Abstract
Background
This was an open-label, phase I, nonrandomized, single-sequence, crossover study to evaluate the effect of concomitant administration of multiple doses of clarithromycin on the single-dose exposure, safety, and tolerability of apixaban in healthy subjects.
Methods
In total, 19 subjects received a single oral dose of apixaban 10 mg on day 1. On day 4, subjects began receiving oral clarithromycin immediate release (IR) 500 mg twice daily (bid) for 4 days. On day 8, subjects received oral apixaban 10 mg and oral clarithromycin IR 500 mg bid. Oral clarithromycin IR 500 mg bid was given alone on days 9 and 10.
Results
Compared with apixaban alone, coadministration of apixaban with clarithromycin resulted in increased apixaban exposure. The adjusted geometric mean ratio (GMR) was 1.299 (90% confidence interval [CI] 1.220–1.384) for peak plasma concentration (Cmax), whereas the adjusted GMR for the area under the concentration curve (AUC(INF)) was 1.595 (90% CI 1.506–1.698). The mean half-life and median time to Cmax of apixaban were comparable with and without concomitant administration of clarithromycin. Administration of apixaban and clarithromycin concomitantly did not result in increased adverse events compared with administration of either agent alone. All adverse events were mild in intensity.
Conclusions
Apixaban Cmax and AUC(INF) increased 30% and 60%, respectively, when multiple doses of clarithromycin were coadministered with apixaban versus administration of apixaban alone. The increase in apixaban Cmax and AUC(INF) with concomitant clarithromycin was less than that which has been observed when apixaban was given with ketoconazole. Administration of apixaban alone and in combination with clarithromycin bid was safe and generally well-tolerated by the healthy adult subjects in this study.
Clinical Trial Registration
ClinicalTrials.gov identifier number NCT02912234.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Apixaban peak plasma concentration (Cmax) and area under the concentration curve in the presence of clarithromycin increased to a lesser extent than coadministration of ketoconazole, i.e., 30% and 60%, respectively; time to Cmax and elimination half-life were unchanged. |
Apixaban 10 mg alone and in combination with twice-daily clarithromycin immediate release 500 mg was safe and generally well-tolerated by healthy adult volunteers. |
1 Introduction
Apixaban is an oral, selective, direct-reversible inhibitor of coagulation factor Xa that is approved in a number of countries for thromboprophylaxis in patients who have undergone elective hip or knee replacement surgery [1,2,3], for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation [4, 5], for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and to reduce the risk of recurrent DVT and PE following initial therapy [6, 7].
Apixaban pharmacokinetics are characterized by an oral bioavailability of ∼ 50%, linear pharmacokinetics, dose proportionality across the clinically relevant dose range, and no clinically significant food effect. Apixaban is eliminated by renal and nonrenal mechanisms, including metabolism, biliary excretion, and direct intestinal excretion. It has a half-life of 12 h, and renal clearance accounts for ∼ 27% of total systemic clearance [8,9,10,11,12]. Apixaban is a substrate for P‐glycoprotein (P‐gp) and breast cancer resistance protein and is predominantly metabolized by cytochrome P450 3A4 (CYP3A4), with minor contributions from CYP1A2, 2C8, 2C9, 2C19, and 2J2 [13, 14]. Its metabolism is also characterized by subsequent sulfation of oxidative metabolites by sulfotransferases [13, 14]. Because of the multiple elimination pathways for apixaban, concomitant administration of medications expected to classically interact with it have limited impact on apixaban exposure. In addition, the impact of mild-to-moderate renal impairment is also minimized by the availability of multiple metabolic pathways. Exposure approximately doubled when apixaban was coadministered with ketoconazole (a strong inhibitor of both CYP3A4 and P‐gp), compared with administration of apixaban alone [15]. A 50% decrease in exposure was observed after coadministration with rifampin, a strong inducer of both CYP3A4 and P‐gp [10] compared with administration of apixaban alone.
Regulatory bodies consider both clarithromycin and ketoconazole to be strong inhibitors of both CYP3A4 and P-gp, but in vivo and in vitro data demonstrate that the inhibitory effect of clarithromycin is generally lower than that of ketoconazole. Such contrasts between the effects of two inhibitory medications underline the importance of clinical interaction studies. Clinical drug–drug interaction (DDI) studies showed that clarithromycin increased the area under the plasma concentration–time curve (AUC) of midazolam, a sensitive CYP3A4 substrate, by 260–860% [16, 17], and ketoconazole increased the AUC of midazolam by 420–1500% [18,19,20]. In addition, in vitro data show that ketoconazole is a more potent inhibitor of P-gp, as half maximal inhibitory concentration (IC50) values for ketoconazole are approximately tenfold smaller than those of clarithromycin [21]. The interaction of concomitant apixaban and clarithromycin was predicted to be less than the twofold observed when apixaban was coadministered with ketoconazole. These differences between clarithromycin and ketoconazole suggest that medicines considered to have similar interaction profiles may have dissimilar effects and, as such, indicate the need to study different agents during the development program of a new drug. The aim of this study was to evaluate the impact of concomitant clarithromycin administration on the exposure and safety of apixaban in healthy adult subjects.
2 Methods
2.1 Study Design
This was an open-label, nonrandomized, single-sequence, crossover study in healthy adult subjects to determine the effect of multiple-dose clarithromycin on the single-dose pharmacokinetics of apixaban. Subjects underwent screening evaluations to determine eligibility within 21 days before study treatment administration.
2.2 Study Population
Healthy male or female subjects, as determined by medical history, physical examination, 12-lead electrocardiogram (ECG), vital signs, and clinical laboratory evaluations, including coagulation parameters, who were aged 18–45 years (inclusive), with a body mass index of 18.0–30.0 kg/m2 (inclusive), and who had signed the informed consent form were eligible for inclusion in the study. Female subjects (women of childbearing potential [WOCBP]) could not be nursing or pregnant and were also required to have a negative pregnancy test within 24 h before study drug administration. WOCBP and men who were sexually active with WOCBP were required to use an acceptable method of contraception. Prohibited and/or restricted treatments included any prescription drugs or over-the-counter acid controllers within 4 weeks prior to study treatment, except those cleared by the medical monitor; other drugs, including over-the-counter medications and herbal preparations, within 2 weeks prior to study treatment except those cleared by the medical monitor; oral, injectable, or implantable hormonal contraceptive within 3 months of study treatment administration; and any agent, including but not limited to aspirin, nonsteroidal anti-inflammatory drugs, anticoagulants, fish oil capsules, and gingko, which are known to increase the potential for bleeding, within 2 weeks prior to study treatment.
Exclusion criteria included any acute or chronic illness; clinically significant laboratory, ECG, or physical examination findings; or history or evidence of abnormal bleeding or coagulation disorders, menorrhagia, intracranial hemorrhage, or abnormal bleeding or a family history of bleeding diathesis in a first-degree relative. In addition, subjects with a history of gastrointestinal disease or surgery that could impact the absorption of study drug were excluded. Subjects were not permitted to consume any food or beverages containing grapefruit or citrus juice, caffeine, or alcohol from 3 days prior to study start until study completion. Subjects were also not permitted to consume tobacco or nicotine-containing products throughout the study.
All subjects provided informed consent prior to the initiation of the study. The study was conducted in accordance with the principles stated in the Declaration of Helsinki and was consistent with the International Conference on Harmonization Good Clinical Practice and in accordance with the ethical principles underlying European Union Directive 2001/20/EC and the US Code of Federal Regulations, Title 21, Part 50 (21CFR50). The protocol, amendments, and subject informed consent received appropriate approval by the Institutional Review Board/Independent Ethics Committee (IntegReview IRB, Austin, TX, USA) before initiation of study at the site (PPD Development, LP, Austin, TX, USA).
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or the EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer. Bristol-Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The study design schematic is presented in Fig. 1. The approximate duration of this study was 33 days, including a 21-day screening period. Subjects entered the clinical facility 1 day prior to dosing and were confined for the duration of the study until discharge on day 12. Subjects received a single oral dose of apixaban 10 mg (2 × 5 mg) on day 1. On day 4, subjects began receiving twice-daily (bid) oral doses of clarithromycin immediate release (IR) 500 mg (BIAXIN® Filmtab) for 4 days to allow clarithromycin exposures to reach steady state. On day 8, subjects received an oral dose of apixaban 10 mg (2 × 5 mg) and oral doses of clarithromycin IR 500 mg 12 h apart. Oral clarithromycin IR 500 mg bid were given alone on days 9 and 10. At the time of dosing, 240 mL of water was given to the participant along with their dose of study treatment. Subjects were required to fast (nothing to eat or drink except water) for 8 h before and until 4 h after study treatment administration on days 1 and 8. On all other days, subjects were required to fast from 2 h before and until 2 h after study treatment administered in the morning. Subjects were not permitted to drink water 1 h before and after study treatment administration except with dosing. End of trial was defined as the date of the last health status follow-up contact made to a subject discharged from the study.
2.3 Sample Collection and Analysis
Blood and urine samples were obtained for clinical laboratory tests at screening, days -1, 7, and before study discharge on day 12.
Blood samples were collected for the assessment of apixaban concentrations up to 72 h after dosing with apixaban on study days 1–4 and days 8–11. Sample collection times included predose and 1, 2, 3, 4, 6, 8, 12, 24, 36, 48, 60, and 72 h post-dose. Approximately 200 mL of blood was drawn from each subject during the study.
Blood samples for pharmacokinetic (PK) analysis were collected either via direct venipuncture or indwelling catheter, in a 1.8 mL collection tube containing 3.2% sodium citrate as the anticoagulant. Within 15 min of collection, each sample was centrifuged at room temperature to separate plasma. The plasma was stored within 1 h of collection at or below − 20 °C until shipped on dry ice to the analysis laboratory at the end of the study. Samples were analyzed at PPD (Richmond, VA, USA).
The plasma samples were analyzed for apixaban using a previously reported validated liquid chromatography-tandem mass spectrometry method [22]. The calibration curves had a nominal range of 1.00–1000 ng/mL. The precision of the assay for the analytical quality control samples of apixaban was ≤ 5.48% coefficient of variation (CV) and ≤ 8.85% CV for between-run and within-run, respectively.
2.4 Safety Assessments
Physical examinations, vital sign measurements, 12-lead ECGs, and clinical laboratory tests were performed at selected times throughout the study. Subjects were closely monitored for adverse events (AEs) throughout the study. Safety assessments were based on medical review of AE reports and the results of clinical laboratory tests, vital sign measurements, 12-lead ECG measurements, and physical examinations and measurements. The incidence of reported AEs was tabulated and reviewed for potential significance and clinical importance. The collection of nonserious AE information began at initiation of investigational product. All serious AEs were to be collected from the date of subject’s written consent until 30 days after discontinuation of dosing, or subject’s participation in the study if the last scheduled visit occurred at a later time.
2.5 Pharmacokinetic Analysis
The PK-evaluable population included only subjects who received at least 1 dose of apixaban. Individual subject PK parameter values were derived using noncompartmental methods by a validated PK program, Phoenix® WinNonlin®, version 6.4 (Certara, Princeton, NJ, USA) using plasma concentration versus actual time data. PK parameters assessed included the maximum plasma concentration (Cmax), time to Cmax (tmax) obtained from observed data, AUC from zero to last quantifiable concentration (AUC(0–T)), AUC from zero extrapolated to infinite time (AUC(INF)), and terminal half-life; all were derived from noncompartmental methods. For the purpose of calculating PK parameters, pre-dose concentrations that were less than the lower limit of quantification (LLOQ) and concentrations before the first quantifiable concentration that were less than LLOQ were set to zero, and all other concentrations less than LLOQ were set to missing.
2.6 Statistical Analysis
SAS® software version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses, tabulations, and graphical presentations of biostatistical data, and version 9.3 was used for all PK analyses, tabulations, and graphical presentations. Descriptive summaries were presented for continuous variables using number of subjects, mean, standard deviation (SD), median, minimum, and maximum. Analyses were performed on the natural logarithms of Cmax, AUC(INF), and AUC(0–T) using a linear mixed-effects model with treatment (apixaban alone, apixaban with clarithromycin) as a fixed effect and measurements within participants as repeated measures. Point estimates and 90% confidence intervals (CIs) for treatment differences on the log-scale derived from the model were exponentiated to obtain estimates for geometric mean ratios (GMRs) and CIs on the original scale.
2.7 Planned Sample Size
Sample size determination was based on consideration of the precision of the estimate of the GMRs of Cmax, AUC(INF), and AUC(0–T) of apixaban with and without clarithromycin. With 18 evaluable subjects, there was an 80% probability that the 90% CI of apixaban Cmax GMR would be within 91–110% of the point estimate. For example, if the point estimate of apixaban Cmax GMR was 1, 1.25, or 1.5, there would be an 80% probability that the 90% CI would be within 0.91–1.10, 1.14–1.38, or 1.37–1.65, respectively. With 18 evaluable subjects, there was an 80% probability that the 90% CI of apixaban AUC(INF) and AUC(0–T) GMRs would be within 93–107% of the point estimate. These precision estimates were based on the assumptions that Cmax, AUC(INF), and AUC(0–T) of apixaban were lognormally distributed, with intrasubject SDs of 0.209, 0.156, and 0.153, respectively, as estimated from a bioavailability study [23, 24]. To allow for dropouts, 20 subjects were planned to be dosed with apixaban on day 1.
3 Results
3.1 Study Population
In total, 19 subjects entered the treatment period of the study after screening; 18 of these completed the study (94.7%). One subject was discontinued from the study on day 7 because of an AE of generalized urticaria (mild, related to study drug) reported during bid clarithromycin treatment on day 6. All 19 subjects had adequate PK profiles and were included in the evaluable PK population. The population was 52.6% female, with a mean ± SD age of 31.3 ± 7.92 years and body weight of 72.4 ± 11.3 kg (range 54.5–96.9). Demographic characteristics are provided in Table 1.
3.2 Pharmacokinetic Results
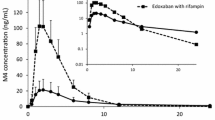
Concentration–time profiles of apixaban on day 1 (apixaban alone) and day 8 (apixaban coadministered with clarithromycin) on a linear scale are provided in Fig. 2. Apixaban reached a maximum concentration at 3 h after dosing. Mean apixaban concentrations declined with multiexponential elimination. Apixaban concentrations were increased when coadministered with clarithromycin compared with administration of apixaban alone, but the disposition curve for the combination of apixaban and clarithromycin remained parallel to the curve for apixaban alone. Summary statistics for the PK parameters and the GMRs with the 90% CIs are provided in Table 2. When apixaban was coadministered with clarithromycin, the adjusted GMR for Cmax was increased by 30%, and the AUC(0–T) and AUC(INF) were both increased by 60%, compared with administration of apixaban alone, with 90% CI wholly above 125%, indicating an increase in apixaban exposure in the presence of clarithromycin. There was no change observed in the tmax or half-life of apixaban with or without concomitant administration of clarithromycin.
Apixaban concentration–time profile with and without concomitant administration of clarithromycin. Symbols indicate the mean and standard deviation (error bars) data points from the measured apixaban concentration with clarithromycin (squares indicate treatment C: apixaban plus clarithromycin) and without clarithromycin (circles indicate treatment A: apixaban alone)
3.3 Safety Results
There were no bleeding-related AEs, deaths, or serious AEs. One subject (5.3%) was discontinued from the study on day 7 because of an AE of generalized urticaria (mild, related to study drug) reported during clarithromycin bid treatment on day 6. Overall, 9 of 19 subjects (47.4%) reported at least one AE during the study: one subject (5.3%) after apixaban treatment (not related to study drug), and eight subjects (42.1%) during clarithromycin treatment, of which five (26.3%) were considered related to study drug. Four (22.2%) of the 18 subjects who received treatment with apixaban and clarithromycin together experienced additional AEs during this phase of the study (all had experienced an AE in an earlier phase), and two (11.1%) AEs were considered related to study drug. All AEs were classified as mild in intensity. With the exception of one subject who received a heating pad as medical treatment for an AE of back pain (mild, not related to study drug) during treatment with clarithromycin bid, all other AEs resolved without treatment. Overall, the most commonly reported AEs assessed as related to the study drug were classified as gastrointestinal disorders (six subjects; 31.6%). One subject had a clinical laboratory test result that met the predefined marked abnormality criteria for low neutrophils, which resolved at post-discharge follow-up; this result was not reported as an AE. No notable clinical laboratory tests, ECG, vital signs, or physical examination results were observed.
4 Discussion
This study was designed as a crossover study to minimize the impact of inter-subject variability and assess the impact of coadministration of clarithromycin with apixaban relative to administration of apixaban alone. The study showed that concomitant administration of clarithromycin, a strong CYP3A4 inhibitor and inhibitor of P-gp, resulted in a 30% increase in Cmax and a 60% increase in apixaban AUC(INF). The modest effect on apixaban exposure is consistent with the multiple elimination pathways of apixaban, including hepatic metabolism and renal, biliary, and direct intestinal excretion. Similar to the impact of either ketoconazole or diltiazem on apixaban exposure [15], clarithromycin had little to no impact on tmax or half-life. Assuming that clarithromycin did not impact the volume of distribution of apixaban, the results suggest that inhibition of CYP3A4 and P-gp efflux by clarithromycin resulted in an increase in apixaban exposure by increasing the bioavailability of apixaban in addition to modestly reducing elimination.
During the apixaban drug development program, multiple DDI studies with modulators of CYP3A4 or P-gp were conducted, and the effect on apixaban PK was dependent on the strength of the modulators [15, 25]. While both clarithromycin and ketoconazole could be considered strong dual inhibitors of CYP3A4 and P-gp, the current study showed that the inhibitory effect of clarithromycin is generally smaller than that of ketoconazole. The results of this study are similar to those reported with other direct oral anticoagulants evaluating the impact of clarithromycin on exposure. Mueck et al. [26] showed that coadministration of clarithromycin 500 mg bid with rivaroxaban 10 mg resulted in AUC(INF) and Cmax values of 1.54 (90% CI 1.44–1.64) and 1.40 (90% CI 1.30–1.52), respectively, compared with those seen when rivaroxaban was administered alone, whereas coadministration of ketoconazole 400 mg once daily with rivaroxaban resulted in AUC(INF) and Cmax values of 2.58 (90% CI 2.36–2.82) and 1.72 (90% CI 1.61–1.83), respectively, compared with administration of rivaroxaban alone. Despite both clarithromycin and ketoconazole being considered strong dual inhibitors of CYP3A4 and P-gp, the interplay between the enzyme transporter influencing absorption (P-gp) and metabolizing enzyme influencing elimination (CYP3A4) may result in different effects on the PK profile, since an agent may not have the same inhibition potency on the enzymes effecting metabolic elimination and transports effecting absorption [27].
The clinical relevance of any DDI depends not only on the change in exposure but also on the assessment of the benefit–risk profile of the compound and the overall therapeutic window. In contrast to warfarin, which is characterized by numerous DDIs and a narrow therapeutic window [28], apixaban has multiple clearance pathways and demonstrated a favorable benefit–risk profile with fixed-dose regimen without monitoring in multiple indications [8, 29]. Considering a modest increase in apixaban exposure, no dose adjustment for apixaban is required when coadministered with clarithromycin [8, 30]. Prescribers should consider the clinical relevance of this and any other DDI in the context of the full clinical picture, including comorbidities, frailty, body size, renal fuction, or other coadministered medications that may also affect bleeding risk.
In conclusion, Apixaban Cmax and AUC(INF) in the presence of clarithromycin increased to a lesser extent than coadministration of ketoconazole, i.e., 30% and 60%, respectively; tmax and elimination half-life were unchanged.
References
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594–604.
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807–15.
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487–98.
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek E, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699–708.
Bristol-Myers Squibb Company PI. Eliquis (apixaban) prescribing information. 2018. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed 6/14/2018.
Frost C, Yu Z, Nepal S, Bragat A, Moore K, Shenker A, et al. Apixaban, a direct factor Xa inhibitor: single-dose pharmacokinetics and pharmacodynamics of an intravenous formulation [abstract 148]. J Clin Pharmacol. 2008;48:1132.
Vakkalagadda B, Frost C, Byon W, Boyd RA, Wang J, Zhang D, et al. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa. Am J Cardiovasc Drugs. 2016;16(2):119–27.
Wang L, He K, Maxwell B, Grossman SJ, Tremaine LM, Humphreys WG, et al. Tissue distribution and elimination of [14C]apixaban in rats. Drug Metab Dispos. 2011;39(2):256–64.
Zhang D, Frost CE, He K, Rodrigues AD, Wang X, Wang L, et al. Investigating the enteroenteric recirculation of apixaban, a factor Xa inhibitor: administration of activated charcoal to bile duct-cannulated rats and dogs receiving an intravenous dose and use of drug transporter knockout rats. Drug Metab Dispos. 2013;41(4):906–15.
Wang L, Zhang D, Raghavan N, Yao M, Ma L, Frost CE, et al. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos. 2010;38(3):448–58.
Zhang D, He K, Herbst J, Kolb J, Shou W, Wang L, et al. Characterization of efflux transporters involved in distribution and disposition of apixaban. Drug Metab Dispos. 2013;41(4):827–35.
Frost CE, Byon W, Song Y, Wang J, Schuster AE, Boyd RA, et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol. 2015;79(5):838–46.
Yeates RA, Laufen H, Zimmermann T. Interaction between midazolam and clarithromycin: comparison with azithromycin. Int J Clin Pharmacol Ther. 1996;34(9):400–5.
Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA, O’Mara EM Jr, Hall SD. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64(2):133–43.
McCrea J, Prueksaritanont T, Gertz BJ, Carides A, Gillen L, Antonello S, et al. Concurrent administration of the erythromycin breath test (EBT) and oral midazolam as in vivo probes for CYP3A activity. J Clin Pharmacol. 1999;39(12):1212–20.
Olkkola KT, Backman JT, Neuvonen PJ. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther. 1994;55(5):481–5.
University of Washington. Metabolism & Transport Drug Interaction Database. 2012. http://www.druginteractioninfo.org. Accessed 4/9/2019.
Cook JA, Feng B, Fenner KS, Kempshall S, Liu R, Rotter C, et al. Refining the in vitro and in vivo critical parameters for P-glycoprotein, [I]/IC50 and [I2]/IC50, that allow for the exclusion of drug candidates from clinical digoxin interaction studies. Mol Pharm. 2010;7(2):398–411.
Pursley J, Shen JX, Schuster A, Dang OT, Lehman J, Buonarati MH, et al. LC-MS/MS determination of apixaban (BMS-562247) and its major metabolite in human plasma: an application of polarity switching and monolithic HPLC column. Bioanalysis. 2014;6(15):2071–82.
Song Y, Chang M, Suzuki A, Frost RJ, Kelly A, LaCreta F, et al. Evaluation of crushed tablet for oral administration and the effect of food on apixaban pharmacokinetics in healthy adults. Clin Ther. 2016;38(7):1674–85.
Diletti E, Hauschke D, Steinijans VW. Sample size determination for bioequivalence assessment by means of confidence intervals. Int J Clin Pharmacol Ther Toxicol. 1991;29(1):1–8.
Frost C, Shenker A, Gandhi MD, Pursley J, Barrett YC, Wang J, et al. Evaluation of the effect of naproxen on the pharmacokinetics and pharmacodynamics of apixaban. Br J Clin Pharmacol. 2014;78(4):877–85.
Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76(3):455–66.
Zhang L, Zhang Y, Huang SM. Scientific and regulatory perspectives on metabolizing enzyme-transporter interplay and its role in drug interactions: challenges in predicting drug interactions. Mol Pharm. 2009;6(6):1766–74.
Di MA, Frigerio B, Spadarella G, Ravani A, Sansaro D, Amato M, et al. Old and new oral anticoagulants: food, herbal medicines and drug interactions. Blood Rev. 2017;31(4):193–203.
Byon W, Sweeney K, Frost C, Boyd R. Population pharmacokinetics, pharmacodynamics, and exploratory exposure-response analyses of apixaban in subjects treated for venous thromboembolism. CPT Pharmacometrics Syst Pharmacol. 2017;6(5):340–9.
European Medicines Agency. Eliquis (apixaban) summary of product characteristics. 2015. https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf. Accessed 4/9/2019.
Acknowledgements
The authors acknowledge Robert Adamczyk and Brenda Cirincione for their support and contributions to the development of this publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Bristol-Myers Squibb and Pfizer Inc. Rob Coover, MPH, and Andrew Shepherd, PhD, employees of Caudex, provided editorial assistance, which was funded by Bristol-Myers Squibb and Pfizer Inc.
Conflict of interest
WB is an employee of Pfizer, Inc., and owns stock/stock options. DM is an employee of Bristol-Myers Squibb. SG, EM, XL, and BM are employees of Bristol-Myers Squibb and own stock/stock options.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Garonzik, S., Byon, W., Myers, E. et al. The Effects of Clarithromycin on the Pharmacokinetics of Apixaban in Healthy Volunteers: A Single-Sequence Crossover Study. Am J Cardiovasc Drugs 19, 561–567 (2019). https://doi.org/10.1007/s40256-019-00348-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-019-00348-2