Abstract
As a result of electrochemical conversion of carbon dioxide (CO2), value-added chemicals like as synthetic fuels and chemical feedstocks can be produced. In the current state of the art, copper-based materials are most widely used being the most effective catalysts for this reaction. It is still necessary to improve the reaction rate and product selectivity of CuOx for electrochemical CO2 reduction reaction (CO2RR). The main objective of this work was synthesized and evaluate the copper oxide electrocatalyst combined with silver (CuO 70% Ag 30%) for the conversion of carbon dioxide into synthetic fuels. The catalysts have been prepared by the oxalate method and assessed in a flow cell system. The results of electrochemical experiments were carried out at room temperature and at different potentials (-1.05 V–0.75 V vs. RHE in presence of 0.1 M KHCO3) and gas and liquid chromatographic analysis are summarized. The CuOx-based electrodes demonstrated the selective of ~ 25% at -0.55 V for formic acid (HCOOH) and over CuO -Ag and selective of ethylene at ~ 20% over CuOx at -1.05 V. Other products were formed as ethylene, ethanol, and propanol (C2H4, EtOH, PrOH) at more positive potentials. On the other hand, carbon monoxide, acetate, ethylene glycol, propinaldehyde, glycoaldehyde and glyoxal (CO, CH3COO, C2H6O2, C3H6O, C2H4O2, C2H2O2) have been formed and detected. Based on the results of these studies, it appears that the formation of synthetic fuels from CO2 at room temperature in alkaline environment can be very promising.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is an urgent need for technological solutions to remove carbon dioxide (CO2) from the atmosphere to combat global warming, which is caused by an increase in the amount of carbon dioxide that is being emitted into the atmosphere. Innovative solutions are essential to achieving global energy and climate change goals [1]. It is essential that both existing technologies and those not yet on the market are deployed as soon as possible. During this decade, major efforts must be made in the area of innovation as well as deployment of these new technologies in order to bring them to market in time. By next twenty years it is expected that most of the CO2 emissions from the world’s energy sector will be reduced using new technologies that are readily available today [2,3,4,5,6,7,8]. To reduce CO2 emissions, and to store renewable energy, it is necessary to use renewable energy to convert carbon dioxide and water into synthetic fuels and chemical feedstocks [9, 10]. An abundance of evidence suggests that carbon dioxide can be converted into organic compounds using electrochemical cells with active electrocatalysts at the cathode of the cells [11,12,13]. . However, the carbonaceous synthetic fuels can be applied in several energy technologies like combined heat and power systems [14, 15]. There has been considerable interest in the development of novel, structured materials from non-noble and non-critical raw materials in recent years [16,17,18]. There are a wide variety of electrocatalysts that can be used for the CO2RR, and reduction products are highly dependent on the electrocatalyst used [19, 20]. In electrochemical CO2RR, three steps are involved, which begin with the adsorption of carbon dioxide on catalyst surfaces. A second step in carbon dioxide reduction involves activating and reducing CO2 molecules. Generally, electron transfer is the rate-determining step in creating CO2 intermediates, since it imposes a high energy barrier. Finally, the catalyst surface is recovered for further reactions after desorption of products. As an important intermediate in carbon dioxide reaction reduction, CO2 plays a significant role in determining how final products are distributed [21, 22]. It should be noted that copper-based catalysts perform differently depending on the state of oxidation of the catalyst. There is a direct relationship between the catalytic performance of an electrocatalyst and its structure and active site. Other study by Zheng et al. [23], examined the connection between the fundamentals of the reaction and the effectiveness of electrocatalysts in their critical assessment of CO2 reduction to C2 products by focusing on the fundamentals of the reaction. During a comprehensive discussion of the mechanistic aspects of the C2 reactions under electrocatalytic conditions, copper-based catalysts are discussed in terms of both mechanics and practical aspects under electrocatalytic conditions. The authors also visualized the roadmap for generating C2 products by demonstrating the advantages of integrating theoretical calculations, surface characterization, and electrochemical measurements into one process. Concerning Gao et al. [24]. , have been demonstrated that selected geometries and compositions of catalysts in combination with a carefully selected electrolyte are responsible for the enhanced selectivity of C2+ in the reaction. As it is known, copper-based catalysts exhibit different performance for carbon dioxide reduction depending on their oxidation state. Hori et al., investigated in aqueous inorganic electrolytes the acid-base equilibrium between bicarbonate and CO2 reactant involving H+ (CO2 (g) + H2O (l) + H2CO3 (aq) + H+ (aq)), whereas reduction entails protonation. As a result, H+ concentration on the surface of catalysts plays an important role in deterring their product selectivity. The C2H4 is favored when the electrolyte concentration is low (0.1 M) whereas CH4 and H2 are favored when the electrolyte concentration is high [25]. However, there is a limitation on the carbon dioxide conversion rate of electrocatalysts due to their structure and oxidation state, but it is possible to optimize their electrocatalytic performance. In general, surface vacancies are also important factors that influence electrochemical performance. Copper catalysts are one of the most commonly used electrocatalysts in electrochemical CO2 reduction [26,27,28,29] by multiple electron transfer reactions. To achieve high efficiency in CO2 reduction, however, it is necessary to have a sufficient number of active sites. It can electrochemically convert CO2 into different products, such as hydrocarbons and alcohols, due to its electrochemical properties [11, 30,31,32]. Selectivity of the products is directly related to the active copper species and the morphology, however, an irregular surface structure can be present on the catalyst surface, where wires, particles, and aggregates can be distributed in a nearly random manner. Despite this severe heterogeneity, multiple factors still support the hypothesis that high FE values are determined by local pH levels and the surface of the catalyst is covered with Cu species [33], under the surface of the catalyst, oxygen levels are monitored. The reduction of carbon dioxide involves a set of steps described as follows: dissolution, adsorption, activation, multiple electron/proton transfers, as well as desorption of carbon products from the catalyst after it has been activated. The temperature can also affect carbon dioxide solubility in aqueous solutions, as reported by several researchers [34, 35]. CO2 reduction efficiency and selectivity depend also on electrodes and reactor design in addition to the catalyst itself. Special attention should be given to the synthesizes and materials used for carbon dioxide conversion. A simple method was used to synthesize nanoparticles copper - based materials that are most widely used being the most effective catalysts for carbon dioxide reduction. With the utilization of copper nanoparticles synthesized via the oxalate method, in this work we demonstrate the selectivity CO2 conversion to multi carbon compounds in a flow cell at room temperature. We report the effect of Ag in combination with CuO at different potentials (-0.75, -0.85, -0.95, -1.05 V vs. RHE in 0.1 M KHCO3), the liquid phase outlet streams were collected and analyzed using gas and liquid chromatography.
Materials and methods
Cleaning procedure
There was a 12 - hours soak required before each experiment was conducted for all glassware and the PEEK H-cell in an acid solution containing 0.5 M H2SO4 and 1 g / L KMnO4 was conducted. To remove any remaining manganese oxide, the glassware and H - cell were rinsed and soaked in a solution of H2O2 and H2SO4. A subsequent rinse with ultrapure water was performed along with three boils in Milli - Q before the glassware and H - cell were cooled down (≥ 18.2 M Ω cm ) ultrapure water [36].
Electrode preparation
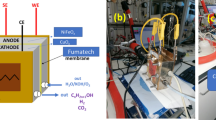
Copper oxide powder (CuO or CuO -Ag) was drop-cast onto a graphite disk. Beforehand, an ink of the respective powder was prepared by mixing 1 mg cm-2 of powder mixed with isopropanol. The ink was then dried in ambient air for at least 30 min. The resulting electrode was partially used as the working electrode. It is worth mentioning that the electrode was reduced by sweeping the potential from − 0.1 V to -1.0 V for 20 cycles at a scan rate of 50mV/s.
Electrolysis
All electrolysis experiments in this study were conducted using a custom-made PEEK H - type cell. The working electrode was used as mentioned before (3.2. Electrode preparation). The counter electrode was a dimensionally stable anode (DSA), while the reference electrode was a leak-free mini HydroFlex ® hydrogen electrode ( Gaskatel ) [36]. An anion-exchange membrane (Selemion AMVN, AGC) was used to facilitate the separation of the working electrode and the counter electrode compartment. The compartments were filled with 6 mL of electrolyte. CO2RR was carried out in 0.1 M CO2 - saturated KHCO3 (pH = 6.8) electrolyte. CO2 gas flow the purge was conducted continuously over test into the electrolyte at a rate of 15 mL / min. All potentials ( -0.75, -0.85, -0.95, -0.105 V vs. RHE in 0.1 M KHCO3) were controlled using an Ivium potentiostat ( Ivium Technologies ). During the experiment, impedance spectroscopy (EIS) was utilized to determine the resistances, and 85% ohmic drop compensation was applied (graph not shown). A sample of the gas was taken every 10 min during the experiment and analyzed using a Micro-GC (Agilent), which was equipped with two thermal conductivity detectors (TCD). One TCD was equipped with a CP-SIL 5B column to separate CO2, CH4, and C2H4, while the other TCD used a combination of MS5A and CP - PORABOND Q columns to separate H2, O2, N2, CH4, and CO. Liquid products were analyzed using high-performance liquid chromatography (HPLC, Shimadzu) with an Aminex HPX-87 H column from BioRad, equipped with a refractive index detector (RID).
Synthesis of catalysts
A copper-based cathodes electrocatalyst was synthesized according to the procedure described previously in the paper [37]. It involved dissolving copper nitrate in distilled water and neutralizing it with NaOH in order to mix copper nitrate with oxalic acid at pH 6.5. The molar ratio of the metal and chelating agent was 10. After forming a metal complex, hydrogen peroxide was applied at 80 ° C to decompose it, resulting in a precipitate, which was filtered, washed, and dried at 100 °C for 24 h. The raw powders were subsequently calcined at 350 ° C for 120 min. The Ag catalyst was impregnated onto CuO and was successively treated in diluted 5% H2 / 95% He atmosphere to form metallic silver nanoparticles. On the prepared catalyst, ball milling at 160 rpm was performed during 24 h on the 70% CuO and 30% Ag specimens.
Physicochemical characterisation
Using a D 8 Advance diffractometer, the structure and crystallinity of the cathode electrocatalyst were determined (Bruker AXS, Germany) equipped with Ni β - filtered Cu - Kα radiation (λ = 1.5406 Å), for the purpose of this experiment, the following parameters were used: 2θ range 20–100 °, 40 kV and 40 mA, scan step of 0.03° s− 1. To obtain the micrographs, scanning electron microscopy (SEM) were used to obtain a series of images from the sample in an Apreo SEM (Thermo Fisher Scientific) with an acceleration voltage of 15 kV and an electron beam current of 0.4 nA. Electrode chemical composition was investigated by energy dispersive X - Ray spectrometry (EDX) using an Oxford Instruments X -Max N 150 Silicon Drift detector coupled to the Apreo SEM. EDX data processing was carried out with the Pathfinder™ X - ray Microanalysis software v 1.3. A chemical composition of the electrode has been calculated using the chemical compositions of ten different regions on the electrode. To determine the surface chemical composition of the samples as well as the chemical environment of the samples, X-ray photoelectron spectroscopy (XPS) was used. A Physical Electronics GMBH PHI 5800-01 spectrometer was used for this analysis (Physical Electronics GmbH, Munich, Germany), which was equipped with a monochromatic Al - Ka source (1486.6 eV) and a 300 W power beam.
Results and discussion
The copper oxide catalyst was prepared by oxalate method as active phase with small particles as a way of increasing the number of active sites on the surface of the cathode catalyst by maximizing their concentration. Both catalysts were subjected to a preliminary study to determine phase purity and morphology. X-ray diffraction studies prove that the obtained CuO is a face-centered cubic phase in agreement with standard powder diffraction. A comparison of the crystal reflection of CuO and CuO-Ag specimens is shown in Fig. 1, along with typical patterns for CuO (JCPDS card n◦ 05–0661) and metallic Ag for reference (JCPDS card n◦ 04 -0783). No phase impurities were present in the diffractogram and Scherrer equation applied to the FWHM of CuO and Ag peaks showed that, and the crystallite size was determined to be 8 nm (CuO) and 17 nm (Ag). Other investigation was conducted by SEM analyses (Fig. 2) of powders supported carbon papers. It is intended to illustrate the morphology of CuO and Ag electrocatalysts and indicating that there is little agglomeration between the crystallites of CuO. To determine the elements in the sample, elemental analysis was performed the presence of Ag was confirmed by EDX (Fig. 2a).
In the following analysis regarding the scanning electron microscopy (SEM), the images showed in the Fig. 2, representing the CuO nanoparticles with basically spherical and clustered morphology combined with silver with predominant tetrahedron structure.
XPS spectra of Ag and CuO to determine their surface valence state. Figure 3 shows the Survay of CuO -Ag and CuO. Copper oxide and silver surface atomic ratios derived from XPS were similar to those obtained from XRD, with slight oxide enrichment. To determine the atomic composition of the surface, an XPS analysis was conducted. As can be seen in Fig. 3, both catalysts showed an enhanced signal from carbon. As a support for the powder analysis, carbon paper was used during electrode assembly. The atomic composition was 0.67% silver, 18.32% copper, 36.6% oxygen, 44.7% carbon for sample (a) and 25.62% copper, 45.24% oxygen, 29.14% carbon for sample (b). There were no signs of contamination on the surfaces of the materials examined.
In Fig. 4, the spectra of Ag 3d (a) and Cu 2p (b) are reported. The binding energy position of Ag 3d 5/2 ( 368.52 eV ) and 3d 3/2 ( 374.51 eV ) ( Fig. 4a ) proves the metallic state of silver ( Ag 0 ) at the surface of the catalyst [38]. The spectrum of Cu 2p (Fig. 4b) consists of two spin-orbit components at binding energies of 933.72 eV (2p 3/2) and 953.44 eV ( 2 p 1/2 ) and two Cu 2+ shake-up satellites. Spectral shapes and peak positions indicate the presence of Cu (II) species at the surface of CuO - Ag catalyst, in addition to being consistent with the structure of cupric oxide (CuO) [16, 39, 40].
The Fig. 5. shows the results in a flow cell in a 0.1 M KHCO3 solution, two different cathodes were evaluated for electrochemical carbon dioxide reduction reactions. Two main formulations of catalysts have been developed as a result of the synthesis of oxalate, copper oxide (CuO) and copper oxide mixed with silver (CuO -Ag 70% : 30%). The CO2 reduction reaction over two different cathodes was investigated at different potentials − 0.75, -0.85, -0.95, -0.105 V vs. RHE. The composites exhibit good catalytic activity due to their synergistic properties provided by their electronic and geometric structures. It was observed that the selectivity increased for hydrogen (H2) and formic acid (HCOOH) formation at -0.85 V over CuO - Ag. Following, C2+ products were formed ethylene, ethanol, and propanol (C2H4, EtOH, PrOH) at -0.75 V. On the other hand, carbon monoxide, acetate, ethylene glycol, propinaldehyde, glycoaldehyde and glyoxal (CO, CH3COO, C2H6O2, C3H6O, C2H4O2, C2H2O2) have been detected in poor concentration. In comparison to the materials investigated by Jeon et al. [41]. , in a full flow single cell the electrocatalytic performances of Cu Ag combination catalysts of varying compositions was identified. The authors observed that at 2.2 V voltage, C2H4 faradaic efficiency appears to be 1.5 times higher in the Cu90 Ag10 than the bare Cu and has a higher current density in C2H4 relative to the bare Cu. The authors observed the synergistic effect between Cu and Ag, the Cu90 Ag10 exhibits improved performance due to the appropriate ratio between Cu and Ag between the two alloys.
On the investigated electrodes containing only CuOx, Fig. 6, ethylene formation (~ 20% of C2H4) occurred at -1.05 V vs. RHE compared than composite catalyst CuO - Ag (~ 10% of C2H4). In the experiments acetate formation seems increased at the − 0.85 V potential range basically only for both copper oxide catalysts and the conversion decreased for hydrogen. Poor FEs were observed for the CuOx electrodes at more reductive potentials.
According to Kim et al. [11]. , is possible to form at low overpotential C2-C3 products using an combination of Cu nanoparticles. The redox mechanisms during electrochemical operation result in enhanced selectivity for C2 products from copper catalysts essentially based on copper oxide. In particular, the state of oxidation of Cu and the shape of its surface determines the selectivity and productivity. It is important to note that the carbon dioxide reaction involves the transfer of multiple protons as well as multiple electrons (2 electrons, 4 electrons, 6 electrons, or 8 electrons). Despite the challenges that remain, Cu-based electrocatalysts will continue to receive considerable attention in the literature for targeted practical applications.
Conclusions
Clearly, there has been a lot of progress made on electrochemical carbon dioxide reduction reactions by the development of new electrocatalytic materials that are both more efficient and more stable, or by developing new methodologies that prevent electrochemical carbon dioxide reduction reactions from occurring within the first place. Although some advances have been made in understanding how the reaction works, there is still a lot to be understood about its mechanism. In this work two different cathodes were synthesized by oxalate method and evaluated for carbon dioxide conversion in a flow cell configuration. By enhancing CO2 reduction, Ag can mitigate the H2 evolution reaction, thereby providing a competitive advantage over carbon-based fuels at more negative potentials. Two different cathodes were evaluated for CO2RR in an alkaline environment. Using the CuOx and CuO-Ag composite catalysts, C2+ products (C2H4, EtOH and PrOH), in 0.1 M KHCO3 have been formed. These results appear very promising for alternative and innovative green fuel production from CO2 conversion over copper oxide catalysts prepared by the oxalate method.
References
Cheng, W., Dan, L., Deng, X., Feng, J., Wang, Y., Peng, J., et al.: Global monthly gridded atmospheric carbon dioxide concentrations under the historical and future scenarios. Sci. Data. 9, 83 (2022)
Sikora, A.: European Green deal – legal and financial challenges of the climate change. ERA Forum (2020)
Sosa-Nunez, G.: Relationship Between Emissions Trading System and the 2030 Agenda for Sustainable Development. Springer Climate2022. pp. 285–303
Union, E.: Opinion of the European Committee of the regions — stepping up Europe’s 2030 climate ambition towards COP26. Official J. Eur. Union (2021)
Hoegh-Guldberg O, Jacob D, Taylor M, Bolaños TG, Bindi M, Brown S, et al. The human imperative of stabilizing global climate change at 1.5°C. Science. 2019;365:eaaw6974.
Parker, C.F., Karlsson, C., Hjerpe, M.: Assessing the European Union’s global climate change leadership: From Copenhagen to the Paris Agreement. J. Eur. Integr. 39, 239–252 (2017)
Jones, M.W., Peters, G.P., Gasser, T., Andrew, R.M., Schwingshackl, C., Gütschow, J., et al.: National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Sci. Data. 10, 155 (2023)
Larson, E.J.L., Portmann, R.W.: Anthropogenic aerosol drives uncertainty in future climate mitigation efforts. Sci. Rep. 9, 16538 (2019)
Ullah Khan, I., Hafiz Dzarfan Othman, M., Hashim, H., Matsuura, T., Ismail, A.F., Rezaei-DashtArzhandi, M., et al.: Biogas as a renewable energy fuel – A review of biogas upgrading, utilisation and storage. Energy. Conv. Manag. 150, 277–294 (2017)
Lo Faro, M., Zignani, S.C., Trocino, S., Antonucci, V., Aricò, A.S.: New insights on the co-electrolysis of CO2 and H2O through a solid oxide electrolyser operating at intermediate temperatures. Electrochim. Acta. 296, 458–464 (2019)
Kim, D., Kley, C.S., Li, Y., Yang, P.: Copper nanoparticle ensembles for selective electroreduction of CO(2) to C(2)-C(3) products. Proc. Natl. Acad. Sci. U S A. 114, 10560–10565 (2017)
Zhang, L., Merino-Garcia, I., Albo, J., Sánchez-Sánchez, C.M.: Electrochemical CO2 reduction reaction on cost-effective oxide-derived copper and transition metal–nitrogen–carbon catalysts. Curr. Opin. Electrochem. 23, 65–73 (2020)
Kang, X., Li, L., Sheveleva, A., Han, X., Li, J., Liu, L., et al.: Electro-reduction of carbon dioxide at low over-potential at a metal–organic framework decorated cathode. Nat. Commun. 11, 5464 (2020)
Lo Faro, M., Antonucci, V., Antonucci, P.L., Aricò, A.S.: Fuel flexibility: A key challenge for SOFC technology. Fuel. 102, 554–559 (2012)
Ruth, J.C., Stephanopoulos, G.: Synthetic fuels: What are they and where do they come from? Curr. Opin. Biotechnol. 81, 102919 (2023)
Zignani, S.C., Lo Faro, M., Palella, A., Spadaro, L., Trocino, S., Lo Vecchio, C., et al.: Bifunctional CuO-Ag/KB Catalyst for the Electrochemical reduction of CO2 in an alkaline solid-state Electrolysis Cell. Catalysts. 12, 293 (2022)
Chan, K.: A few basic concepts in electrochemical carbon dioxide reduction. Nat. Commun. 11, 5954 (2020)
Lo Faro, M., Campagna Zignani, S., Vecino-Mantilla, S., Monforte, G., Aricò, A.S.: Co-electrolysis of CO2 and H2O using an Exsoluted Perovskite Layer. ECS Trans. (2023)
Vasileff, A., Xu, C., Jiao, Y., Zheng, Y., Qiao, S.-Z.: Surface and Interface Engineering in copper-based bimetallic materials for selective CO2 Electroreduction. Chem. 4, 1809–1831 (2018)
Bagger, A., Ju, W., Varela, A.S., Strasser, P., Rossmeisl, J.: Electrochemical CO2 reduction: A classification problem. ChemPhysChem. 18, 3266–3273 (2017)
Yan, Y., Ke, L., Ding, Y., Zhang, Y., Rui, K., Lin, H., et al.: Recent advances in Cu-based catalysts for electroreduction of carbon dioxide. Mater. Chem. Front. 5, 2668–2683 (2021)
Zignani, S.C., Lo Faro, M., Carbone, A., Pallela, A., Spadaro, L., Aricò, A.S.: Alkaline electrolysis using CuOx cathode for the conversion of carbon dioxide into liquid fuels. Mater. Renew. Sustainable Energy. 12, 141–146 (2023)
Zheng, Y., Vasileff, A., Zhou, X., Jiao, Y., Jaroniec, M., Qiao, S.-Z.: Understanding the Roadmap for Electrochemical reduction of CO2 to Multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 141, 7646–7659 (2019)
Gao, D., Arán-Ais, R.M., Jeon, H.S., Roldan Cuenya, B.: Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2, 198–210 (2019)
Hori, Y., Murata, A., Takahashi, R.: Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1: Phys. Chem. Condens. Phases. 85, 2309–2326 (1989)
Grosse, P., Yoon, A., Rettenmaier, C., Herzog, A., Chee, S.W., Roldan Cuenya, B.: Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nat. Commun. 12, 6736 (2021)
Wang, X., Klingan, K., Klingenhof, M., Möller, T., Ferreira de Araújo, J., Martens, I., et al.: Morphology and mechanism of highly selective Cu(II) oxide nanosheet catalysts for carbon dioxide electroreduction. Nat. Commun. 12, 794 (2021)
Yang, Y., Louisia, S., Yu, S., Jin, J., Roh, I., Chen, C., et al.: Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature. 614, 262–269 (2023)
Kim, J., Choi, W., Park, J.W., Kim, C., Kim, M., Song, H.: Branched copper oxide nanoparticles induce highly selective Ethylene production by Electrochemical Carbon Dioxide Reduction. J. Am. Chem. Soc. 141, 6986–6994 (2019)
Birdja, Y.Y., Pérez-Gallent, E., Figueiredo, M.C., Göttle, A.J., Calle-Vallejo, F., Koper, M.T.M.: Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy. 4, 732–745 (2019)
Roy, A., Jadhav, H.S., Park, S.J., Seo, J.G.: Recent advances in the possible electrocatalysts for the electrochemical reduction of carbon dioxide into methanol. J. Alloys Compd. 887, 161449 (2021)
Zhang, W., Hu, Y., Ma, L., Zhu, G., Wang, Y., Xue, X., et al.: Progress and Perspective of Electrocatalytic CO(2) reduction for renewable carbonaceous fuels and chemicals. Adv. Sci. (Weinh). 5, 1700275 (2018)
Hongrutai, N., Watmanee, S., Pinthong, P., Panpranot, J.: Electrochemical reduction of carbon dioxide on the oxide-containing electrocatalysts. J. CO2 Utilization. 64, 102194 (2022)
Zhong, H., Fujii, K., Nakano, Y.: Electroactive species study in the electrochemical reduction of CO2 in KHCO3 solution at elevated temperature. J. Energy Chem. 25, 517–522 (2016)
Gawel, A., Jaster, T., Siegmund, D., Holzmann, J., Lohmann, H., Klemm, E., et al.: Electrochemical CO(2) reduction - the macroscopic world of electrode design, reactor concepts & economic aspects. iScience. 25, 104011 (2022)
Marques da Silva, A.H., Raaijman, S.J., Santana, C.S., Assaf, J.M., Gomes, J.F., Koper, M.T.M.: Reprint of Electrocatalytic CO2 Reduction to C2 + Products on Cu and CuxZny Electrodes: Effects of Chemical Composition and Surface Morphology. ELSEVIER SCIENCE SA, Journal of Electroanalytical Chemistry (2021)
Arico, A.S., Gullo, L.R., Rosa, D.L., Siracusano, S., Tavares, A.B.L.C., Xicola, A.S.: Solid oxide fuel cell with Cermet Cu/Ni alloy anode. WO2004049491A1 WIPO (PCT) [(2004). https://patentimages.storage.googleapis.com/c9/63/3d/4f11029d899718/WO2004049491A1.pdf]
Corro, G., Vidal, E., Cebada, S., Pal, U., Bañuelos, F., Vargas, D., et al.: Electronic state of silver in Ag/SiO2 and Ag/ZnO catalysts and its effect on diesel particulate matter oxidation: An XPS study. Appl. Catal. B. 216, 1–10 (2017)
Crisafulli, R., de Barros, V.V.S., Rodrigues de Oliveira, F.E., de Araújo Rocha, T., Zignani, S., Spadaro, L., et al.: On the promotional effect of Cu on Pt for hydrazine electrooxidation in alkaline medium. Appl. Catal. B. 236, 36–44 (2018)
Monteiro, M.C.O., Philips, M., Schouten, K.J., Koper, M.T.M.: Energy efficient CO2 reduction to CO on Gold Gas Diffusion electrodes in Acidic Media. ECS Meeting Abstracts. MA2021–02, 1854 (2021)
Jeon, Y.E., Ko, Y.N., Kim, J., Choi, H., Lee, W., Kim, Y.E., et al.: Selective production of ethylene from CO2 over CuAg tandem electrocatalysts. J. Ind. Eng. Chem. 116, 191–198 (2022)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
The authors acknowledge the financial support provided by the EU H2020 LOTERCO2M project “CRM-free Low Temperature Electrochemical Reduction of CO2 to Methanol” Grant Agreement number: 761093 and by the EU H2020 GREEN DEAL “ECO2FUEL” project “Large-scale low temperature electrochemical CO2 conversion to sustainable liquid fuels” Grant Agreement number: 20 101037389. Dr Sabrina C. Zignani acknowledges financial support from the CNR - Short Term Mobility 2022 program. The authors also acknowledge the Prof. Dr. Marc T. M. Koper and Dr. Alisson H. M. da Silva for the support during the experiments in the laboratory at Leiden University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zignani, S.C., Aricò, A.S. CO2 conversion to synthetic fuels using flow cell reactor over Cu and Ag based cathodes. Mater Renew Sustain Energy 13, 233–241 (2024). https://doi.org/10.1007/s40243-024-00263-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-024-00263-w