Abstract
Methanolysis of yellow grease (YG) was performed to synthesize its corresponding methyl ester (YGME) using BaO loaded on ZSM-5 (BaO/ZSM-5) as a heterogeneous base catalyst that was prepared via metallic solution hydrolysis method and characterized using N2 adsorption–desorption (BET), surface basicity, XRD, TGA/DTA, SEM, FTIR and Raman techniques.### The Taguchi design approach was utilized to optimize the transesterification process factors, and among the parameters studied, calcination temperature was found to have a significant influence on YGME yield. At 70 ℃ for 3 h, a YGME yield of 95.9 \(\pm 0.94\)% was obtained using a methanol/YG molar ratio of 15:1 and 1 g (2 wt.% of YG used) of BaO/ZSM-5 sample calcined at 700 ℃. The BaO/ZSM-5 catalyst was reused six times with only a 15% decrease in activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quest for renewable and alternative fuel has dominated global scene due to the petroleum price hike, high demand for energy, environmental concerns and increase in the number of industries. Biodiesel is a suitable alternative fuel because it has physicochemical properties similar to fossil diesel, is nontoxic and biodegradable. It is produced through the transesterification of triglyceride-containing feedstock using liquid or solid catalyst with simple alcohol (methanol, ethanol and propanol). Utilizing homogeneous catalysts such as mineral acid (H2SO4, HCl), alkali (KOH and NaOH) or methoxides (CH3ONa and CH3OK) has numerous drawbacks including soap formation, high molar ratio of alcohol to oil, environmental contamination, increased separation costs and excessive reactant usage [1]. Hence, intensification of biodiesel or fatty acid methyl esters (FAME) production by utilizing solid catalysts in the form of heterogeneous base catalysts to conduct transesterification reaction would immensely enhance process economic and efficiency. This is because of the fact that heterogeneous base catalysts are reusable, recoverable, less corrosive and produce no soap [2]. Additionally, no washing of product is required which would prevent generation of wastewater.
Numerous solid catalysts have been utilized in transesterification of bio-oils, including metal (alkaline earth or transition) oxides [3, 4], alkaline metal carbonates [5] and metal dispersed on supporting materials [6, 7]. Heterogeneous catalysts are either basic or acidic, with the former exhibiting higher activity than the latter, requiring only mild operating conditions to produce biodiesel while allowing for easy catalyst regeneration [7]. Metal oxides from the alkaline earth group (BaO, CaO, MgO and SrO) have the potential to be used in alcoholysis reactions. In particular, BaO has been widely used in biodiesel synthesis due to its non-toxicity, high basic strength and ability to facilitate faster reaction rate [8]. Nonetheless, pristine BaO has significant downside, including a small surface area, a tendency to leach and a poor level of stability [9]. Therefore, research on the binding of active species on structured supports has been encouraged to enhance textural qualities, reduce leaching, increase basicity and stability, and guarantee the catalyst’s recyclability [10, 11]. Various catalytic supports, such as silica [12], alumina [13], clay [14] and zeolite [15], have been impregnated with active phase previously during transesterification reaction.
Due to its larger surface area, improved thermal stability and accessible pore volume, zeolite has been reported to be one of the best catalyst supports [16]. Lawan et al. investigated a zeolite-supported CaO catalyst for biodiesel production from waste lard (WL) using a microwave reactor. The biodiesel yield of 90.89% was achieved using methanol/WL molar ratio of 30:1 with catalyst dosage of 8% wt./vol and microwave power of 595 W for 1.25 h [15]. Wu et al. compared the activities of supported CaO catalysts with different zeolites (NaY, KL and NZSM-5) and pristine CaO in methanolysis of soybean. The CaO/NaY showed the best activity for soybean conversion into biodiesel (95%) at 65 °C for 3 h using methanol/oil molar ratio of 9:1 and 30 wt.% of CaO/NaY dosage [17]. Methanolysis of sunflower oil to biodiesel over zeolite-based catalyst was investigated, and a methyl ester yield of 93.5 wt.% was achieved at 60 °C using sodium bentonite-modified zeolite X [18]. At 120 °C and 24 h, ET 10 zeolite catalyst resulted in 80.7% conversion of soybean into biodiesel [19]. However, information is currently lacking on optimization of biodiesel production from yellow grease over BaO-modified ZSM zeolite catalyst.
The search for inexpensive feedstock for synthesis of biodiesel has motivated studies on the use of non-edible oil, algae and waste oil as starting materials for the transesterification process. The use of waste materials significantly reduces the cost of biodiesel production while producing high-quality desired product [20, 21]. Yellow grease (YG), also known as used cooking oil, used vegetable oil or waste vegetable oil, is a food waste collected from households, eateries, restaurants and hotels [22]. Safe disposal of this household waste is a cause for concern due to its environmental effect. Meanwhile, the utilization of YG as a biodiesel feedstock could contribute to industrial symbiosis and circular economy by converting waste to green fuel and lowering biodiesel production costs.
In this study, ZSM-5 (zeolite)-supported barium oxide was synthesized using a metallic solution hydrolysis method and used for methanolysis of YG to identify a suitable solid catalyst that could outperform a homogeneous catalyst. Different calcination temperatures were considered during catalyst formulation. Furthermore, Taguchi optimization was used to optimize various process factors (namely reaction temperature, catalyst dosage, methanol/oil molar ratio and calcination temperature) affecting biodiesel production.
Materials and method
Materials
Methanol (CH3OH, 99.5%), sodium carbonate (Na2CO3, 97%), barium nitrate ((Ba(NO3)2, 95%), zeolite socony mobil-5 (ZSM-5) and n-hexane (96%) were acquired from Sigma-Aldrich Chemical Company, India. Yellow grease (also known as used cooking oil) was collected from a campus canteen, Indian Institute of Petroleum, Dehradun, India, and the characteristics of the feedstock have previously been reported [23].
Synthesis of BaO/ZSM-5 catalyst
The ZSM-5-supported BaO (BaO/ZSM-5) catalyst was formulated via a metallic solution hydrolysis method reported by Olutoye et al. [14]. Aqueous solution of Ba(NO3)2 and 0.6 M Na2CO3 solution were added at the same time into 150 mL distilled water, stirred at 300 rpm, heated at 60 °C for 8 h and aged overnight at ambient temperature. The obtained solution is referred to as pillaring solution. Then, a weighed amount of ZSM-5 (20 g) with barium in distilled water was also prepared and kept overnight. The ZSM-5 suspension was mixed with pillaring solution for 30 min and aged at 50 °C for 5 h. The resultant mixture was dried in an oven overnight and calcined at three different temperatures (600, 700 and 800 °C) for 4 h. Finally, the obtained calcined catalysts were ground and immediately used for methanolysis reaction.
Analysis of ZSM-5 and BaO/ZSM-5 catalyst
Crystallographic structures of ZSM-5 and BaO/ZSM-5 catalyst were determined using X-ray diffractometer (Bruker AXS, Germany). The XRD was scanned at a rate of 0.02°/min with Cu k \(\propto\) (\(\lambda\) = 1.54 Å) radiation. The functional groups on the two sample surfaces were evaluated using FTIR spectrophotometer (Perkin Elmer, USA) and the infrared spectra were investigated in the range 4000–400 cm−1. The external morphologies of the ZSM-5 and BaO/ZSM-5 samples were evaluated using field emission scanning electron microscope (Quanta 200F, FEI, The Netherlands). Furthermore, Micromeritics surface area analyzer (Model ASAP 2010) was used for determining the specific surface area, average pore volume and pore diameter of the ZSM-5 and BaO/ZSM-5 catalyst based on N2 adsorption–desorption isotherms acquired at − 196 ℃ (77 K). Determination of specific surface area and pore sizes was achieved via Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BDJ) methods, respectively. Additionally, surface basicity of the as-prepared BaO/ZSM-5 catalyst was analyzed by ion-exchange titration technique as previously reported. The thermal stability evaluation of the raw catalyst sample was carried out under N2 gas at 20 ℃/min and 60 mL/min over a temperature range of 30–900 °C using a thermogravimetric analyzer (Shimadzu DTG 60, Japan). Moreover, the chemical structure, crystallinity and molecular interaction of the catalyst sample were analyzed using UV Raman spectrometer (HORIBA, France SAS).
Taguchi design of experiment
Herein, Taguchi optimization approach was used in optimizing the transesterification of YG into biodiesel. To investigate the effect of operating variables on the yellow grease biodiesel (YGB) yield, four main controllable factors were selected: reaction temperature, T oC; methanol/YG molar ratio, M mol/mol; calcination temperature, CT ℃; and catalyst dosage, D wt.%. Each investigated parameter has three testing conditions (denoted by levels L1, L2 and L3, as illustrated in Table 1). As depicted in Table 1, an experimental design of four controllable factors with three levels would require a total of nine experiments.
The signal-to-noise ratio (S/N) is a logarithmic parameter that is used to compare the response to the desired value [24]. The signal and noise in this study represent desirable and undesirable values, respectively. The S/N ratio is divided into three groups: larger is better (LB), smaller is better (SB) and nominal is better (NB). The experimental results from the transesterification experimental runs were analyzed using the LB criterion (Eq. 1) to estimate the optimum process conditions and to investigate the significance of individual parameters influencing the methanolysis process [25].
where \({y}_{i}\) and n are the measured response and number of repetitions under similar experimental conditions, respectively.
Two-step transesterification studies
The feedstock was first esterified by reacting 200 mL of it with 100 mL of methanol in the presence of concentrated H2SO4 (0.2 mL) at 65 ℃ for 1.5 h in a two-neck round bottom flask equipped with a condenser and thermometer. Following the reaction, the excess methanol was evaporated and the esterified product was thereafter washed to remove dissolved H2SO4. The FFA content of the YG was once again determined via the titration method and its value was found to be less than 3.0 wt.% (minimum FFA content required for transesterification to occur). Subsequently, the esterified YG was transesterified into biodiesel using the same experimental devices as mentioned above. In the transesterification process, the required amounts of methanol and BaO/ZSM-5 catalyst were mixed and shaken at 55 °C for 20 min to generate methoxide, which was later added to the esterified YG, and the reactions were carried out based on the process conditions investigated (see Table 2).
After the methanolysis reaction was completed, the products formed were separated by centrifugation and the biodiesel, which was obtained as the top product, was heated in a vacuum evaporator to get rid of excess methanol. After that, the FAME content in the produced biodiesel was determined using GC (Agilent GC 7890A), flame ionization detector and J & W DH-5HT capillary column of 15 m \(\times\) 0.32 mm \(\times\) 0.1 mm. The internal standard and carrier gas used were methyl heptadecanoate and helium gas, respectively. The FAME content (Y) was calculated using Eq. (2).
where \(w_{i}\) and \(w_{b}\) are the weights of the internal standard and biodiesel sample, respectively, while \({A}_{i}\) and \({A}_{b}\) signify the total peak areas of the internal standard and biodiesel sample, respectively.
Results and discussion
Optimization of the transeterification process by the Taguchi approach
The effects of the reaction temperature, methanol/YG molar ratio, calcination temperature and catalyst dosage on yellow grease methyl ester (YGME) yield were evaluated via the Taguchi optimization method. The L9 orthogonal experimental design, YGME yields and S/N ratios are presented in Table 2. Based on the obtained results, the highest YGME yield (95.4%) was achieved at 60 ℃, 12:1, 700 ℃ and 2.0 wt.% for the reaction temperature, methanol to YG molar ratio, calcination temperature and catalyst dosage, respectively.
Figure 1 displays the plots of mean S/N ratio of YGME yield against the various independent process factors (reaction temperature, methanol/YG molar ratio, calcination temperature and catalyst dosage). Figure 1a shows that the YGME yield attained a maximum at a middle level of methanolysis temperature (70 °C). At 60–70 °C, methanol begins to boil and this enhances the probability of collisions between molecules, thereby promoting complete reaction [20]. However, at higher temperature far exceeding methanol’s boiling point, lower YGME yields and higher glycerol yields were obtained, whereby gasification of methanol resulted in bubbles which inhibited the reaction on the three-phase interface [14, 20]. Figure 1b reveals that maximum YGME yield was achieved at high level of methanol to YG molar ratio, which suggested that excessive alcohol was required to shift the transesterification toward equilibrium to produce more desired product and enhance YG conversion [26].
Figure 1c shows an improvement in YGME yield when BaO/ZSM-5 catalyst calcined at 700 °C (middle level of calcination temperature) was used to catalyze YG conversion to YGME. This can be due to the fact that the BaO/ZSM-5 catalyst was homogeneously dispersed and the interaction between the zeolite and barium metal became stronger at 700 °C [27]. Moreover, the higher catalytic performance exhibited by the catalyst sample calcined at 700 °C might be due to the well-inserted barium metal in the framework of the zeolite, which improved the surface basicity of the catalyst. According to Refaat [28], the base sites over heterogeneous catalysts are active centers for transesterification reaction. Therefore, the influence of basicity of catalyst on catalytic activity could be correlated. However, low yield of YGME reported at calcination temperature above 700 °C, as seen in Fig. 1c, was as a result of collapse of the catalyst structure which resulted in agglomeration of the catalyst particle, thereby reducing the surface area of the catalyst and consequently lowering the YGME yield [24]. Figure 1d shows the impact of the catalyst dosage on the synthesis of YGME via transesterification process, which revealed the formation of more biodiesel at 2.0 wt.% (middle level of catalyst dosage). This finding suggested that moderate BaO/ZSM-5 catalyst quantity could enhance the interaction between the methanol and the catalyst. However, at higher catalyst dosage (> 2.0 wt.%), the YGME yield reduced probably due to soap formation that was caused by the saponification reaction between the remaining FFA and the base catalyst, thus making the separation of the biodiesel difficult from the product mixture [23]. Consequently, the YGME yield decreased as the BaO/ZSM-5 dosage increased.
Thus, the optimum condition levels for the maximum YGME yield, as indicated in overall results (Fig. 1), were 70 °C methanolysis temperature, 15:1 methanol/YG molar ratio, 700 °C calcination temperature and 2.0 wt.% catalyst dosage. At these established optimum conditions, the predicted YGME yield was 97.9% (S/N ratio = 39.8). Following the establishment of the optimal levels, methanolysis experiments were carried out to validate the predicted output parameter, and the average maximum experimental YGME yield and corresponding S/N ratio were determined to be 95.9 \(\pm 0.94\)% and 42.6, respectively. When the aforementioned optimum condition (70 °C, 15:1, 700 °C and 2 wt.%) is compared to the reaction condition (60 °C, 12:1,700 °C and 2 wt.%) that provided the highest YGME yield in run 7 (see Table 2), it can be assumed that the former (optimum condition) is more favorable to biodiesel yield, most likely because methanol had already vaporized at 70 °C, facilitating mass transfer of reactants and dispersion of the catalyst particles [14]. In addition to this, excessive methanol shifted the equilibrium to form higher YGME yield. These findings indicated that the Taguchi method is very capable of identifying the right optimum condition even when it is not on the design matrix.
ANOVA for transesterification of YG over the BaO/ZSM-5 catalyst
ANOVA was used as a statistical tool to confirm the significance of each of the investigated variables, which was necessary to understand their contribution. Table 3 lists the ANOVA results for the YGME yield, including the F value, p value and contribution factor (CF). Based on the ANOVA results, the most significant influence on YG conversion into YGME was the calcination temperature, which had a CF (estimated using Eq. 3) of 72.1%. Furthermore, the S/N ratio ranges for the examined parameters were estimated to determine their ranks. According to Table 4, the first (1st) rank indicated that the calcination temperature had the greatest impact on heterogeneous catalyzed transesterification reaction, followed by methanol/YG molar ratio, while the methanolysis temperature had the least impact on YGME production rate. This was expected because the range of values of temperature investigated was within the boiling point of methanol.
where \({\text{SS}}_{i}\) is the sum of squares of a certain parameter and \(\sum {\mathrm{SS}}_{i}\) is the total sum of squares of all the parameters.
Analysis of BaO/ZSM-5 catalyst
Having proved through catalytic activity studies that BaO/ZSM-5 catalyst calcined at 700 °C showed the best performance among the three catalyst samples investigated, various analyses (TGA/DTA, BET, surface basicity, SEM, XRD, FTIR and Raman spectroscopy) were carried out so as to gain insight into the catalyst properties.
The textural characteristics and basic strength of the support (ZSM-5) and barium-modified ZSM-5 (BaO/ZSM-5) catalyst were determined, and the obtained findings are displayed in Table 5. The specific surface area, total pore volume and average pore diameter reduced as barium metal was dispersed on the support, pointing to the partial blockage of the catalyst pores by the multilayer dispersion of the active ingredient [14, 15]. A similar observation was reported during barium metal loading on montmorillonite K10 as reported by Olutoye et al. [14]. However, BaO/ZSM-5 catalyst had higher surface basicity compared to structure support (ZSM-5) which suggested that the surface of the former was dominant of basic sites, corresponding to the good catalytic activity [29].
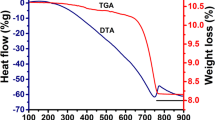
The low activity observed for those BaO/ZSM-5 samples calcined at 600 and 800 °C was due to the catalyst pore saturation with adsorbed gases (such as COX, NOX), which prevented the available pores for methanolysis and only removed at 700 °C. After surface modification and calcination, the zeolite developed a distinctive spectroscopic pattern and activity performance. This is in accordance with SEM micrographs at 25,000x magnifications as revealed in Fig. 2 in which agglomerated small particles earlier observed in Fig. 2a transformed into large agglomerates with void and some big aggregations after modification with barium and calcination at 700 °C. The raw catalyst decomposed into mixed oxides and gases. The adsorbed gases were liberated at the temperature around 700 °C [14], as indicated by the obtained data from TGA analysis (see Fig. 3). Additionally, to buttress the supported catalyst behavior, the TGA/DTA plot displayed three main decomposition stages at around 35 °C and 700 °C, as shown in Fig. 3. The first stage of the thermal degradation, which occurred at temperature between 35 and 326 °C, with 7.9% of mass loss, was due to the removal of adsorbed water molecules from the composite [30]. Over the temperature range of 326–550 °C, a 12.1% weight loss was observed in the second stage, which could be attributed to the degradation and interfering of Ba(NO3)2 with aluminosilicate layer of the ZSM-5, similar to the results reported by Hassanpour and Taghizadeh [31]. In addition, the third stage of thermal degradation, which occurred between 550 and 696 °C, corresponded to the evolution of adsorbed gases from the catalyst surface [14, 32].
These findings were corroborated by the XRD analysis results (Fig. 4) which indicated that the diffractogram of BaO/ZSM-5 catalyst displayed peaks (23.8°, 26.2°, 27.9°, 32.4°) attributed to the BaO phases (JCPDS card No. 00-074-1228), thus confirming decomposition of nitrate, hydroxide and carbonate compounds [32, 33]. More so, other peaks belonging to SiO2 (39.5°, 60.2° and 67.6°), Al2O3 (68.4°), AlSi2(PO4)3 (60.7°) and Ca2AlSiO2 (48.1° and 52.7°) were detected in the supported catalyst due to the combination of Ba metal and zeolite [30], which rendered the catalyst active in the YG conversion into YGME.
The surface functional groups on the ZSM-5 and BaO/ZSM-5 samples were analyzed through FTIR analysis. The spectra of the two studied samples are displayed in Fig. 5. The presence of absorption bands at 3512 cm−1 and 3521 cm−1 in the spectra of the support and Ba-loaded ZSM-5 catalyst, respectively, confirmed the presence of H2O molecules, and they were attributed to the stretching modes of outer and inner O–H groups, align with FTIR data reported by Lawan et al. [15]. However, the broadness of band in the spectrum of ZSM-5 reduced upon loading of barium metal on it, attributed to water desorption after calcination [1]. The Al–OH bending vibration in the spectra of both structured support and supported catalyst appeared as absorption bands in the range of 1600–1500 cm−1 [15], and the characteristic peaks at 1030–900 cm−1 were attributed to the metal-hydroxyl (M+-OH) functional group [32]. The peaks observed on both spectra of zeolite and Ba-loaded zeolite at around 700–600 cm−1, 500 cm−1 and 400 cm−1 represented Al–Al–OH, Si–O–Al, Al–O and Si–O stretching vibrations, respectively [33]. The obtained FTIR data were corroborated by the Raman spectrum of BaO/ZSM-5 catalyst shown in Fig. 6 which displays prominent bands at 307 cm−1, 481 cm−1, 596 cm−1 and 963 cm−1 that were ascribed to the T–O–T bending mode [34], Si–O bending mode [25], Al–O–Si stretching mode [35] and T–O stretching mode [36], respectively, thus confirming that the catalyst support (ZSM-5) was high in silica and alumina as T is referred to as Si or Al atoms [37]. More so, a peak at 136 cm−1 was associated with O–Ba–O symmetric stretching mode [38] which reaffirmed successful loading of BaO on ZSM-5 zeolite.
Fuel properties of biodiesel obtained from YG
Table 6 shows the results of fuel analysis conducted on the biodiesel produced from yellow grease. The acid value of the YG biodiesel was 0.18 mg KOH/g, which was in line with the ASTM D6751 specification and suggested that the two-step transesterification technique employed to convert high FFA feedstock to methyl esters was highly effective. After converting YG to YGME, the kinematic viscosity and specific gravity values reduced and conformed to the ASTM D6751 standard, thus reaffirming a successful transesterification process. Moreover, other properties of the YG biodiesel, including flash point, cloud point and pour point which were obtained to be 147 °C, 8 °C and 6 °C, respectively, conformed to the ASTM D6751 specification. These values indicated that the produced YG biodiesel possessed better cold flow properties and was safe to store and transport [39].
BaO/ZSM-5 catalyst reusability study
The stability of the BaO/ZSM-5 catalyst was evaluated in six consecutive transesterification experiments by using the same feedstock at optimum operating conditions (70 °C transesterification temperature, 15:1 methanol/YG ratio, calcination temperature of 700 °C and BaO/ZSM-5 dosage of 2 wt.%). After each experiment, the used BaO/ZSM-5 catalyst was recovered through washing with n-hexane and oven-drying at 90 °C for 8 h. As shown in Fig. 7, YGME yield was greater than 80% even after the sixth cycle, suggesting that the heterogeneous base catalyst was stable and could be recycled for a minimum number of six times. The slight decrease in BaO/ZSM-5 activity observed might be attributed to surface poisoning caused by triglyceride molecules in YG and esters formed [11, 20]. Overall, this finding demonstrated that the BaO/ZSM-5 composite was highly stable, with only minor decay in catalytic activity.
Conclusions
The conversion of yellow grease into biodiesel over the BaO/ZSM-5 catalyst was studied. Thermal treatment of Ba-modified ZSM-5 at 700 °C was found to be critical in achieving solid catalyst with high activity for transesterification of YG to produce its corresponding methyl esters. The Taguchi optimization method was used to estimate YGME yield as a function of operating variables. The optimal methanolysis temperature, methanol/YG molar ratio, calcination temperature and catalyst dosage were 70 °C, 15:1, 700 °C and 2.0 wt.%, leading to YGME yield of 95.9 \(\pm 0.94\)%. The recovered BaO/ZSM-5 catalyst demonstrated excellent activity for six consecutive cycles under those conditions.
References
Olutoye, M.A., Hameed, B.H.: A highly active clay-based catalyst for the synthesis of fatty acid methyl ester from waste cooking palm oil. Appl. Catal. A 450, 57–62 (2023)
Yusuff, A.S., Adeniyi, O.D., Olutoye, M.A., Akpan, U.G.: A review on application of heterogeneous catalysts in the production of biodiesel from vegetable oils. J Appl Sci Process Eng 4(2017), 142–157 (2017)
Liu, X., He, H., Wang, Y., Zhu, S., Piao, X.: Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 87, 216–221 (2008)
Yoo, S.J., Lee, H.S., Vriansyah, B., Kim, J., Kim, J.D., Lee, Y.W.: Synthesis of biodiesel from rapeseed oil using supercritical method with metal oxide catalysts. Biores. Technol. 101, 8686–8689 (2010)
Dai, Y.M., Lin, J.H., Huang, S.T., Lee, W.L.W., Hsieh, C.H., Chen, F.H., Chen, C.C.: Natural soil and lithium carbonate as economical solid-base catalysts for biodiesel production. Energy Rep. 6, 2743–2750 (2020)
Wan Omar, W.N.N., Amin, N.A.S.: Biodiesel production from waste cooking oil over alkaline modified zirconia catalyst. Fuel Process. Technol. 92, 2397–2405 (2011)
Farooq, M., Ramli, A., Subbarao, D.: Biodiesel production from waste cooking oil biofunctional heterogeneous solid catalysts. J. Clean. Prod. 59, 131–140 (2013)
Fard, R.G.Z., Jafari, D., Palizian, M., Esfandyari, M.: Biodiesel production from beef tallow using the barium oxide catalyst. React. Kinet. Mech. Catal. 128(5), 723–738 (2019)
Maneechakr, P., Karnjanakom, S.: Systematic production of biodiesel fuel from palm oil over porous K2O@ CaO catalyst derived from waste chicken eggshell via RSM/kinetic/thermodynamic studies. J. Environ. Chem. Eng. 9(6), 106542 (2021)
Tang, Z.-E., Lim, S., Pang, Y.-L., Ong, H.-C., Lee, K.-T.: Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: state of the art and fundamental review. Renew. Sustain. Energy Rev. 92, 235–253 (2018)
Yusuff, A.S., Gbadamosi, A.O., Popoola, L.T.: Biodiesel production from transesterified waste cooking oil by zinc-modified anthill catalyst: parametric optimization and biodiesel properties improvement. J. Environ. Chem. Eng. 9(2), 104955 (2021)
Macario, A., Giordano, G., Onida, B., Cocina, D., Tagarelli, A., Giuffre, A.M.: Biodiesel production process by homogeneous/heterogeneous catalytic system using an acid-base catalyst. Appl. Catal. A 378(2010), 160–168 (2010)
Taufiq-Yap, Y.P., Abdullah, N.F., Basri, M.: Biodiesel production via transesterification of palm oil using NaOH/Al2O3 catalysts. Sains Malays 40, 587–594 (2011)
Olutoye, M.A., Wong, S.W., Chin, L.H., Amani, S.W., Asif, M., Hameed, B.H.: Synthesis of fatty acid methyl esters via the transesterification of waste cooking oil by methanol with a barium-modified montmorillonite K10 catalyst. Renew. Energy 86, 392–398 (2016)
Lawan, I., Garba, Z.N., Zhou, W., Zhang, M., Yuan, Z.: Synergies between the microwave reactor and CaO/zeolite catalyst in waste lard biodiesel production. Renew. Energy 145, 2550–2560 (2020)
Bare, S.R.: Surface science surface analysis of zeolite: an XPS, variable kinetic energy XPS, and low energy ion scattering study. Surf. Sci. 648, 376–382 (2016)
Wu, H., Zhang, J., Wei, Q., Zheng, J., Zhang, J.: Transesterification of soybean oil to biodiesel using zeolite supported CaO as strong base catalysts. Fuel Process. Technol. 109, 13–18 (2013)
Ramos, M.J., Casas, A., Rodriguez, L., Romero, R., Perez, A.: Transesterification of sunflower oil over zeolites using different metal loading. A case of bleaching and agglomeration studies. Appl Catal A Gen. 346, 79–85 (2008)
Supple, B., Holward-Hildige, R., Gonzalez-Gomez, E., Leashy, E.J.J.: The effect of stream treating waste cooking oil on the yield of methyl ester. J. Am. Oil Chem. Soc. 79, 175–178 (2002)
Tan, T.H., Abdullah, M.O., Nolasco-Hipolito, C., Taufiq-Yap, Y.H.: Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: catalyst characterization and biodiesel yield performance. Appl. Energy 160, 58–70 (2015)
Hums, M.E., Cairncross, R.A., Spataris, S.: Life-cycle assessment of biodiesel produced from grease trap waste. Environ. Sci. Technol. 50, 2718–2726 (2016)
Rosnelly, C.M., Sofyana, D., Sarah, S.: The processing of used cooking oil (yellow grease) using combination of adsorption and ultrafiltration membrane process. IOP Conf Ser Mater Sci Eng 334, 012066 (2018)
Yusuff, A.S., Popoola, L.T., Adeniyi, O.D., Olutoye, M.A.: Coal fly ash supported ZnO catalyzed transesterification of Jatropha curcas oil: Optimization by response surface methodology. Energy Convers Manag X. 16, 100302 (2022)
Tan, Y.H., Abdullah, M.O., Nolasco-Hipolito, C., Ahmed Zauzi, N.S.: Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by soild ostrich and chicken-eggshell derived CaO. Renew. Energy 114, 437–447 (2017)
Yusuff, A.S., Tanko, N.L., Azeez, T.M., Obende, A.O.: Methanolysis of fresh and used soybean oil to biodiesel under mild conditions: process optimization, fuel quality characterization and thermal stability studies. Chem. Eng. Process. 182, 109177 (2022)
Suwanno, S., Rakkan, T., Yunu, T., Nisa, P.N., Kimtun, P., Poonsuk, P., Sangkharak, K.: The production of biodiesel using residual oil from palm oil mill effluent and crude lipase from oil palm fruit as alternative substrate and catalyst. Fuel 195, 82–87 (2012)
Abdullahi, K., Ojonugwa, S.S., Yusuff, A.S., Umaru, M., Mohammed, I.A., Olutoye, M.A., Aberuagba, F.: Optimization of biodiesel production from Allamanda seed oil using design of experiment. Fuel Commun 14, 100081 (2023)
Refaat, A.A.: Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 8(1), 203–221 (2011)
Tan, Y.H., Abdullah, M.O., Kansedo, J., Mubarak, N.M., Khalid, M., Abdullah, E.C., Nolasco-Hipolito, C.: Biodiesel production from used cooking oil using green solid catalyst derived from calcined fusion waste chicken and fish bones. Renew. Energy 139, 696–706 (2019)
AlSharifi, M., Znad, H.: Development of a lithium based chicken bone (Li-Cb) composite as an efficient catalyst for biodiesel production. Renew. Energy 136, 856–864 (2019)
Hassanpour, S., Taghizadeh, M., Yaripour, F.: Preparation, characterization and activity evaluation of H-ZSM-5 catalysts in vapor-phase methanol dehydration to dimethyl ether. Ind. Eng. Chem. Res. 49, 4063–4069 (2010)
Yusuff, A.S., Kumar, M., Obe, B.O., Mudashiru, L.O.: Calcium oxide supported on coal fly ash (CaO/CFA) as an efficient catalyst for biodiesel production from Jatropha curcas oil. Top. Catal. (2021). https://doi.org/10.1007/s11244-021-01478
Yusuff, A.S., Bhonsle, A.K., Trivedi, J., Bangwal, D.P., Singh, L.P., Atray, N.: Synthesis and characterization of coal fly ash supported zinc oxide catalyst for biodiesel production using used cooking oil as feed. Renew. Energy 170, 302–314 (2021)
Auerbach, A.S., Carrado, K., Dutta, P.: Handbook of zeolite science and technology. CRC Press, New York (2003)
Dutta, P.K., Puri, M.: Synthesis and structure of zeolite ZSM-5: a Raman spectroscopy study. J. Phys. Chem. 91, 4329–4333 (1987)
Belaabel, R., Elabed, S., Addaou, A., Laajab, A., Rodrigeuez, M.A., Lahsni, A.: Synthesis of LTA zeolite for bacteria adhesion. Bol. la Soc. Esp Ceram, Vidr. 55, 152–158 (2016)
Attila, O., King, N., Meirer, F., Weckhuysen, B.M.: 3-D Raman spectroscopy of large zeolite ZSM-5 crystals. Chem-A Eur J. 25(29), 7158–7167 (2019)
de Waal, D., Range, K.J., Konigstein, M., Kiefer, W.: Raman spectra of the barium oxide peroxide and strontium oxide peroxide series. J. Raman Spectrosc. 29, 109–113 (2019)
Yusuff, A.S., Dada, T., Olateju, I.I., Azeez, T.M., Azeez, S.O.: Experimental investigation of influence of methyl, ethyl and methyl-ethyl ester blends of used cooking oil on engine performances and emissions. Energy Conv Manag X 17, 10034 (2023)
Funding
The authors received no financial supports for this research or publication of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Ethical approval
The research involved no human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yusuff, A.S., Onibonoje, M.O. Biodiesel production from transesterified yellow grease by ZSM-5 zeolite-supported BaO catalyst: process optimization by Taguchi’s experimental design approach. Mater Renew Sustain Energy 12, 199–208 (2023). https://doi.org/10.1007/s40243-023-00240-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-023-00240-9