Abstract
Background
Compounded cantharidin has been used for decades to treat molluscum contagiosum but lacks rigorous clinical evidence to support its safety and efficacy. VP-102 is a shelf-stable drug–device combination product that contains topical cantharidin (0.7% weight/volume [w/v]) and is being evaluated for the treatment of molluscum.
Objectives
Our objective was to present pooled safety and efficacy analyses of VP-102 in the treatment of molluscum compared with vehicle.
Methods
Participants aged ≥ 2 years were randomized 3:2 to topical administration of VP-102 or vehicle in two randomized, double-blind, vehicle-controlled phase III trials. Study drug was applied to all baseline and new lesions once every 21 days until clear or for a maximum of four applications. Assessors blinded to treatment counted all lesions at each study visit. All adverse events (AEs) were documented. Data were pooled for analyses.
Results
In total, 310 participants received VP-102 and 218 received vehicle. Mean age was 7.5 years (range 2–60) for VP-102 and 6.8 (2–54) for vehicle. Complete clearance of all molluscum lesions at day 84 occurred in 50% of VP-102 participants and 15.6% of vehicle recipients (p < 0.0001). Mean molluscum lesion counts decreased 76% for VP-102 and 0.3% for vehicle at day 84 (p < 0.0001). The most common AEs in the VP-102 group were application site blistering, pruritus, pain, and erythema, which were generally mild or moderate in severity.
Conclusions
Pooled analyses showed a significantly higher percentage of participants with complete molluscum lesion clearance and larger reductions in lesion counts with VP-102 than with vehicle. AEs were anticipated because of the pharmacodynamic properties of cantharidin.
Trial Registration
ClinicalTrials.gov identifiers: NCT03377790 (first posted 19 December 2017) and NCT03377803 (first posted 19 December 2017).
Video abstract: Pooled Results of Two Randomized Phase III Trials Evaluating VP 102, a Drug Device Combination Product Containing Cantharidin 0.7% (w/v) for the Treatment of Molluscum Contagiosum (MP4 131293 KB)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This manuscript presents the pooled safety and efficacy outcomes from two phase III trials using VP-102 to treat molluscum contagiosum. |
The results of these studies provide robust efficacy and safety data that support the use of VP-102 for the treatment of molluscum in patients aged ≥ 2 years. |
These data may also assist healthcare professionals and caregivers with expectations of molluscum contagiosum treatment outcomes. |
1 Introduction
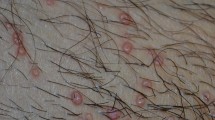
Molluscum contagiosum (molluscum) is a common cutaneous infection caused by a DNA poxvirus that primarily affects children. The molluscum contagiosum virus is trophic to epidermal keratinocytes, where it replicates, forming hyperplastic epithelial cells organized into focal lesions. The period from inoculation to presentation of lesions on the epithelial surface has been estimated at 2–8 weeks [1, 2].
With a prevalence of 5.1–11.5% in children aged 0–16 years [3], molluscum accounts for roughly 1% of all diagnosed skin disorders [4] and ranks in the top 50 most common causes of disease [5]. Molluscum is the third most common viral skin infection, behind herpes simplex virus and human papilloma virus, in children and one of the five most prevalent skin diseases worldwide but may be under-recognized [6]. Atopic dermatitis (AD), immune deficiency, and sexual activity are additional risk factors [7]. Patients with AD or a family history of atopy are at higher risk for molluscum infection [8], and the virus can also trigger the onset of AD and/or AD flares [9].
Molluscum is easily transmitted to other individuals. A reported 42% of pediatric patients spread the disease to a sibling [9]. Molluscum spreads to other skin sites via autoinoculation and to other people via skin-to-skin contact, co-bathing, or via fomites [10].
Molluscum is often described as benign and self-limited following acquisition of host immunity [2, 7, 11, 12]. However, the average duration of infection in untreated children aged 4–15 years was 13.3 months in the largest cohort study in the UK, including 306 patients. At 24 months, 13% of cases remained unresolved [13].
Treatment guidelines for molluscum have not been established. A 2003 survey of US physicians across specialties found that practices and preferences for the treatment of molluscum varied widely [11]. No medications for this condition have yet been approved by the US FDA. Many clinicians recommend mechanical or pharmacologic methods not approved by the FDA for molluscum or an “active nonintervention” approach to allow for eventual spontaneous resolution [11]. Active treatment is most often recommended to alleviate symptoms, minimize autoinoculation and transmission to others, improve cosmetic appearance, and prevent scarring [4, 12, 14].
Although no treatment for molluscum has been approved by the FDA, compounded cantharidin has been used to treat molluscum and warts for more than 60 years [15]. The exact mechanism of action for treating molluscum with cantharidin is unknown, yet it is hypothesized that cantharidin’s vesicant properties cause weakening and degradation of keratinocyte desmosomes, with associated blister formation, promoting shedding of infected keratinocytes and viral clearance [16, 17]. Compounded cantharidin is typically formulated with volatile compounds and stored in screw-top bottles, which can lead to changes in cantharidin concentration once prepared [18]. Guidance for the use of cantharidin is limited, and access to compounded cantharidin has been restricted [19, 20]. In addition to lack of access, few controlled studies have evaluated compounded cantharidin. Smaller studies have highlighted the need to identify an optimal dosing strategy and emphasize the problems with inconsistent formulations that contribute to efficacy and safety concerns, especially in sensitive skin areas [14, 19, 20]. Finally, compounded cantharidin is commonly applied using rudimentary, imprecise tools (such as cotton-tipped applicators) that could lead to inadvertent treatment of unaffected skin. Thus, there is an unmet need for a treatment that can leverage the unique mechanism of action of cantharidin but overcome obstacles of the compounded version, including a consistent and precise application method containing a controlled formulation with a proven dosing schedule, with testing completed in large populations of patients with molluscum.
VP-102 is a shelf-stable, proprietary drug–device combination product containing cantharidin (0.7% weight/volume [w/v]) and the inactive ingredients acetone, gentian violet, and denatonium benzoate in a film-forming topical solution. The applicator device containing the solution is composed of a glass ampule containing 450 µL of solution within a single-use applicator device that allows for a stable concentration of solution, along with a filter and precision (1 mm) applicator tip opening that allows for topical application of the solution to treatable molluscum lesions by a healthcare professional. The applicator allows for shelf stability, consistent formulation of the solution, and precise application to affected skin. Once applied, the acetone evaporates quickly, leaving a thin dry film that is then washed off within roughly 24 h. Gentian violet is a surgical dye inside the film that allows for distinction between treated and untreated lesions during application. Denatonium benzoate, one of the most bitter agents on the planet, is included to deter oral ingestion [21].
We present pooled analyses of two large vehicle-controlled phase III clinical trials, CAMP (Cantharidin Application in Molluscum Patients)-1 and -2, that were conducted to evaluate the safety and efficacy of VP-102 compared with vehicle in participants aged ≥2 years with molluscum. In addition, pooling data from these two large-scale phase III trials allows for a reduction in the variability seen in individual trials and may assist healthcare professionals make decisions about treatments and set expectations of safety and efficacy for their patients [22].
2 Methods
2.1 Study Design
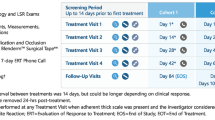
This pooled analysis included data from two randomized, double-blind, vehicle-controlled, identically designed phase III trials (CAMP-1 and CAMP-2) conducted to evaluate VP-102 in molluscum (detailed study design previously published [21]). Participants aged ≥2 years with at least one treatable molluscum lesion were eligible for inclusion. Participants with active AD, non-mucosal genital-area lesions and inflamed lesions were included. Full exclusion criteria have been described previously [21]. Participants were required to refrain from other treatments for molluscum within 14 days before and throughout the duration of the study. Independent, blinded investigators assessed total lesion counts prior to treatment at each study visit and at the end of study (EOS) visit (day 84). Individual lesions were not tracked. Safety evaluations were completed at 24 h and 7 and 14 days after each treatment as well as at clinic visits starting at visit 2 until the EOS visit. Participants were randomized at baseline to receive VP-102, a drug–device combination product containing cantharidin 0.7% (w/v), or vehicle, which included the same applicator and formulation of VP-102 without cantharidin. Study drug (VP-102 or vehicle) was applied to every lesion deemed treatable by the investigator at each visit (visit 1/day 1, visit 2/day 21, visit 3/day 42, and visit 4/day 63) when lesions were present, with a follow-up visit at day 84 (the EOS visit) for safety and efficacy. Participants were instructed to wash off the study drug with soap and water approximately 24 h after each application or earlier if significant blistering, pain, or other AEs occurred.
2.2 Endpoints
The primary efficacy endpoint was the proportion of VP-102-treated participants achieving complete clearance of all treatable (baseline and new) molluscum lesions relative to vehicle-treated participants at EOS visit/day 84 in the trials’ intent-to-treat (ITT) populations (i.e., all randomized participants). Secondary efficacy outcomes included the proportion of participants that achieved complete clearance of all baseline and new molluscum lesions at visit 1/day 1, visit 2/day 21, visit 3/day 42, and visit 4/day 63. The percentage change of lesion counts from baseline to each visit was assessed as an exploratory prespecified endpoint.
Safety assessments included monitoring of treatment-emergent adverse events (TEAEs) and local skin reactions (LSRs) expected because of the pharmacodynamic action of cantharidin as a vesicant. Detailed methods for safety monitoring can be found in Eichenfield et al. [21]. In summary, participants were queried about the incidence and severity of LSRs (blistering, pain, erythema, etc.) at 24 h and 7 and 14 days after treatment as well as at clinic visits beginning at visit 2/day 21. Tolerability was determined based on discontinuation of study medication because of AEs.
2.3 Statistical Analysis
Determination of the sample size per trial was based on Pearson’s χ2 test with a two-sided significance level of α = 0.05 to give ≥ 95% power to detect treatment differences in proportion of participants with complete clearance of all baseline and new molluscum lesions at the EOS visit/day 84 (the primary efficacy endpoint) for the individual trials.
All data were pooled by group (VP-102 or vehicle) from CAMP-1 and CAMP-2 for analyses that were prespecified in the statistical analysis plan. For all efficacy variables, data were summarized using descriptive statistics or counts and percentages for the ITT population. The primary endpoint (percentage of participants with complete clearance at EOS) and other binary endpoints (percentage of participants with complete clearance at days 21, 42, and 63) were tested with Pearson’s χ2 test. All statistical tests were two-sided with a significance level of α = 0.05.
Participants who did not have a status assessment of complete clearance of all treatable lesions at day 84 were considered to have missing data for the primary endpoint. Participants with missing clearance data at day 84 were considered as not having achieved complete clearance. Continuous endpoints (percentage change in lesion count from baseline) were analyzed with an analysis of covariance model. Statistical analyses were performed with the SAS statistical software package (version 9.3, SAS Institute Inc., Cary, NC, USA). Pooled safety was evaluated by severity and for total incidence by group for both TEAEs and LSRs. Baseline characteristics were compiled using the ITT population (any participant who was randomized), and safety analyses were completed using the safety population (any participant receiving at least one dose of study drug).
3 Results
3.1 Participants
A total of 529 participants were enrolled in the two trials. One subject was removed from the study after not meeting inclusion/exclusion criteria after enrollment, prior to treatment, resulting in 310 participants receiving treatment with VP-102 and 218 participants receiving vehicle (safety population). The complete participant flow CONSORT diagram with reasons for discontinuation has been previously published [21]. The overall study discontinuation rate for any reason was 6.4% in the VP-102 group and 4.6% in the vehicle group. The demographics and molluscum medical histories at baseline were similar, with no significant differences between groups in the individual trials (Table 1). The mean ± standard deviation (SD) ages were 7.5 ± 6.7 and 6.8 ± 5.8 years for the VP-102 and vehicle groups, respectively. Mean ± SD days since clinical diagnosis was 123 ± 200.9 (range 1–1247) for VP-102-treated participants and 126 ± 198.7 (range 1–1302) for the vehicle-treated participants. The proportion of all participants who had previously received one or more treatments for molluscum was 28.7% in the VP-102 and 33% in the vehicle groups. The percentage of participants with active AD (as determined by concomitant usage of topical corticosteroids, topical calcineurin inhibitors, or phosphodiesterase-4 inhibitors during the study) was 7.4% (23/310) in the VP-102 group and 9.2% (20/218) in the vehicle group. The mean ± SD number of treatable molluscum lesions present was 20.5 ± 23.1 (range 1–184) in the VP-102 group and 22.5 ± 22.3 (range 1–110) in the vehicle group.
3.2 Primary Endpoint: Percentage of Participants with Complete Clearance of Molluscum Lesions at Day 84 (End of Study)
The percentage of participants with complete clearance at EOS was 50% (155/310) with VP-102 and 15.6% (34/218) with vehicle (p < 0.0001) (Fig. 1).
Percentage of participants with complete clearance of baseline and new molluscum lesions (CAMP-1 and CAMP-2). Intent-to-treat population; VP-102-treated participants (n = 310) and vehicle-treated participants (n = 218). The percentage of participants with complete clearance was statistically significantly higher in the VP-102-treated participants starting after the first treatment (visit 2/day 21) and persisted through the end of the study (EOS visit/day 84). Participants who achieved complete clearance at earlier visits were required to be clear at the EOS visit/day 84 to be counted as achieving clearance at that visit
3.3 Secondary Endpoints: Percentage of Participants with Complete Clearance of Molluscum Lesions at Earlier Visits
Statistically significant differences in favor of VP-102 in percent of participants with complete lesion clearance were observed after a single treatment at treatment visit 2/day 21 (p = 0.0002) and for all subsequent timepoints (visit 3/day 42, visit 4/day 63, and the EOS visit/day 84; all p < 0.0001; Fig. 1).
3.4 Exploratory Endpoints: Percent Change in Lesion Counts from Baseline to Study Visits
At EOS/day 84, VP-102-treated participants experienced a statistically significant decrease in the number of baseline and treatable new lesions compared with vehicle-treated participants (− 76 vs. − 0.3%, respectively; p < 0.0001; Fig. 2). Earlier time points were also significant starting after the first treatment (visit 2/day 21 through visit 4/day 64) (Fig. 2).
Percentage change from baseline in molluscum lesion counts after treatment with VP-102 or vehicle (CAMP-1 and CAMP-2). Intent-to-treat population; VP-102-treated participants (n = 310) and vehicle-treated participants (n = 218). VP-102-treated participants had a statistically significantly larger decrease in mean percentage change in lesions from baseline visit compared with vehicle-treated participants at each visit beginning after the first treatment through the EOS visit (day 84). Overall, VP-102-treated participants had a 76% decrease in lesions whereas vehicle-treated participants had a decrease of 0.3%. EOS end of study. *p < 0.0001
3.5 Safety Outcomes
Most AEs were considered mild or moderate in severity. AEs were primarily LSR TEAEs and were expected because of the pharmacodynamic properties of cantharidin, a vesicant (Table 2). The most frequently reported TEAEs in the VP-102-treated participants were application site vesicles, pain, pruritus, erythema, and scab.
A total of 6.4% (20/311) of VP-102-treated participants and 4.6% (10/216) of vehicle-treated participants discontinued the study, with the majority of participants withdrawing due to parent/guardian request (Table 3). VP-102 was well-tolerated, as evidenced by a 1.9% (6/311) drug discontinuation rate due to AEs in the VP-102 group and 0.5% (1/216) for vehicle-treated participants [21] (Table 3). No deaths or serious AEs related to treatment occurred during the studies. More information about discontinuations and TEAEs can be found in Eichenfield et al. [21].
4 Discussion
Currently there is an unmet need for a safe and effective treatment for molluscum. Practice patterns vary widely as there is no consensus on optimal treatment [15], likely because of the lack of level 1 data [19] and FDA-approved treatment options [11, 14, 15]. Despite compounded cantharidin’s long history and widespread use, formulations can only be obtained outside the USA (which may violate US law) or through US compounding pharmacies. Compounding requires adherence to local guidance and carries uncertainties with regards to safety, efficacy, formulation stability, and concentration of the active ingredient [18]. VP-102 is a proprietary drug–device combination product with an applicator that contains a 0.7% w/v cantharidin formulation manufactured under good manufacturing practice conditions along with visualization and bittering agents. The formulation is contained in a single-use applicator with a 1-mm tip to ensure precision application. The cantharidin used in VP-102 is ˃ 99% pure. VP-102 was used in these two large phase III trials with a consistent wash-off time and application schedule.
Pooling analyses increases sample size and statistical power. This is important when comparing treatments, especially in populations with a potential for high variability in baseline characteristics. Pooling data also assists practitioners in understanding expected outcomes of a treatment for their patients in a larger cohort [22]. The pooled analyses from CAMP-1 and CAMP-2 demonstrated that the treatment of molluscum with VP-102 resulted in a statistically significantly higher percentage of participants with complete clearance of lesions and a higher percentage reduction in lesions over time than vehicle treatment. In addition, the pooled efficacy outcomes were similar to those in the individual trials [21].
Complete clearance of lesions in the vehicle-treated participants was consistent with findings in large-scale molluscum trials of similar duration [23]. The low clearance rate in the vehicle-treated participants (15.6%) was consistent with the prolonged length of time molluscum can take to resolve without intervention. This clearance rate also rationalizes the need for treatment to reduce autoinoculation, spread to others, and potential pain and infection that has been documented in patients with molluscum. The vehicle was not expected to have a therapeutic effect on study participants because of the low amount of gentian violet in the solution, the limited amount of time the flexible film was present on the skin, and lack of active ingredient in the solution [21]. The vehicle did not contain cantharidin, but vehicle-treated participants has an 29.2% incidence of blistering. This phenomenon has been observed by other studies using cantharidin [24], and it is hypothesized that the medication’s film on a lesion could be misconstrued for a popped blister. In addition, inflamed lesions may appear similar to blisters.
Cantharidin’s pharmacodynamic action as a vesicant results in anticipated LSRs such as vesicles, erythema, pain, and scab in those participants treated with VP-102. Application site reactions were primarily mild or moderate in intensity. LSRs were also noted in the vehicle-treated participants, suggesting that these side effects may be part of the natural progression of molluscum.
VP-102 was well-tolerated in both trials, as demonstrated by the rate of discontinuation of study medication due to AEs in this largely pediatric population where all molluscum lesions present were treated at each visit. The overall discontinuation rate of the study was also low, suggesting a patient/caregiver population that was highly motivated to treat molluscum.
The studies had several limitations that are mentioned in detail in Eichenfield et al. [21] and summarized here. Individual molluscum lesions were not tracked, as only a total body lesion count was used, so the number of treatments needed for clearance per lesion is unknown. Given that new lesions can develop 2–8 weeks after exposure, autoinoculation was likely to occur in our study population during the trial. Lesions could have developed later in the trial or after treatment was completed but before the EOS visit, which could influence the status of complete clearance. While cantharidin is commonly administered until the patient is clear of all lesions, this study limited the number of treatments to a maximum of four. The studies did not include participants who had lesions on mucosal openings, which could limit the extrapolation of data to patients with molluscum with sexually transmitted disease. Most participants were Caucasian, potentially limiting generalizability to other ethnicities. Finally, most participants in the studies were aged < 18 years, requiring further studies in adults before strong conclusions can be made for this population.
5 Conclusion
The CAMP trials were the first large vehicle-controlled studies using VP-102, a drug–device combination product containing a consistent formulation of cantharidin (0.7% w/v) in an applicator that allows for precise treatment for molluscum lesions through a uniform application and dosing schedule. The pooled results of the CAMP studies provide robust efficacy and safety data that support the use of VP-102 for the treatment of molluscum in patients aged ≥2 years and may assist healthcare professionals and caregivers with expectations of treatment outcomes.
References
Bugert J. Genus molluscipoxvirus. Poxviruses Birkhäuser advances in infectious diseases: Birkhauser Basel; 2007. pp. 89–112.
Hanna D, Hatami A, Powell J, Marcoux D, Maari C, Savard P, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23(6):574–9.
Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130–6.
Leung AKC. The natural history of molluscum contagiosum in children. Lancet Infect Dis. 2015;15(2):136–7.
Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Investig Dermatol. 2014;134(6):1527–34.
Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201–52.
Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13(10):877–88.
Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102(3):191–4.
Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43(3):503–7.
Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104(5):301–5 (E1; E2).
Hughes CM, Damon IK, Reynolds MG. Understanding U.S. healthcare providers’ practices and experiences with molluscum contagiosum. PLoS ONE. 2013;8(10):e76948.
Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5(8):505–12.
Olsen JR, Gallacher J, Finlay AY, Piguet V, Francis NA. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15(2):190–5.
van der Wouden JC, van der Sande R, Kruithof EJ, Sollie A, van der Suijlekom-Smit LW, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017;5:CD004767.
Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34(5):504–15.
Guzman AK, Schairer DO, Garelik JL, Cohen SR. Safety and efficacy of topical cantharidin for the treatment of pediatric molluscum contagiosum: a prospective, randomized, double-blind, placebo-controlled pilot trial. Int J Dermatol. 2018;57(8):1001–6.
Cathcart S, Coloe J, Morrell DS. Parental satisfaction, efficacy, and adverse events in 54 patients treated with cantharidin for molluscum contagiosum infection. Clin Pediatr (Phila). 2009;48(2):161–5.
Del Rosso JQ, Kircik L. Topical cantharidin in the management of molluscum contagiosum: preliminary assessment of an ether-free, pharmaceutical-grade formulation. J Clin Aesthet Dermatol. 2019;12(2):27–30.
Pompei DT, Rezzadeh KS, Viola KV, Lee DH, Schairer DO, Chismar LA, et al. Cantharidin therapy: practice patterns and attitudes of health care providers. J Am Acad Dermatol. 2013;68(6):1045–6.
Agnetta V, Torres A, Desai S, Hebert A, Kircik L. Compounding in dermatology update. J Drugs Dermatol. 2020;19(2):JOS0220.
Eichenfield LF, McFalda W, Brabec B, Siegfried E, Kwong P, McBride M, Rieger J, Willson C, Davidson M, Burnett P. Safety and efficacy of VP-102, a proprietary drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized, double-blind, vehicle-controlled trials. JAMA Dermatol. 2020. https://doi.org/10.1001/jamadermatol.2020.3238.
van der Steen JT, Kruse RL, Szafara KL, Mehr DR, van der Wal G, Ribbe MW, et al. Benefits and pitfalls of pooling datasets from comparable observational studies: combining US and Dutch nursing home studies. Palliat Med. 2008;22(6):750–9.
Hebert AA, Siegfried EC, Durham T, de Leon EN, Reams T, Messersmith E, et al. Efficacy and tolerability of an investigational nitric oxide-releasing topical gel in patients with molluscum contagiosum: a randomized clinical trial. J Am Acad Dermatol. 2020;82(4):887–94.
Coloe Dosal J, Stewart PW, Lin JA, Williams CS, Morrell DS. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31(4):440–9.
Acknowledgements
The authors thank all the investigators and the participants and their caregivers who made these studies possible. We also express appreciation to Dr. Jessica McLin from Versant Learning Solutions for her graphical assistance and to Dr. Jennifer Andres from Verrica Pharmaceuticals Inc. for her work with editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Trials were supported by Verrica Pharmaceuticals Inc.
Conflict of interest
Lawrence F. Eichenfield, MD, received funds for research to his institution as a study center and consulting fees and stock options from Verrica Pharmaceuticals Inc. He is also on the board of directors for Verrica Pharmaceuticals Inc. Elaine Siegfried, MD, received funds for research to her institution as a study center and consulting fees from Verrica Pharmaceuticals Inc. She has also received consulting fees and funds as a research center for Novan. Pearl Kwong, MD, received funds for research as a study center and consulting fees from Verrica Pharmaceuticals Inc. Mark McBride, PhD, was paid as a consultant to run analyses on the statistics for the study by Verrica Pharmaceuticals Inc. Dr. McBride is also a consultant for Novan. Jayson Rieger, PhD, was an employee of Verrica Pharmaceuticals, holds company stock from Verrica Pharmaceuticals Inc., and holds a patent related to the work. David Glover, PhD, is an employee of PBM Capital Group. Cynthia Willson, RN, BSN, is an employee of Verrica Pharmaceuticals and holds company stock. Matthew Davidson, PhD, was an employee of, received personal fees from, and holds stock of Verrica Pharmaceuticals Inc. He also holds patents related to the research. Patrick Burnett, MD, PhD, was an employee of Verrica Pharmaceuticals and holds company stock. Melissa Olivadoti, PhD, is an employee of Verrica Pharmaceuticals.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Conceptualization: MD, JR, ES, CW, DG. Methodology: MD, JR, MM, DG. Formal analysis and investigation: MD, JR, MM, PB, CW, LE, ES, PK. Writing—original draft preparation: MO. Writing—review and editing: JR, MD, MM, PB, CW, LE, ES, PK, DG, MO. Funding acquisition: JR, MD. Supervision: PB, CW, JR, DG, MD.
Ethics approval
Local institutional review boards or ethics committees at each trial center approved the protocol and consent form, oversaw trial conduct, and maintained documentation.
Consent to participate
Written informed consent was obtained from the 17 adult participants and the parents or guardians of participants aged < 18 years as per the local state regulations.
Consent for publication
Not applicable as no patient-related data were used.
Additional information
Digital features
This article is published with digital features, including a video abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13117736.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eichenfield, L.F., Siegfried, E., Kwong, P. et al. Pooled Results of Two Randomized Phase III Trials Evaluating VP-102, a Drug-Device Combination Product Containing Cantharidin 0.7% (w/v) for the Treatment of Molluscum Contagiosum. Am J Clin Dermatol 22, 257–265 (2021). https://doi.org/10.1007/s40257-020-00570-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-020-00570-8