Abstract
Triboelectric nanogenerators (TENGs), a new technology for gathering sustainable energy, have attracted much scientific interest. In this study, we describe a unique method for modifying the triboelectric power generation performance of Polyvinyl Alcohol (PVA) by adding ZnO–CuO–AgO (ZCA) trimetallic nanoparticles to improve the performance of TENGs and answer the requirement for ecologically benign and biodegradable materials. Hydrothermal synthesis adopted to create ZnO–CuO–AgO trimetallic nanoparticles ensures a distinctive structure with a large surface area, essential for enhancing triboelectric power generation. From the AFM results, it is evident that 1% PVA/ZCA showed the highest output voltage of 0.27V. Despite following the general trend, at higher concentrations of ZCA nanofiller in the PVA matrix, the enhancement of output voltage is not observed, which can be attributed to the non-uniform distribution. The effect of spin-coated film thickness and nanoparticle concentration on the triboelectric performance of the PVA nanogenerator is studied by monitoring the open-circuit voltage in response to various mechanical stimuli. Finally, the developed biodegradable nanogenerators in this study can be used for sustainable energy harvesting applications such as wearable electronics, self-powered sensors, and environmental monitoring systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triboelectric nanogenerators (TENGs) have emerged as sustainable energy-gathering tools in self-powering devices and electronics [1, 2]. These devices possess many characteristics: lightweight, high flexibility, environmental friendliness, strong robustness, and low manufacturing cost. However, the developed TENGs show poor and insufficient electrical output for portable electronic applications, which demands other materials modification strategies [3]. This includes incorporating nanoparticles into polymer-based triboelectric materials [4], selecting optimal opposite triboelectric materials [5], and modifying their surfaces [6]. Such modification strategies significantly raise manufacturing costs and limit the use of these materials in commercial applications. In this situation, simpler ways of TENG fabrication are necessary to target highly efficient and flexible TENGs.
Polyvinyl alcohol (PVA) is notable for its exceptional water solubility, biodegradability, and biocompatibility. This polymer is widely used in biomedical applications and as a contact layer within TENGs [7]. PVA is blended with several nanomaterials and polymers to modulate the mechanical characteristics and biodegradability, extending its usefulness and limiting the expensive processing [8]. Patnam et al. [8] combined polyvinylidene fluoride (PVDF) with microstructure-aligned PVA to develop respective negatively and positively polarized triboelectric surfaces. They could achieve 230 V maximum peak-to-peak open-circuit voltage and 6 μA short-circuit current, with 3.1 W/m2 instantaneous output power density. In another study by Chen et al. [9] PVA was doped with phenyl phosphonic acid (PPA)- branched polyethyleneimine (PEI) to achieve 28.8 ± 0.4 V maximum open circuit voltage, 62.3 ± 5.4 μA short-circuit current, and 228.8 μW power. At 10 wt% of PPA-PEI, the composite also showed significantly reduced flammability.
TENGs operate by coupling electrostatic induction and contact electrification [10]. These devices typically consist of electrode-coated positive and negative tribo-materials on the back. Due to the unique triboelectric polarity of these two surfaces, electrons are transferred from positive to negative during friction, causing an equal number of electrons to flow through the external circuit. The output voltage, current, and power depend on the triboelectric charge density, which can be efficiently attained by coupling the two materials with the highest differing charge affinities. Polymers like PVA are coupled with numerous nanomaterials of metal oxides, carbon, and inorganic fillers to increase the electron transferring capability and thereby target high triboelectric output [4]. Wang and co-workers [11] demonstrated a wind-driven self-powered sensor system that detects NO2 gas in the atmosphere using PVA/silver nanofibers-based TENG. The device's peak-to-peak open-circuit voltage and power density were 530 V and 359 mW/m2, respectively. In another work, electrospun fibers of casein/PVA demonstrated ∼ 195 mV output voltage and ∼ 0.018 μA current using the standard testing method, whereas ∼ 20 V output voltage and ∼ 37 μA current by repeated finger tapping on the device.
Though the published results explain significantly high triboelectric voltage generation [12], a thorough analysis of how molecular and ionic engineering affects the triboelectric performance of PVA blends is still unclear. The rational design and holistic engineering of triboelectric devices that efficiently use biocompatible materials are severely hampered by this knowledge gap. Numerous nanomaterials and varieties of fabrication strategies are applied to develop ultimately high triboelectric power generation capacity and high efficiency for the materials. This work demonstrates a simple film formation technique using the Dr. Blade approach, which is common in laboratories to develop PVA thin films for TENGs. A trimetallic nanomaterial, ZnO–CuO–AgO (ZCA) is used as the reinforcement to generate the PVA composites. The use of various components in a composite can result in synergistic effects. The incorporation of ZnO, CuO, and AgO into a PVA polymer matrix may improve the nanogenerator's overall triboelectric performance. Each material may have distinct qualities, and their combination may result in enhanced charge creation and storage. Silver oxide (AgO) is a metal oxide, whereas copper oxide (CuO) is a semiconductor. The combination of these materials with zinc oxide (ZnO) can produce a variety of electrical conductivities, enabling effective electron transport during the triboelectric process. The overall performance of the TENG is anticipated to improve with the inclusion of ZCA nanofiller in the PVA matrix. The ZCA nanocomposite is a metal alloy that provides additional benefits, such as enhanced charge dissipation at the surface of PVA/ZCA nanocomposite and the generation of a high amount of triboelectric charge. Moreover, the nanocomposite is compatible with other materials, and studies show that the designed nanocomposite is mechanically stable. A hydrothermal approach was followed for the nanomaterial synthesis, and simple solution mixing was done for the PVA composite manufacture. In addition to opening up a brand-new avenue for improving the electrical output performance of nanogenerators through surface modification, this research also provides a sustainable and eco-friendly energy alternative for portable electronic gadgets.
Experimental techniques
Reagents and chemicals
Polyvinyl alcohol (PVA) was purchased from Merck Schuchart, Germany, of molecular weight Mw = 78,000 g/mol. Zinc acetate dihydrate, Sodium hydroxide, Copper (II) acetate, Hydrochloric acid, and silver nitrate were obtained from Sigma Aldrich. All the experiments were carried out using distilled water. The chemicals involved are all > 97% purity and were not subjected to any additional purification methods before use.
Synthesis of ZnO–CuO–AgO trimetallic oxide nanoparticle
This study employs a novel hydrothermal approach to synthesize a pure zinc oxide-copper oxide-silver oxide nanocomposite. The synthesis proceeds via two steps with the modified synthesis from a study [13]. The first step comprises the synthesis of the ZnO–CuO, which is followed by the doping of silver. About 2 g of precursor Zinc acetate dihydrate (Zn (CH3COO)2·2H2O) was dissolved in 50 ml double distilled water and stirred for 10 min using a magnetic stirrer. 1.6 g of NaOH was dissolved in 44 ml water and stirred for 10 min. The NaOH solution was added dropwise into the zinc acetate solution, stirring vigorously for 20 min to produce a gelatinous precipitate. To this white precipitate, 7% of Copper (II) acetate monohydrate solution was added and stirred at 70 °C for half an hour until it became metallic grey. The solution was placed in an autoclave and kept in a furnace at 160 °C for 5 h. The autoclave was allowed to cool at room temperature, and the precipitate formed was centrifuged. It was then rinsed thoroughly with ethanol and distilled water three times to remove residual sodium acetate. The product was dried at 110 °C for 2 h using a hot plate to obtain ZnO–CuO nano powder. After that, to obtain a ZnO–CuO–AgO composite, Ag dopant was added in proportion. For 2 g of ZnO–CuO nano powder, 7% AgNO3 was added, and a similar procedure was followed for hydrothermal synthesis, centrifugation and washing to get the final product. The formed ZnO–CuO–AgO (ZCA) nanomaterial was annealed at 350 °C for 2 h.
Preparation of PVA/ZCA polymer nanocomposite
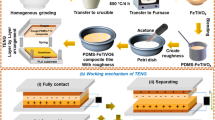
Pure PVA film and films doped with ZCA nanoparticles at different concentrations were prepared by the solution mixing process. The PVA neat solution was prepared by dissolving 1.0 g PVA in 10 ml distilled water, followed by magnetic stirring at 70 °C for 5 h to create a transparent solution. Further, ZCA nanoparticles with different weight percentages (1 wt.%, 2 wt.%, 3 wt.%) dispersed in the distilled water by sonication were added to the PVA by magnetic stirring overnight (i.e., 1% PVA/ZCA, 2% PVA/ZCA, 3% PVA/ZCA). The sample preparation is demonstrated in Fig. 1A.
Fabrication of PVA/ZCA coated thin films
The films were coated on aluminum foil wrapped in a glass substrate using the Dr. Blade (DB) coating technique, with adjustable land length and gap height. The precursor solution (PVA/ZCA) was added dropwise to the substrate and swiped linearly by a metal blade as shown in Fig. 1B. By swiping the precursor solution with the blade at a speed of 10 mm s−1, the optimized films were blade coated on aluminum foil substrates. The distance between the glass substrate and the blade was 200 μm. Two different thin films were fabricated following the same procedure. The first film was only developed using the neat PVA solution. Whereas the second film was obtained by coating neat PVA solution and the PVA/1wt.% ZCA nanocomposite solution layer-by-layer. Similarly, by varying the filler concentration, different thin films were fabricated. Before starting the tests, the films were annealed immediately at 100 °C for 20 min in a vacuum oven.
Characterization techniques
X-ray diffraction analysis (PAN analytical, Empyrean, Royston, United Kingdom) X′-pert Pro Cu (Ka) with a scanning rate of 2°/min and scanning angle ranging between 10° ≤ 2θ ≤ 90° was used to examine the phases of synthesized ZCA and modified PVA/ZCA particles. Fourier transformation infrared spectrophotometer (FTIR, PerkinElmer Spectrum 400) in transmission mode addressed the phase variation of ZCA particles and PVA/ZCA polymer composites in a range of 500–4000 cm−1 with the resolution of 2 cm−1. The surface morphology of the ZCA and PVA/ZCA was studied by a scanning electron microscope (SEM-Nova Nano-450, Netherlands) and transmission electron microscopy (TEM, FEI, TALOS F200X, New York, NY, USA). Atomic force microscopy (AFM) analysis of PVA/ZCA was carried out to study the surface topography and roughness (MFP-3D, Asylum Research, USA). The thermal stability of PVA/ZCA was investigated using a TGA synchronization analyzer (PerkinElmer, TGA 4000, Boston, MA, USA) at temperatures ranging from 30 °C to 600 °C at a heating rate of 10 °C/minute. The PVDF/ZCA samples' crystallinity was determined using a Perkin Elmer DSC4000 differential scanning calorimeter (DSC). The samples were heated between 20 º C and 200 °C at 10 °C/min−1 under a nitrogen atmosphere. The triboelectric studies were done by arranging a specific setup of a resistance box connected to an NI (National Instrument) and computer. The samples were electroded, and the composite layer and the PVA neat layer faced each other for triboelectric power generation. One layer was made static, and the other kept rubbing over it with the help of a rolling motor. Both sides of the triboelectric layer surfaces were connected with the resistor box, and the corresponding output voltage was monitored using the computer.
Results and discussions
Structural and morphological elucidation of ZCA nanoparticle
XRD and FTIR studies determine the crystallinity and structural properties of the hydrothermally synthesized ZCA nanomaterials, as illustrated in Fig. 2. The deviation in peaks at 2θ values, 34.17 º and 81.55 º, corresponding to (002) and (014) crystalline planes in Zn, is due to the presence of dopants and according to JCPDS-89–0510. The peaks 2θ values 38.15º, 44.33º, and 77.42º are related to the respective crystal planes of (111), (002), and (113) and are due to the presence of Ag. The diffraction peaks at 2θ values 64.47º, 64.64º, and 76.98º confirm the (022) plane in Cu present in the ZCA nanomaterial [14, 15]. However, slight shifts in the 2θ values represent the modification of the parent material to the final desired product (Fig. 2a). Infrared spectroscopy analysis is used to analyse the vibration bands due to the Zn–O bond, and the formation of new chemical bonds and their distinct vibrational behaviour of the dopants are identified (Fig. 2b). The broad spectrum at 3380 cm−1 in the transmittance spectrum of the synthesized sample is related to water molecules. The band at 1648 cm−1 is due to the stretching vibration of water molecules [16]. The bands at 1470 cm−1 and 1380 cm−1 show the vibration distortion of the O–H group due to the presence of Cu and Ag respectively [17, 18]. The metal–oxygen (MO) stretching bands at 650 cm−1 and 770 cm−1 confirm the existence of Cu–O [19]. And the broader peaks at 547 cm−1 exhibit ZnO–CuO coupling [20,21,22,23,24,25,26].
SEM and EDAX mapping further characterize the ZCA nanomaterial, as illustrated in Fig. 3. The surface morphology of the ZCA nanomaterial is visualized as flakes or layers from the SEM image (Fig. 3a) with very few nm thickness. The distribution is uniform, but the layers are grown in one another, showcasing the intermixing patterns. This ensures a nanocomposite structure and allows the interaction of the materials in the nanoscale, leading to high mechanical, electrical and electronic properties. This peculiar structure is further evident from the EDS mapping image in Fig. 3b. The elemental mapping visualizes the spatial distribution of CuO, ZnO and AgO within the ZCA nanomaterial. All elements are distributed uniformly in the sample, although the atomic percentages differ (Fig. 3c). The absence of any other elements confirms the sample's purity. Furthermore, the chemical composition of the samples is shown in the insets of Fig. 3c, confirming the structural integrity of the sample.
The particle size and morphology were analysed in detail using the TEM images provided in Fig. 4. Different resolutions, 50 nm, 200 nm, and 500 nm are provided to demonstrate the particle size and layer structure in ZCA nanomaterials. The formation of layers in one another, as proposed by the SEM image, is clear in TEM as well. ZnO–CuO–AgO trimetallic nanomaterials show amazing morphology that can help in the uniform distribution of nanomaterials within the polymer medium. The HRTEM image provided in Fig. 4d shows the crystalline nature of the sample, and the corresponding d-spacing of 0.34 nm was obtained.
Morphological and structural elucidation of PVA/ZCA nanocomposite
Atomic force microscopy (AFM) was used to investigate the surface morphology of neat PVA with different weight percentages of ZCA. The images were evaluated using the tapping mode AFM for neat PVA, 1% PVA/ ZCA, 2% PVA/ZCA, and 3% PVA/ ZCA, as shown in Fig. 5a–d. The neat PVA has a smoother surface than the other PVA/ZCA polymer nanocomposites with different weight percentages, as seen in the images. With an increase in ZCA weight percentages from 1wt.% to 3wt.%, the statistical analysis findings demonstrate an increasing trend in average surface roughness (Ra), from 1.60 nm to 13.03 nm, leading to an eight-fold improvement in surface roughness.
Table 1 shows a similar trend for root mean square surface roughness (Rm) ranging from 2.03 nm to 18.13 nm for polymer composites ranging from neat PVA to 1% -3% PVA/ZCA. As seen in Fig. 5a–d, the surface roughness is highlighted by the increasing size of spherical structures due to the agglomeration of nanofillers at higher concentrations.
The diffraction patterns for Neat PVA and PVA/ZCA nanocomposite films of various concentrations are given in Fig. 6a. The PVA film diffractogram exhibits peaks in 2θ value19.8º, 22.9º and 40.6º which are in clear agreement to the crystalline PVA diffractogram pattern reported [27]. With the introduction of nanofillers ZCA of various concentrations, new peaks in the diffractogram are observed at 2θ value 31.8º, 47.6º and 68.9º with respective planes (100), (102) and (201) corresponding to the cubic ZnO phase. The diffraction peaks were observed at 2θ values 32.5 º, 38.2 º and 54.8 º and with respective planes (111), (020), and (202) corresponding to the cubic Ag2O phase [28]. The diffraction peaks present at 2θ value 36.2º, 38.1º with respective planes (002), (111) corresponding to the CuO phase confirms the presence of CuO dopant also in the PVA matrix [29]. The FTIR spectroscopy was used to obtain insight into the various structural features corresponding to the functional groups present in the neat PVA and PVA/ZCA nanocomposite film in Fig. 6b. The FTIR spectra of neat PVA shows too high intense absorption peaks at 3267 cm−1 and 1083 cm−1 corresponding to the O–H stretching vibrations and C–O stretching vibrations respectively. There was no major shift in these peak positions with increased dopant concentration. The band observed at 2921 cm−1 corresponds to the C–H asymmetric stretching vibration of the PVA. With the addition of ZCA nanofiller, this peak is slightly shifted to 2934 cm−1 for 1% doped PVA/ZCA [30]. However, with the increase in nanofiller percentage, the peak position remains almost the same. A low intense peak at 1751 cm−1 corresponding to the C=O is observed in all the samples at similar positions. In the fingerprint region, two major peaks are observed at 672 cm−1 and 830 cm−1 for the neat PVA, and there is a slight shift of the peak observed at 830 cm−1 upon adding 1% ZCA filler. The new peak is now observed at 840 cm −1, indicating some interference of the nanofiller with the surface functional groups of PVA [31]. The chemical bonding or weak Van der Waals interactions can alter the way the nanoparticles adhere to the surface of the polymer. Possible hydrogen bonding interactions between the surface OH functional groups on the PVA and oxygen atoms of the ZCA nanoparticle surfaces might enhance adhesion. This strong interfacial bonding can facilitate charge transfer during triboelectric processes.
Thermal stability and Crystallinity of PVA/ZCA nanocomposite
To investigate the effect of ZCA on PVA polymer properties, the thermal properties of PVA and PVA/ZCA nanocomposites were tested using TGA in a nitrogen flow. The TGA and relevant DTG curves are shown in Fig. 7a. All samples of neat PVA and its nanocomposites exhibit a three-step weight loss process, which includes (i) the first weight loss occurring at about 50–160 °C, which is attributed to the evaporation of the residual solvent; (ii) the second stage observed at about 200–250 °C, which is primarily due to the thermal decomposition of PVA side chains; and (iii) the third weight loss occurring at around 300–350 °C, which can be attributed to the decomposition of the PVA main chains [32]. With the increase in the filler concentration, the decomposition temperature is slightly decreased compared to the neat PVA, as shown in Fig. 7b.
Dielectric properties of PVA/ZCA polymer nanocomposites
Broadband dielectric spectroscopy is a useful tool for investigating the molecular dynamics of polymer nanocomposites since significant information about these processes may be collected over a wide frequency range (milli to mega) hertz at ambient temperature. The dielectric constant and dielectric loss values for PVA/ZCA nanofilm are represented in Fig. 8. The dielectric constant of neat PVA film was observed to be of value 13, with the increase in the concentration of filler ZCA with different weight percentages the dielectric constant value also increases correspondingly due to the Maxwell–Wagner–Sillars (MWS) interfacial polarization effect [33]. Compared to the neat PVA, the dielectric constant of 3 wt.% of PVA/ZCA increases 4 times, as shown in Fig. 8a. Similar trend is also observed for PVA/CuO-ZnO reported by Chandegara. et al. [34]. The large enhancement in the dielectric constant of the PVA/ZCA matrix arises from the large ionic polarization in the ZnO [35]. Such a significant rise indicates an increase in the number of charge carriers in the PVA matrix due to incorporating ZnO, which improves conductivity [36]. The dielectric loss is a portion of the energy lost in a dielectric medium from an applied electric sinusoidal source. Figure 8b shows the frequency-dependent dielectric loss of neat PVA and different nanofiller weight percentages. The dielectric loss also increased by 4 times by incorporating nanofillers ZCA into the PVA matrix. However, there is a negligible change in the dielectric loss values with respective changes in the concentration of ZCA from 1 wt.% to 3 wt%.
The mobility of charge carriers in a polymer matrix induces conductivity depending on the weight percentage of nanofillers introduced into the PVA matrix. Figure 8c depicts the influence of nanofiller concentration on the conductivity of PVA/ZCA nanocomposite with the respective applied field frequency. It is well known that the conductivity of polymeric nanocomposite is greatly influenced by the nature and composition of nanofillers dispersed [37]. As shown in Fig. 8c, the conductivity values of nanocomposites PVA/ZCA remain unchanged up to 100Hz. With a further increase in the frequency, there is a slight increase in the conductivity with an increase weight percentage of nanofiller as in the inset in Fig. 8c. Some charge transfer complexes are formed in the polymer chains as the composition of the polymer changes. As a result, polymer chains become more flexible, and the conductivity increases [38]. The conductivity of neat PVA at 10Hz was calculated as 1.59 S/cm (× 10–11) with the introduction of 1 wt.% of ZCA into the PVA matrix causing a rise in the conductivity value of 0.48 S/cm. With further increase of the weight percentage of filler ZCA, there was a negligible change in the conductivity. This increase is due to increased charge carriers' mobility [39]. According to Gavade et al. the improvement in conductivity is attributable to the creation of conductive channels in the nanofiller aggregates and the decrease in potential barrier width in the bulk area of the PVA nanocomposites [37].
Triboelectric power generation in PVA/ZCA nanocomposites
The polymer composite films with various weight percentages of ZCA were used to fabricate by triboelectric nanogenerators (TENG). The nanocomposite films were fabricated using Dr Blade (db) coating technique with a dimension of (2 × 2 cm2) as well as the solution drop casting method with a dimension of (1 × 2 cm2) with working parameters of frequency 1.2 kHz and resistance 3 MΩ generated a typical AC-type signal. The fabricated films were used in investigating the effect of the fabrication method on the triboelectric properties. TENG tests were done by applying mechanical force by gently rubbing the films with the fingertips. Consistency in rubbing was maintained by conducting the experiment in the same manner each time, with the same procedure and by the same individual. The resulting output voltage obtained by the application of mechanical force was measured periodically, and the peak-to-peak output voltage was recorded with the instrumental setup as shown in Fig. 9. Using the Dr Blade (db) coating technique, the average thickness of substate films raises from 10.6 μm to 14.4 μm thick by incorporation of 3 wt.% ZCA nanofiller in PVA matrix. In the solution drop casting method, the average thickness of all the polymer nanocomposite films rises from 110 μm to 260 μm with the introduction of 3 wt.% ZCA nanofiller in PVA matrix.
As shown in Fig. 9, the Dr. Blade-coated polymer films of 1% PVA/ZCA showed the highest output voltage of 0.27V. Contrary to the general trend, a further increase in the weight percentage of the nanofiller doesn't result in the enhancement of output voltage. This can be attributed to the non-uniform distribution of ZCA nanofiller in the PVA matrix at higher concentrations, as observed from the AFM. The neat PVA film produced an output voltage of 0.06V, 4.5 times lower than the output voltages observed for 1wt% PVA/ZCA nanocomposite film obtained using the Dr.Blade technique. It has been observed earlier that the introduction of inorganic nanofillers aids in the facile transportation of electrons to the surface of the nanocomposite films, contributing to the enhancement of output voltage and triboelectric properties. Similarly, we attribute increased output voltage with 1% PVA/ZCA nanofilm to the corporative effect of ZnO–CuO–AgO metal oxides contribution in facilitating the electrons. Indeed, factors such as chemical bonding or weak Van der Waals interactions can influence how nanoparticles adhere to the polymer surface. The potential hydrogen bonding interactions between surface OH functional groups on the PVA and oxygen atoms of the ZCA nanoparticle surfaces might enhance adhesion. This strong interfacial bonding can facilitate charge transfer during triboelectric processes. Additionally, nanoparticles, especially those with rough surfaces, have the potential to increase the effective contact area at the interface. This increased contact area can impact the charge transfer kinetics and, consequently, the triboelectric response. Chandegara et.al. also observed enhanced output voltage with ZnO-CuO/PVA nanocomposite [34]. For all composites, reliable and stable results were observed for up to 2 min of continuous mechanical force, demonstrating stability and repeatability in triboelectric power generation.
To investigate the effect of the thickness of the films on the triboelectric properties of the nanocomposite films, we have prepared and tested the triboelectric power generation from the thicker films fabricated using the solution drop casting method. The output performance observed for these thicker films is depicted in Fig. 10. As observed with the thin films prepared from Dr.Blade technique, the thin film of 1% PVA/ZCA prepared from solution drop casting exhibited the best triboelectric generation in comparison to 2% and 3% PVA/ZCA nanocomposite films. To our surprise, there was no improvement in the triboelectric performance with the increase in nanofiller concentration in the PVA matrix.
Figure 11 illustrates the schematic representation of triboelectric voltage generation for the PVDF/ZCA composite. The composite films are prepared as two layers, upper and lower, which are subjected to mechanical rubbing. Electrodes are applied to both layers to electronically protect them. In the initial state, charges are uniformly distributed in the layers, as shown in the figure. However, when both layers come into contact and slide over each other, the distribution of charges is affected. In a typical triboelectric power generation scenario, rubbing induces a charge flow, resulting in the generation of output voltage. This type of nanocomposites are valuable for self-powering devices.
Conclusions
This study addressed the potential of enhancing the triboelectric power generation performance for biodegradable PVA-based nanocomposites. Trimetallic nanoparticles of ZnO–CuO–AgO were integrated into PVA by simple Dr Blade method, and the thin films developed were analyzed for structural, morphological, and thermal characteristics. Trimetallic nanomaterials were synthesized so that the different metal oxides are grown in one another to form a nanoarchitecture. The sample composite thin films were electroded, made into a triboelectric charge layer, and paired with neat PVA thin films to design the nanogenerator. A maximum output voltage of approximately 0.3 V was achieved for the ZCA nanocomposite containing 1 wt.% filler, and the result was promising and notable in this low level of nanomaterials used. Charge transfer happens within the biodegradable polymer composites containing the filler combination. This study explored the potential of special nanoarchitecture in very low concentrations for maximizing the functional performance of polymer thin films.
Data availability
Data will be made available on request.
References
Yadav, P., Sahay, K., Verma, A., Maurya, D.K., Yadav, B.C.: Applications of multifunctional triboelectric nanogenerator (TENG) devices: materials and prospects. Sustain. Energy Fuels 7(16), 3796–3831 (2023)
Wang, W., Yu, A., Zhai, J., Wang, Z.L.: Recent progress of functional fiber and textile triboelectric nanogenerators: towards electricity power generation and intelligent sensing. Adv. Fiber Mater. 3(6), 394–412 (2021)
Yang, W., Li, X., Han, X., Zhang, W., Wang, Z., Ma, X., Li, M., Li, C.: Asymmetric ionic aerogel of biologic nanofibrils for harvesting electricity from moisture. Nano Energy 1(71), 104610 (2020)
Zhang, R., Örtegren, J., Hummelgård, M., Olsen, M., Andersson, H., Olin, H.: A review of the advances in composites/nanocomposites for triboelectric nanogenerators. Nanotechnology 33(21), 212003 (2022)
Gong, J., Xu, B., Tao, X.: Breath figure micromolding approach for regulating the microstructures of polymeric films for triboelectric nanogenerators. ACS Appl. Mater. Interfaces 9(5), 4988–4997 (2017)
Liu, Y., Fu, Q., Mo, J., Lu, Y., Cai, C., Luo, B., Nie, S.: Chemically tailored molecular surface modification of cellulose nanofibrils for manipulating the charge density of triboelectric nanogenerators. Nano Energy 1(89), 106369 (2021)
Luo, X., Zhu, L., Wang, Y.C., Li, J., Nie, J., Wang, Z.L.: A flexible multifunctional triboelectric nanogenerator based on MXene/PVA hydrogel. Adv. Func. Mater. 31(38), 2104928 (2021)
Patnam, H., Dudem, B., Graham, S.A., Yu, J.S.: High-performance and robust triboelectric nanogenerators based on optimal microstructured poly (vinyl alcohol) and poly (vinylidene fluoride) polymers for self-powered electronic applications. Energy 15(223), 120031 (2021)
Chen, X., Yusuf, A., del Rio, J.S., Wang, D.Y.: A facile and robust route to polyvinyl alcohol-based triboelectric nanogenerator containing flame-retardant polyelectrolyte with improved output performance and fire safety. Nano Energy 1(81), 105656 (2021)
Wu, C., Wang, A.C., Ding, W., Guo, H., Wang, Z.L.: Triboelectric nanogenerator: a foundation of the energy for the new era. Adv. Energy Mater. 9(1), 1802906 (2019)
Wang, D., Zhang, D., Guo, J., Hu, Y., Yang, Y., Sun, T., Zhang, H., Liu, X.: Multifunctional poly (vinyl alcohol)/Ag nanofibers-based triboelectric nanogenerator for self-powered MXene/tungsten oxide nanohybrid NO2 gas sensor. Nano Energy 1(89), 106410 (2021)
Wang, R., Mu, L., Bao, Y., Lin, H., Ji, T., Shi, Y., Zhu, J., Wu, W.: Holistically engineered polymer–polymer and polymer–ion interactions in biocompatible polyvinyl alcohol blends for high-performance triboelectric devices in self-powered wearable cardiovascular monitorings. Adv. Mater. 32(32), 2002878 (2020)
Abinaya, C., Marikkannan, M., Manikandan, M., Mayandi, J., Suresh, P., Shanmugaiah, V., Ekstrum, C., Pearce, J.M.: Structural and optical characterization and efficacy of hydrothermal synthesized Cu and Ag doped zinc oxide nanoplate bactericides. Mater. Chem. Phys. 184, 172–182 (2016)
Abinaya, C., Marikkannan, M., Manikandan, M., Mayandi, J., Suresh, P., Shanmugaiah, V., Ekstrum, C.: Structural and optical characterization and efficacy of hydrothermal synthesized Cu and Ag doped zinc oxide nanoplate bactericides. Mater. Chem. Phys. 184, 172–182 (2016)
Sharwani, A.A., Narayanan, K.B., Khan, M.E., Han, S.S.: Photocatalytic degradation activity of goji berry extracts synthesized silver-loaded mesoporous zinc oxide (Ag@ZnO) nanocomposites under simulated solar light irradiation. Sci. Rep. 12, 10017 (2022)
Yogamalar, N.R., Bose, A.C.: Tuning the aspect ratio of hydrothermally grown ZnO by choice of precursor. J. Solid State Chem. 184, 12–20 (2011)
Vijayakumar, T.S., Karthikeyeni, S., Vasanth, S., Ganesh, A., Bupesh, G., Ramesh, R., Manimegalai, M., Subramanian, P.: Synthesis of silver-doped zinc oxide nanocomposite by pulse mode ultrasonication and its characterization studies. J. Nanosci. 1–7, 785064 (2013)
Ashok kumar, M., Muthukumaran, S.: Microstructure, optical and FTIR studies of Ni, Cu co-dopped ZnO particles by co-precipitation method. Opt. Mater. 37, 671–678 (2014)
Selvanathan, V., Aminuzzaman, M., Tey, L.-H., Razali, S.A., Althubeiti, K., Alkhammash, H.I., Guha, S.K., Ogawa, S., Watanabe, A., Shahiduzzaman, Md., Akhtaruzzaman, Md.: Muntingia calabura leaves mediated green synthesis of CuO nanorods: exploiting phytochemicals for unique morphology. Materials 14(21), 6379 (2021)
Shanmugaiah, H., Harikrishnan, N.S., AlHarbi, K., Shine, J.M., Khaled, N., Balasubramanian, G.: Shyamkumar, facile synthesis of silver nanoparticles using streptomyces sp, vsmgt 1014 and their antimicrobial efficiency. Dig. J. Nanomet. Bios. 10, 179–187 (2015)
Zhang, Z., Yi, J.B., Ding, J., Wong, L.M., Seng, H.L., Wang, S.J., Tao, J.G., Li, G.P., Xing, G.Z., Sum, T.C., Huan, C.H.A., Wu, T.: Cu-doped ZnO nanoneedles and nanonails: morphological evolution and physical properties. J. Phys. Chem. C 112, 9579–9585 (2008)
Marikkannan, M., Vishnukanthan, V., Vijayshankar, A., Mayandi, J., Pearce, J.M.: A novel synthesis of tin oxide thin films by the sol-gel process for optoelectronic applications. AIP Adv. 5, 027122–027130 (2015)
Thanh, N.T.K., Maclean, N., Mahiddine, S.: Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 114, 7610–7630 (2014)
Zeferino, R.S., Flores, M.B., Pal, U.: Photoluminescence and raman scattering in Ag-doped ZnO nanoparticles. J. Appl. Phys. 109, 014308–014314 (2011)
Chow, L., Lupan, O., Chai, G., Khallaf, H., Ono, L.K., Roldan Cuenya, B., Tiginyanuf, I.M., Ursaki, V.V., Sontea, V., Schulte, A.: Synthesis and characterization of Cu-doped ZnO one-dimensional structures for miniaturized sensor applications with faster response. Sens. Actuators A 189, 399–408 (2013)
Gandhi, V., Ganesan, R., Syedahamed, H.H.A., Thaiyan, M.: Effect of cobalt doping on structural, optical, and magnetic properties of ZnO nanoparticles synthesized by coprecipitation method. J. Phys. Chem. C 118, 9715–9725 (2014)
Pattabi, M., Amma, B.S., Manzoor, K.: Photoluminescence study of PVP capped CdS nanoparticles embedded in PVAmatrix. Mater. Res. Bull. 42(5), 828–835 (2007)
Pandey, M., Singh, M., Wasnik, K., Gupta, S., Patra, S., Gupta, P.S., Pareek, D., Chaitanya, N.S.N., Maity, S., Reddy, A.B., Tilak, R.: Targeted and enhanced antimicrobial inhibition of mesoporous ZnO–Ag2O/Ag, ZnO–CuO, and ZnO–SnO2 composite nanoparticles. ACS Omega 6(47), 31615–31631 (2021)
Udayabhaskar, R., Karthikeyan, B.J.J.O.A.P.: Optical and phonon properties of ZnO: CuO mixed nanocomposite. J. Appl. Phys. 115(15), 154303 (2014)
Aguiar, D.B.D.S.D., Aguiar, D.J.M.D., Paula, J.D.F.P.D., Cintho, O.M.: Obtaining ultrafine dispersions of silver particles in poly (vinyl Alcohol) matrix using mechanical alloying. Polymers 14(17), 3588 (2022)
Jabbar, W.A., Habubi, N.F., Chiad, S.S.: Optical characterization of silver doped poly (vinyl alcohol) films. J. Arkansas Acad. Sci. 64(1), 101–105 (2010)
Dai, Y., Tang, Q., Zhang, Z., Yu, C., Li, H., Xu, L., Zhang, S., Zou, Z.: Enhanced mechanical, thermal, and UV-shielding properties of poly (vinyl alcohol)/metal–organic framework nanocomposites. RSC Adv. 8(67), 38681–38688 (2018)
Kremer, F., Schönhals, A. (eds.): Broadband Dielectric Spectroscopy. Springer Science & Business Media (2002)
Chandegara, V.R., Joshi, P., Chavda, S., Oza, H.M., Parmar, M., Dhruv, D., Solanki, P.S., Pandya, D.D., Joshi, A.D., Shah, N.A., Trivedi, R.: Studies on properties of green synthesised Cuo/Zno Nano particle/nano rod composites in pva matrix. Zno Nano Particle/Nano Rod Composites in Pva Matrix.
Latif, I., AL-Abodi, E.E., Badri, D.H., Al Khafagi, J.: Preparation, characterization and electrical study of (carboxymethylated polyvinyl alcohol/ZnO) nanocomposites. Am. J. Polym. Sci. 2(6), 135–140 (2012)
Soliman, T.S., Rashad, A.M., Ali, I.A., Khater, S.I., Elkalashy, S.I.: Investigation of linear optical parameters and dielectric properties of polyvinyl alcohol/ZnO nanocomposite films. Phys. Status Solidi (a) 217(19), 2000321 (2020)
Gavade, C., Singh, N.L., Singh, D., Shah, S., Tripathi, A., Avasthi, D.K.: Study of dielectrical properties of swift heavy ion induced modifications in metal oxide/PMMA nanocomposites. Integr. Ferroelectr. 117(1), 76–84 (2010)
Bhajantri, R.F., Ravindrachary, V., Harisha, A., Ranganathaiah, C., Kumaraswamy, G.N.: Effect of barium chloride doping on PVAmicrostructure: positron annihilation study. Appl. Phys. A 87, 797–805 (2007)
Ahad, N., Saion, E., Gharibshahi, E.: Structural, thermal, and electrical properties of PVA-sodium salicylate solid composite polymer electrolyte. J. Nanomater. 2012, 94–94 (2012)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yempally, S., Bonthula, S. & Ponnamma, D. Triboelectric power generation performance of polyvinyl alcohol using ZnO–CuO–AgO trimetallic nanoparticles. Mater Renew Sustain Energy 13, 265–277 (2024). https://doi.org/10.1007/s40243-024-00264-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-024-00264-9