayer L, Kappos L, Racke MK, Rammohan K, Traboulsee A, Hauser SL, et al. Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies. Multiple Scler Relat Disord. 2019;30:236–43.
Barclay K, Carruthers R, Traboulsee A, Bass AD, LaGanke C, Bertolotto A, et al. Best practices for long-term monitoring and follow-up of alemtuzumab-treated MS patients in real-world clinical settings. Front Neurol. 2019;10:253.
Fernández O. Best practice in the use of natalizumab in multiple sclerosis. Ther Adv Neurol Disord. 2013;6(2):69–79.
Samjoo IA, Worthington E, Drudge C, Zhao M, Cameron C, Häring DA, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. J Comp Eff Res. 2020;9(18):1255–74.
Biogen Idec. Tysabri® (natalizumab) 150 mg solution for injection in pre-filled syringe summary of product characteristics; 2021. Available from: https://www.medicines.org.uk/emc/product/12443/smpc#about-medicine.
Hoffmann-La Roche. A phase III, non-inferiority, randomized, open-label, parallel group, multicenter study to investigate the pharmacokinetics, pharmacodynamics, safety and radiological and clinical effects of subcutaneous ocrelizumab versus intravenous ocrelizumab in patients with multiple sclerosis (Ocarina II). ClinicalTrials.gov identifier: NCT05232825; 2023. Available from https://classic.clinicaltrials.gov/ct2/show/NCT05232825
Plourde CL, Varnado WT, Gleaton BJ, Das DG. Reducing infusion clinic wait times using quality improvement. JCO Oncol Pract. 2020;16(8):e807–13.
Simacek KF, Ko JJ, Moreton D, Varga S, Johnson K, Katic BJ. The impact of disease-modifying therapy access barriers on people with multiple sclerosis: mixed-methods study. J Med Internet Res. 2018;20(10): e11168.
De Cock E, Pivot X, Hauser N, Verma S, Kritikou P, Millar D, et al. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer. Cancer Med. 2016;5(3):389–97.
Sugalski JM, Kubal T, Mulkerin DL, Caires RL, Moore PJ, Fiorarancio Fahy R, et al. National comprehensive cancer network infusion efficiency workgroup study: optimizing patient flow in infusion centers. J Oncol Pract. 2019;15(5):e458–66.
Lacinova K, Thokala P, Nicholas R, Dobay P, Scalfaro E, Angehrn Z, et al. ENTIMOS: a discrete event simulation model for maximising efficiency of infusion suites in centres treating multiple sclerosis patients. Appl Health Econ Health Policy. 2022;20:731–42.
Multiple Sclerosis Trust. The impact of Covid-19 on MS services. 21 July 2020. Available from: https://mstrust.org.uk/news/impact-covid-19-ms-services.
Portaccio E, Fonderico M, Hemmer B, Derfuss T, Stankoff B, Selmaj K, et al. Impact of COVID-19 on multiple sclerosis care and management: results from the European Committee for treatment and research in multiple sclerosis survey. Mult Scler. 2022;28(1):132–8.
Rath L, Bui MV, Ellis J, Carey J, Baker J, Taylor L, et al. Fast and safe: optimising multiple sclerosis infusions during COVID-19 pandemic. Multiple Scler Relat Disord. 2021;47: 102642.
Tatlock S, Sully K, Batish A, Finbow C, Neill W, Lines C, et al. Individual differences in the patient experience of relapsing multiple sclerosis (RMS): a multi-country qualitative exploration of drivers of treatment preferences among people living with RMS. Patient. 2023;16(4):345–57.
NICE. The National Institute for Health and Clinical Excellence; Summary of product characteristics; Tysabri® natalizumab; Biogen Idec; 2021. Available from https://www.medicines.org.uk/emc/product/12443/smpc.
VandeVrede L, Gibbs DM, Koestler M, La Joie R, Ljubenkov PA, Provost K, et al. Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab. Alzheimer’s Dement (Amst, Netherl). 2020;12(1): e12101.
Franken MG, Kanters TA, Coenen JL, de Jong P, Koene HR, Lugtenburg PJ, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs. 2018;29:791–801.
North RT, Harvey VJ, Cox LC, Ryan SN. Medical resource utilization for administration of trastuzumab in a New Zealand oncology outpatient setting: a time and motion study. Clinicoecon Outcomes Res. 2015;7:423–30.
Perry MC, editor. The chemotherapy source book. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
Schmier J, Ogden K, Nickman N, Halpern MT, Cifaldi M, Ganguli A, et al. Costs of providing infusion therapy for rheumatoid arthritis in a hospital-based infusion center setting. Clin Ther. 2017;39:1600–17.
Acknowledgements
The authors thank Kristyna Lacinova and Dr. Pamela Dobay for their help developing the ENTIMOS model used in this analysis. Dr. Pamela Dobay contributed to the model during her time as an IQVIA employee (until 2020). Medical writing assistance was provided by IQVIA employees Livia Crica, Natasha Pillai, Vibha Dhamija, Kiran Chaudhary and Shala Mhlanga. Funding for medical writing was provided by Novartis Pharma AG (CH-4002 Basel, Switzerland).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Modelling and medical writing support for the manuscript was funded by Novartis Pharma AG (CH-4002 Basel, Switzerland).
Author Contributions
RN, ZA, RB and NA conceptualised the study. RN and RD provided input data. All authors contributed towards output data analysis and interpretation. RN, ES, ZA, RB and NA contributed towards manuscript drafting. RN, RD, ZA, JB, RB and NA critically reviewed the manuscript for important intellectual content. All authors provided their final approval of the manuscript and agreed to be accountable for all aspects of the work.
Conflict of Interest/Competing Interests
RN is a paid employee of Imperial College Healthcare NHS Trust, London, UK, and has acted as a paid consultant in the past for Biogen, Roche and Novartis. He has also received research funds from and worked on clinical trials run by Biogen and Novartis. ES was a full-time employee of IQVIA AG, Basel, which received funding from Novartis Pharma AG to design, conduct and interpret the reported analyses. ZA was a full-time employee of IQVIA AG, Basel, which received funding from Novartis Pharma AG to design, conduct and interpret the reported analyses. ZA owns stocks of pharmaceutical companies. RB is a full-time employee of Novartis Corporate Center Dublin. NA is a full-time employee of Novartis Pharma AG. JB was a full-time employee of Novartis Pharma AG at the time of the analysis. RD has no conflict of interest to declare.
Ethics Approval
Not applicable since patient-level data were not used in this study.
Consent
Not applicable since patient-level data were not used in this study.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analysed during the current study. Data for populating scenario analysis were sourced from publicly available literature or based on assumptions stemming from the clinical experience of two of the co-authors.
Code Availability
The ENTIMOS model is available through Open-Source Model Clearinghouse.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nicholas, R., Scalfaro, E., Dorsey, R. et al. ENTIMOS: Decision Support Tool Highlights Potential Impact of Non-intravenous Therapies for Multiple Sclerosis on Patient Care via Clinical Scenario Simulation. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-024-00493-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-024-00493-8