Abstract
Introduction
Verrucae vulgaris, or common warts, is a common skin condition for which there is no US Food and Drug Administration-approved treatment. Compounded cantharidin has been used to treat warts for years but lacks a controlled formulation, consistent application schedule and methods, and robust safety and efficacy studies. VP-102 is a proprietary drug-device combination product containing a topical formulation of 0.7% (w/v) cantharidin in a single-use delivery device. This objective of the phase 2 study was to evaluate the efficacy, safety, tolerability, and optimal regimen of VP-102 in the treatment of common warts.
Methods
In this open-label trial, participants aged ≥ 2 years with one to six common warts were administered VP-102 topically to treatable common warts once every 14 days (Cohort 1) or once every 21 days in conjunction with paring (Cohort 2), for up to four treatments. Participants were evaluated through to day 84 (Cohort 1) or day 147 (Cohort 2). The primary endpoint was the percentage of participants with complete clearance of all treatable common warts (baseline and new) at day 84. Secondary endpoints included percentage of participants achieving complete clearance of all treatable common warts at other visits. Safety assessments included treatment-emergent adverse events (TEAEs), including local skin reactions (LSRs).
Results
A total of 21 and 35 participants were enrolled in Cohort 1 and Cohort 2, respectively. Complete clearance at day 84 was seen in 19.0% of participants in Cohort 1 and 51.4% of those in Cohort 2. The most common TEAEs were expected LSRs and included application site vesicles, pain, pruritus, erythema, and scab. Most LSRs were mild or moderate in severity.
Conclusion
VP-102 showed efficacy in complete clearance of common warts from baseline to day 84, as well as at follow-up visits. Due to the higher percentage of patients exhibiting complete clearance in Cohort 2, the treatment regimen of Cohort 2 will be pursued in future studies. TEAEs were expected due to the pharmacodynamic action of cantharidin, a vesicant.
Clinical Trials ID: NCT03487549
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Verrucae vulgaris, or common warts, is a common skin condition with no US Food and Drug Administration-approved treatment. |
VP-102 is a proprietary drug-device combination product containing a topical formulation of cantharidin (0.7% w/v) in a proprietary single-use applicator that is under investigation for the treatment of common warts. |
VP-102 under occlusion showed efficacy in complete clearance of common warts from baseline to day 84, as well as at follow-up visits out to day 147. The most common adverse effects were expected, including application site reactions, and were mild to moderate in severity. |
These positive findings warrant future trials to establish the safety and efficacy of VP-102 under occlusion in a larger population of patients with common warts. |
Introduction
Verrucae vulgaris, also called cutaneous viral warts or common warts, are a common benign condition. Common warts affect 10–20% of school-aged children and are especially common in adolescents, with a peak incidence at age 12–16 years [1].
Common warts are caused by the human papillomavirus (HPV). They are contagious and can be spread through direct skin-to-skin contact from person-to-person, autoinoculation, or indirect contact, especially if the skin surface is disrupted through abrasions or infection [2]. Common warts can be found on any skin surface, although they most frequently occur on sites subject to trauma, such as the lower and upper extremities. Generally, common warts appear as a papular growth with an irregular contour and surface. Warts can cause cosmetic disfigurement and discomfort, as well as alter activities of the sufferer due to embarrassment, pain, and discomfort [3].
Most immunocompetent individuals have resolution of common warts within a 24-month timeframe; however for some, common warts may take longer to resolve [4]. Additionally, any time warts are present there is a risk of enlargement and spread.
Numerous treatments have been utilized for common warts; however, none are US Food and Drug Administration (FDA)-approved, and no recent treatment guidelines exist. In a 2012 Cochrane review, no level I recommendations exist for the treatment of common warts [5]. Cure rates of common warts range from 30 to 58% with treatment of duct tape, cryotherapy, salicylic acid, topical 5-fluorouracil, and combined therapy with salicylic acid and cryotherapy [6]. For many dermatologists, cantharidin has been a common choice for the treatment of common warts [7]. Cantharidin is a vesicant that results in blistering of the skin when applied topically, recruiting immune factors and cells to the area and sloughing of the treated skin with the healing process [8]. Currently, cantharidin must be compounded, formulations may vary in concentration, and there is a lack of shelf stability due to the types of containers used; taken together, these factors can result in variable safety and efficacy [9]. Historical use of cantharidin monotherapy for wart treatment recommended occluding treated areas with a bandage due to the hyperkeratotic pathology of the lesions. Occlusion has been used with the goal of increasing the absorption of canthairidin through the skin, improving efficacy of blistering, and reducing transfer of cantharidin to healthy skin [7, 10,11,12]. However, the optimal duration of occlusion and the pharmacodynamics of the use of occlusion in the topical treatment with cantharidin are still unknown.
There have been no large-scale, standardized clinical trials of cantharidin to assess its efficacy and safety in the treatment of common warts. Only four wart studies have been published evaluating cantharidin monotherapy [7, 11,12,13]. These studies treat different types of warts and have vast differences in methodology. Variations include the amount of time between applications, duration of exposure, application methods, and the formulations of cantharidin used [11,12,13,14]. Studies using cantharidin for the treatment of plantar warts often combine the drug with other agents, including podophyllotoxin and/or salicylic acid [15,16,17,18,19]. Due to the variations in study methodology, formulations, and different types of warts treated, it is not possible to draw strong conclusions on the best protocol for cantharidin treatment of common warts [20].
VP-102 is a controlled, shelf-stable drug-device combination product with a topical solution that contains cantharidin (0.7% w/v) manufactured according to Current Good Manufacturing Practices. VP-102 has proven efficacy and safety in two phase 3 trials for the treatment of molluscum contagiosum in participants aged ≥ 2 years [21, 22]. The mechanism of action of VP-102 is unknown; however, the pharmacodynamic action of the active ingredient in VP-102 (cantharidin) is a vesicant [14]. The aim of this phase 2 was to establish the safety, efficacy, and optimal dosing schedule of VP-102 under occlusion in participants aged ≥ 2 years for the treatment of common warts.
Methods
Cantharidin and Occlusion in Verruca Epithelium (COVE-1) was an open-label phase 2 clinical study. The study followed Good Clinical Practice and country-specific laws and regulations. Compliance also conformed with US federal regulatory codes, the Nuremberg Code, and the Declaration of Helsinki [23]. The Copernicus Group Independent Review Board approved the protocol and consent form, oversaw trial conduct, and maintained documentation. Written informed consent/assent was obtained from all adult participants and the parents or guardians of participants younger than 18 years as per the local state regulations.
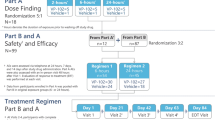
Eligible patients were required to have one to six common warts (excluding genital, palmar/plantar, and subungual warts) measuring ≤ 10 mm in diameter and ≤ 3 mm in height and deemed based upon the clinical judgment of the investigator to be consistent with the inclusion and exclusion criteria of the trial. Pre-study screening for eligibility, provision of informed consent and assent (when applicable), physical exam, and collection of data on demographics, prior and current concomitant medications, and medical history were completed at a baseline visit within 14 days of the first treatment visit. Participants could not have received any type of treatment for common warts within the 14 days prior to the first treatment of the study drug. All participants received topical treatment with approximately 10–20 μL of solution from a VP-102 applicator to all treatable common warts as well as 1–2 mm of surrounding healthy skin, which were then occluded with 3M™ Blenderm™ tape (3M Co., St. Paul, MN, USA). Participants were instructed to slowly remove the tape to prevent unroofing any blister present and wash off VP-102 with soap and water 24 h following treatment. The participant could remove the tape and study drug from an individual common wart without a protocol violation if significant blistering, pain or treatment-emergent adverse events (TEAEs) occurred prior to the 24-h time point. Participants were enrolled into two separate cohorts which varied in treatment schedule and technique. An infographic of the study protocol can be found in Fig. 1. A wart count was taken at each clinic visit and could include common warts present at baseline and any new common warts that appeared during the study and during a follow-up period (if applicable).
Treatment methods for cohorts 1 and 2, including study drug application at study visits and ERT follow-up. Single asterisk indicates that the minimum interval between treatments was 14 days but it could be longer depending on clinical response. Two asterisks indicate that the drug and tape were removed 24 h post-treatment. The dagger symbol indicates that wart paring was performed at any treatment visit when an adherent thick scale was present and the investigator considered it safe to apply. EOS End of study visit, EOT end of treatment visit, ERT evaluation of response to treatment, LSR local skin reaction
The study included two cohorts with different application schedules and methods. Cohort 1 included participants aged ≥ 2 years who were treated with VP-102 until all treatable common warts were clear at each treatment visit every 14 days (visit 1/day 1, visit 2/day 14, visit 3/day 28, and visit 4/day 42), or a maximum of four treatments. An extended treatment interval of > 14 days between treatments could be used if a participant experienced persistent local skin reactions (LSRs) at the study visit. Paring of common warts was not utilized in this cohort. Participants in Cohort 1 were assessed for efficacy and safety at day 84, the end-of-study (EOS) visit.
Cohort 2 included participants aged ≥ 12 years who were treated with VP-102 every 21 days (visit 1/day 1, visit 2/day 21, visit 3/day 42, and visit 4/day 63) for up to four treatments. One additional treatment with VP-102 was applied to a treated area after clearance if clearance occurred prior to four treatments. If adherent scale was present, trained practitioners pared common warts prior to the application of VP-102. Participants in Cohort 2 also had an end-of-treatment (EOT) visit at day 84 with follow-up visits at days 105 and 126, and an EOS visit at day 147.
In both cohorts, the primary efficacy endpoint included the proportion of participants with complete clearance of all treatable common warts (baseline and new) at the day 84 EOT/EOS visit. Primary safety outcomes included incidence of adverse events, physician examinations, concomitant medications, and LSRs. An evaluation of response to treatment (ERT) occurred during treatment visits and via the telephone for both cohorts at 24 h and 7 days after treatment was administered. Clinical response to the treatment of warts was evaluated at each scheduled in-person visit until EOS by counting all remaining warts.
For Cohort 1, the secondary endpoints were to evaluate the efficacy of VP-102 by assessing the change from baseline in the number of treatable common warts (baseline and new) at the EOS visit (day 84), to evaluate the efficacy of VP-102 by assessing the change from baseline in the percentage of clearance of treatable common warts (baseline and new) at the EOS visit (day 84), and to evaluate the efficacy of VP-102 by assessing the proportion of participants exhibiting complete clearance of all treatable common warts (baseline and new) at earlier visits (visit 2/day 14, visit 3/day 28, visit 4/day 42). Exploratory endpoints included percentage change in common warts compared to baseline by visit.
For Cohort 2, the secondary endpoints were to evaluate the efficacy of VP-102 by assessing the change from baseline in the number of treatable common warts (baseline and new) at the EOT visit (day 84), to evaluate the efficacy of VP-102 by assessing the change from baseline in the percentage of clearance of treatable common warts (baseline and new) at the EOT visit (day 84), and to evaluate the efficacy of VP-102 by assessing the proportion of participants exhibiting complete clearance of all treatable common warts (baseline and new) at visit 2/day 21, treatment visit 3/day 42, and visit 4/day 63. Select exploratory endpoints were to evaluate the efficacy of VP-102 by assessing the proportion of participants exhibiting complete clearance of all treatable common warts at follow-up visits on day 105, day 126, and the EOS visit (day 147) as well as the percentage change in common warts compared to baseline at each visit.
Statistics
Although no formal power calculations were performed, it was estimated that a sample size of 20 subjects (Cohort 1) and 35 subjects (Cohort 2) evaluable at the EOS/EOT visit (day 84 for both cohorts) would be informative regarding common wart clearance rates and build a safety profile for VP-102 in common warts. Efficacy outcomes analyses were completed using the intent-to-treat population, which included any participant who was randomized to treatment. Safety and baseline demographic and disease state outcomes were completed using the safety population, which included any participant who received at least one treatment of VP-102. In calculations of complete clearance, participants with missing data on clearance at any time point were considered not completely clear at that visit. Last observation carried forward was used to calculate percentage change of common warts compared to baseline, change from baseline in treatable common warts, and proportion of participants achieving ≥ 50% clearance. Data were summarized using descriptive statistics (sample size, mean, median, standard deviation, minimum, maximum) for continuous variables and frequencies and percentages for discrete variables.
Results
Demographics
The demographics of the participants in this study are given in Table 1. Participants in Cohort 1 (n = 21) had a mean age of 37.9 (range 7–83) years, with a mean wart count of 2.2 (range 1–6) at baseline. Cohort 2 included 35 participants with a mean age of 37.6 (range 12–67) years with a mean wart count of 1.6 (1–5) at baseline. Participant gender was relatively balanced between male and female in both cohorts, with slightly more females in Cohort 2 (52.4 males and 62.9% females in Cohort 1 and 2, respectively).
Full baseline demographics and wart characteristics are given in Table 1.
Efficacy Outcomes
A total of 19.0% (4/21) of Cohort 1 participants and 51.4% (18/35) of Cohort 2 participants had complete clearance of all treatable common warts at the day 84/EOS/EOT visit. In Cohort 2, 40.0% (14/35) of participants maintained clearance for common warts that were treated during the study through to the EOS visit (day 147; Fig. 2).
Percentage of VP-102-treated participants with complete clearance of all common warts (intent-to-treat population). Cohort 1 shows clearance of all treatable common warts in 19.0% of participants at day 84. Cohort 2 shows clearance of all treatable common warts in 51.4% of participants at day 84, with sustained clearance in 40.0% of participants through to day 147. In Cohort 2, two subjects discontinued the study after day 84
The percentage change in number of common warts from baseline to day 84 was a reduction of 43.5% for Cohort 1 (EOS) and 50.9% for Cohort 2 (EOT). Participants in Cohort 2 showed a 45.5% decrease in treated common warts from baseline to the EOS visit (day 147; Fig. 3).
Percentage change in number of common warts from baseline in VP-102-treated participants (intent-to-treat population). A 43.5% reduction in treatable common warts was seen in Cohort 1 at day 84. In Cohort 2, a 50.9% reduction in treatable common warts was seen at day 84, with a 45.5% reduction seen at day 147
Safety Outcomes
For all enrolled participants, 81.0% (17/21) of Cohort 1 participants and 94.3% (33/35) of Cohort 2 participants completed the study. A total of 19.0% (4/21) of participants discontinued in Cohort 1 and 5.7% (2/34) of participants discontinued in Cohort 2. Overall, TEAEs were reported in 95.2% (20/21) of Cohort 1 participants and 94.1% (32/34) of Cohort 2 participants.
Most TEAEs were LSRs, which were expected as part of the study treatment regimen. Non-LSR TEAEs were not deemed related to study drug except for the occurrence of papilloma viral infection, also defined as “ring warts,” in 8.8% of participants in Cohort 2. The most frequently reported LSR TEAEs were vesicles, pain, erythema, pruritus, and scab at the application site. Most TEAEs were reported as mild or moderate; only two participants in Cohort 1 and four participants in Cohort 2 reported severe TEAEs that were LSRs, including application site pain, discoloration, edema, vesicles, erythema, and scab. No serious LSRs occurred during the trial. A full listing of the incidence and severity of TEAEs that occurred in > 2% of participants are shown in Table 2. There were no incidences of death in the studies. There were no TEAEs related to treatment that led to study or drug discontinuation.
Discussion
Common warts are a frequent health concern, yet there is no FDA-approved treatment available. Currently, available treatments vary in their efficacy, and some can be painful. Cantharidin has been used for decades for the treatment of common warts, yet its use is limited due to concerns over safety issues, inconsistencies in application and concentration, and problems with access [9, 12].
Published reports state that common warts which occur in adults and are recalcitrant to prior treatments are not likely to resolve spontaneously. Common warts with a duration of > 2 years are more difficult to clear [4]. Participants in both cohorts had an average duration of warts of > 4 years, with a clinical diagnosis of warts for > 2 years, and many had unsuccessfully previously treated their warts before entering the study. We hypothesize that these characteristics suggest a potentially recalcitrant wart population, which further strengthens the positive efficacy results and reinforces the potential for this treatment in this disease state.
Complete clearance of baseline warts and all new treatable common warts occurred more frequently in participants in Cohort 2, who utilized a 21-day VP-102 treatment interval along with paring. In COVE-1, wart clearance reported by participants in Cohort 2 at day 84 was 51.4%, which is similar to wart clearances reported in the literature with various treatment modalities [6], and these outcomes were well maintained to EOS for treated common warts at day 147 with 40.0% clearance. Percentage change in common warts was − 50.9% for Cohort 2, which was relatively sustained in treated common warts up to EOS (day 147) (− 45.5%). The enduring efficacy data for both complete clearance and percentage change in common warts is a promising finding for treatment with VP-102 and warrants further research.
The reason for why there was higher percentages of clearance of new and baseline warts in Cohort 2 than Cohort 1 is not known with certainty. One possibility is that the treatment for Cohort 2 included the use of paring, which likely would have improved the penetration and absorption of topical VP-102. Another possibility is that the longer duration between treatments in Cohort 2 (21 vs. 14 days) allowed for greater healing and disappearance of local skin reactions, such as reddening, following the previous treatment, making it easier to determine, with greater confidence, that there was complete clearance of the wart.
Most participants in both cohorts reported at least one LSR TEAE. Consistent with VP-102 treatment experience in previous clinical trials, the most frequently reported LSR TEAEs included vesicles, pain, erythema, pruritus, and scab at the application site [21, 22]. The most common LSRs were expected due to the pharmacodynamic properties of cantharidin, which is a vesicant. Ring wart appearance has been documented in patients treated with destructive therapies, such as cryotherapy and cantharidin [7, 11]; however, in this study only 3 (8.8%) participants in Cohort 2 experienced ring warts, suggesting a low incidence with VP-102. Despite the high incidence of application site AEs, no participants discontinued treatment due to TEAEs, which suggests that treatment of common warts with VP-102 was well-tolerated.
Limitations
The study, which was also open-label, included a small number of participants and did not include a vehicle group for comparison. The study did not include a group of participants treated with VP-102 without occlusion, thus it is not possible to know the effects of VP-102 on common warts alone and the incremental benefit that may be provided by occlusion.
Common warts may have received a different number of treatments depending on when they appeared (e.g., present at baseline vs. emerging during the study). Thus, it would be difficult to determine precisely how many treatments are necessary to clear an individual wart. It is possible that recurrence of common warts could occur during the study given that HPV can lay latent in the skin and appear at any time [24], which could potentially explain the reduction in the percentage of participants with complete clearance during the follow-up visits of Cohort 2.
Conclusion
This phase 2 clinical trial demonstrated the safety and efficacy of treatment of common warts with VP-102 in two treatment cohorts. Treatment with VP-102 was efficacious, as demonstrated by the rates of complete clearance of common warts as well as a reduction of the percentage of common warts from baseline to the EOS visit for both cohorts. The most common TEAEs were mild to moderate and included application site vesicles, pain, pruritus, erythema, and scab, which were considered related to the pharmacodynamic action of cantharidin. Due to the higher complete clearance rate of common warts observed in Cohort 2 (51.4% complete clearance at day 84), the treatment regimen and methodology of Cohort 2 will be utilized in future studies. These positive findings warrant future trials to establish the safety and efficacy of VP-102 in a larger population of patients with common warts.
References
Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20(3):268–71.
Stulberg DL, Hutchinson AG. Molluscum contagiosum and warts. Am Fam Physician. 2003;67(6):1233–40.
Ciconte A, Campbell J, Tabrizi S, Garland S, Marks R. Warts are not merely blemishes on the skin: a study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol. 2003;44(3):169–73.
Sterling JC, Handfield-Jones S, Hudson PM, British Association of Dermatology. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144(1):4–11.
Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2012;9:CD001781.
Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165(2):233–46.
Epstein WL, Kligman AM. Treatment of warts with cantharidin. AMA Arch Derm. 1958;77(5):508–11.
Day R, Harbord M, Forbes A, Segal A. Cantharidin blisters: a technique for investigating leukocyte trafficking and cytokine production at sites of inflammation in humans. J Immunol Methods. 2001;257:213–20.
Del Rosso JQ, Kircik L. Topical cantharidin in the management of molluscum contagiosum: preliminary assessment of an ether-free, pharmaceutical-grade formulation. J Clin Aesthet Dermatol. 2019;12(2):27–30.
Panzer HM. Cantharidin—a useful agent in the local treatment of warts. J Germantown Hosp. 1961;2:82–6.
Rosenberg EW, Amonette RA, Gardner JH. Cantharidin treatment of warts at home. Arch Dermatol. 1977;113(8):1134.
Epstein JH, Epstein WL. Cantharidin treatment of digital and periungual warts. Calif Med. 1960;93:11–2.
Kartal Durmazlar SP, Atacan D, Eskioglu F. Cantharidin treatment for recalcitrant facial flat warts: a preliminary study. J Dermatol Treat. 2009;20:114–9.
Bock RH. Treatment of palpebral warts with cantharon. Am J Ophthalmol. 1965;60(3):529–30.
Becerro de Bengoa Vallejo R, Losa Iglesias ME, Gómez-Martín B, Sánchez Gómez R, Sáez CA. Application of cantharidin and podophyllotoxin for the treatment of plantar warts. J Am Podiatr Med Assoc. 2008;98(6):445–50.
Coskey RJ. Treatment of plantar warts in children with a salicylic acid-podophyllin-cantharidin product. Pediatr Dermatol. 1984;2(1):71–3.
Ghonemy S. Treatment of recalcitrant plantar warts with long-pulsed Nd:YAG laser versus cantharidin-podophylline resin-salicylic acid. J Cosmet Laser Therapy. 2017;19(6):347–52.
Kaçar N, Taşlı L, Korkmaz S, Ergin S, Erdoğan B. Cantharidin-podophylotoxin-salicylic acid versus cryotherapy in the treatment of plantar warts: a randomized prospective study. J Eur Acad Dermatol Venereol. 2012;26(7):889–93.
López-López D, Agrasar-Cruz C, Bautista-Casasnovas A, Álvarez-Castro CJ. Application of cantharidin, podophyllotoxin, and salicylic acid in recalcitrant plantar warts. A preliminary study. Gac Med Mex. 2015;151(1):14–9.
Sterling JC, Gibbs S, Haque Hussain SS, Mohd Mustapa MF, Handfield-Jones SE. British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol. 2014;171(4):696–712.
Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156(12):1315–23.
Eichenfield LF, Siegfried E, Kwong P, et al. Pooled results of two randomized phase III trials evaluating VP-102, a drug-device combination product containing cantharidin 0.7% (w/v) for the treatment of molluscum contagiosum. Am J Clin Dermatol. 2021;22:257–65. https://doi.org/10.1007/s40257-020-00570-8.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Leman JA, Benton EC. Verrucas. Guidelines for management. Am J Clin Dermatol. 2000;1(3):143–9.
Acknowledgements
We thank the clinical researchers, support staff, and participants of the study.
Funding
Verrica Pharmaceuticals Inc. provided funding and support for the trials and investigational agent (VP-102), as well as funding for the drafting of the manuscript, rapid service fee, and creation of the figures. Verrica Pharmaceuticals Inc. sponsored the design and conduct of the studies; collection, management, analysis, and interpretation of the data; as well as coordinating the preparation, review, and approval of the manuscript.
Medical Writing and/or editorial assistance
Medical writing assistance was provided by Dr. Jennifer Andres and editorial assistance was provided by Dr. Christine Crosby, both of whom are employees of Verrica Pharmaceuticals Inc. Figures were created and provided by Versant Learning Solutions and funded by Verrica Pharmaceuticals Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Concept and design: C Willson, P Burnett, DK Glover, J Rieger. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: C Willson, P Burnett. Obtained funding: C Willson, P Burnett. Administrative, technical, or material support: all Authors. Supervision: all Authors. List of investigators: S Guenthner, W McFalda, P Kwong, A Mabry.
Disclosures
S Guenthner, W McFalda, and K Eads received funding to their institutions as clinical trial sites for this research from Verrica Pharmaceuticals Inc. P Kwong received funds to her institution as a clinical trial site and has also received honoraria for consulting and advisory boards from Verrica Pharmaceuticals Inc. M McCafferty is a contractor for Verrica Pharmaceuticals Inc. DK Glover and J Rieger are employees of PBM Capital Group, and P Burnett and M Olivadoti were employees of Verrica Pharmaceuticals Inc. at the time of writing the manuscript. P Burnett is now an employee of Arcutis Biotherapeutics and C Willson is a current employee of Verrica Pharmaceuticals Inc.
Compliance with Ethics Guidelines
The study followed Good Clinical Practice and country-specific laws and regulations. Compliance also conformed with US federal regulatory codes, the Nuremberg Code, and the Declaration of Helsinki. The Copernicus Group Independent Review Board approved the protocol and consent form, oversaw trial conduct, and maintained documentation. Written informed consent/assent was obtained from all adult participants and the parents or guardians of participants younger than 18 years as per the local state regulations.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available as they are proprietary and under review with the FDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Guenthner, S., McFalda, W., Kwong, P. et al. COVE-1: A Phase 2, Open-Label Study to Evaluate Efficacy and Safety and the Optimal Regimen of VP-102, a Proprietary Drug–Device Product Containing Topical Cantharidin (0.7% w/v) Under Occlusion for the Treatment of Common Warts. Dermatol Ther (Heidelb) 11, 1623–1634 (2021). https://doi.org/10.1007/s13555-021-00576-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-021-00576-y