Abstract
Nowadays, addressing the drawbacks of liquid electrolyte-based batteries is a hot and challenging issue, which is supposed to be fulfilled through solid electrolyte systems such as polymer electrolytes. Polymer blend electrolytes (PBEs) are widely investigated as viable options to solve the undesired characteristics of their liquid counterparts and also the poor ionic conductivity of homopolymer-based electrolytes. Even though PBEs outperform homopolymer-based electrolytes in terms of performance, the conductivity of pristine PBEs is quite low for practical applications (i.e. below 10–3 S/cm at room temperature). A very promising approach to solve this limitation is to incorporate additives into the electrolyte systems, to select suitable polymeric materials and to employ the desired synthesizing techniques as the performance of PBEs is strongly dependent on the selection of polymeric materials (i.e. on the inherent properties of polymers), the nature and amount of salts and other additives, and also the techniques employed to synthesize the polymer blend hosts and/or polymer blend electrolytes, determining the functionality, amorphousness, dielectric constant, dimensional stability, and, ultimately, the electrochemical performances of the system. This paper reviews the different factors affecting the miscibility of polymer blends, PBEs synthesizing techniques, the thermal, chemical, mechanical and electrochemical characteristics of PBEs, and also the challenges and opportunities of PBEs. Moreover, the paper presents the current progress of polymer blend electrolytes as well as future prospects for advancing polymer blend electrolytes in the energy storage sectors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
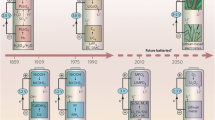
The demand for renewable and sustainable energy sources, as well as the associated energy storage/conversion devices, is significantly growing in light of the increased use of fossil fuels and their negative environmental impact [1,2,3,4]. Energy storage/conversion devices are needed to address the problems of natural intermittency and variability of electricity generated by renewable sources [2, 4, 5]. In this regard, different types of energy storage/conversion devices such as batteries, capacitors, fuel cells, and others have been considered as alternative energy storage/conversion devices because of their excellent electrochemical performances [3, 4]. Among the different forms of battery systems, Li-ion batteries (LIBs) have captured the attention of energy storage manufacturing industries due to their high energy density, good cycle stability, and long cycle life (Fig. 1a)[3, 4, 6, 7]. Even though the disposal of LIBs remains a major concern, the LIB market continues to grow year after year because of the importance of LIBs in our daily lives. The main factors driving the growth of LIB include its low price, the rapid growth of the electric vehicle market, the rapidly growing renewable sectors, and the increased consumer electronics production. Among the various end-user sectors, the automotive market has the most potential for increasing LIB demand in the future since electric vehicles will be used as a means of reducing carbon dioxide emissions, facilitating the disappearance of fossil-fueled vehicles. The battery system in electric vehicles could be constructed either to be charged through the traditional charging system or solar energy. Harvesting solar energy would be accomplished by implanting flexible and wearable battery systems on the appropriate parts of the cars. However, the existing LIBs suffer from serious limitations including flammability, leakage issues, volatility, reactivity with the electrodes, dendrite growth, prone to window potential, and thermally unstable. This made them unsuitable for a wide range of applications needing higher energy density, higher operational temperature, flexibility, and other characteristics [8, 9]. Hence, the majority of the currently conducted research activities focus mainly on the replacement of liquid electrolytes with solid electrolytes such as polymer electrolytes to solve the undesired characteristics of the existing LIBs.
Polymer electrolytes (PEs), which are made up of a wide range of organic compounds and inorganic salts, serve an important role in boosting the power and stability of lithium battery systems [10,11,12,13]. Furthermore, PEs can work as separators between electrodes, simplifying the manufacturing process and offering flexibility to LIBs, allowing PEs-based LIBs to be used in wearable technologies. However, the homopolymer-based electrolytes depicted unsatisfactory ionic conductivity at lower temperatures due to the inherent properties of the polymeric hosts such as structural rigidity that restricts the translational and rotational motions of polymeric chains, impeding ion diffusion [13]. The poor ionic conductivity and other issues of polymeric electrolytes required multidimensional research activities to be conducted on the mechanism of ion transportation and polymer-salt interactions in PEs, allowing the possibility to engineer the characteristics of polymers and salts suitable for the intended applications. This in turn facilitates researchers to focus on creating suitable polymeric membranes and also on the nature of salts to be incorporated. The majority of research on polymeric membranes has focused on lowering the glass transition temperature and structural reorganization tendency of polymeric hosts as well as increasing the functionality of hosts. This is intended to be accomplished through chemical and physical methods like copolymerization, crosslinking, and surface modification reactions, polymer blending, incorporation of fillers, plasticizers, and ionic liquids [14,15,16,17,18,19]. Among these methodologies, the physical means have emerged as an economically feasible approach to improve the performance of pristine homopolymer-based electrolytes which attracts the attention of many research groups and becomes the focus area of this review article. Polymer blend electrolytes (PBEs) for LIBs are in the early stages of development and hence it is vital to gain a thorough understanding of the impact of PBEs on the electrochemical performance of batteries. The review discusses the different parameters affecting the polymer–polymer miscibility and the commonly employed methods to synthesize PBEs. Moreover, it documents the advancements, challenges, and opportunities of PBEs with several proposed research strategies to tackle the limitations of PBEs. The energy density of different batteries, the configuration of PE-based batteries, and the ionic conductivity and mechanical stability of different electrolytes are illustrated in Fig. 1. As demonstrated in Fig. 1c, the ionic conductivity of the electrolytes decreases with increasing structural rigidity of the hosting materials as the system's diffusion and local motion is constrained, yet its mechanical strength rises with increasing structural rigidity.
Effects of polymer miscibility on the polymer blend membranes
In many different fields, homopolymers or copolymers alone may not always be able to satisfy all the requirements for practical usage. As a result, polymer blending, a physical combination of two or more polymers or copolymers without strong chemical bonds, became one of the workable solutions for achieving the desired properties of polymeric materials [7, 24]. In addition to offering the desired properties, synthesizing new polymeric materials by blending polymers or copolymers is more economically feasible than creating new polymers or copolymers by chemical reactions. One of the popular and expanding study areas in polymer technology involves the inexpensively synthesizing of novel materials with improved properties to satisfy unique end-use requirements, increasing and expanding the market for polymer blends steadily. Polymer blends are widely utilized in a variety of industries, including the automobile industry, the electrical and electronic device industry, the packaging industry, and the building and home goods industry.
Polymer blends can generally be divided into miscible and immiscible polymer blends based on their degree of miscibility [24]. A miscible polymer blend is homogenous right down to the molecular level and has a negative free energy mixing value. The participating polymer components in miscible blends lose some of their identity, and the final characteristics are typically averages of the component values, showing a single glass transition temperature (Tg) value that is in between the glass transition temperatures of the constituents and closely correlated to their ratios. In heterogeneous (immiscible) blends, however, the properties of all blend components are present, indicating phase separation between the participating polymers due to a lack of strong adhesion between them. Totally immiscible blends have coarse morphologies, sharp interfaces, and poor adhesion. The Tgs of these blends will vary according to the Tgs of their component polymers. Immiscible blends have achieved commercial success after being efficiently compatibilized with appropriate compatibilizers. The overall properties of immiscible blends depend critically on two demanding structural parameters: a proper interfacial tension that generates a phase size small enough to permit the material to be considered macroscopically homogenous, and an interphase adhesion strong enough to assimilate stresses and strains without disrupting the established morphology. In polymer blending, the advantages of one polymer can partially mask the drawbacks of another.
In polymer blending, the polymer–polymer interaction, which is a determining parameter for their miscibility, is non-covalent bond such as hydrogen bonding, ionic interactions, and/or hydrophobic interactions occurring during processing/blending. The interactions existing between polymers during the blending process are influenced by various factors originating from the polymers themselves, the formulation factors, and processing conditions as shown in Fig. 2. Hence, the miscibility and interaction of polymers are influenced by the following factors (1) polarity – Polymers with similar structural characteristics or with similar polarities are prone to interact and generate miscible blends (2) specific group interaction—the presence of particular group interactions, such as hydrogen bonds, acid–base interactions, charge transfer, ion–dipole interactions, donor–acceptor adducts, or transition metal complexes, between polymers is very likely to result in miscibility (3) molecular weight—A lower molecular weight leads to greater randomization during mixing, thereby increasing entropy, and facilitating miscibility. Surprisingly, a polymer with a similar molecular weight is more miscible than one with a widely different molecular weight, even if they have a similar composition (4) Crystallinity—when a polymer crystallizes, a two-phase system is created. It is therefore extremely rare for two polymers to co-crystallize in a single crystalline phase in a blend, resulting in the formation of two separate crystalline phases when both polymers in the blend crystallize. As a result, a polymer crystallizing in a blend creates another phase in the system (5) Blend Ratio—despite the fact that two polymers appear to be immiscible, small amounts of one polymer can be soluble in large amounts of the other polymer.
Factors affecting the polymer–polymer interactions and hence miscibility of polymers [25]
Methods employed to synthesize PBEs
PBEs are being extensively researched as promising electrolytic systems because of their synergetic effects, which provide an opportunity to synthesize high-performance batteries with better safety characteristics. PBEs can be synthesized using various methods, resulting in polymer membranes with distinct morphologies that will potentially affect the electrochemical performances of the electrolytes. Among the different methods, solution casting [26], phase inversion [27] and electrospinning [28] techniques are the most widely employed methods to synthesize polymer blend electrolytes. For high-performance characteristics, it is crucial that the preparation methods should be optimized to achieve the desired porosity and pore size of the electrolyte.
Solution casting
Solution casting is one of the widely employed polymer film and composite as well as gel-forming methods to fabricate porous membranes, offering a porosity of about 40%. Commonly, the desired polymers are dissolved separately in suitable solvent/s, and then the separately prepared solution is mixed and stirred mechanically or electronically together with salt to prepare the slurry. Fillers and/or plasticizers could be added to the PBE solution under stirring to form hybrid and/or plasticized PBEs. The prepared slurry is then cast on a substrate and subjected to a drying system to obtain the PBEs. For example, Rajendran groups [29] fabricated Poly(methyl methacrylate)/Polyvinylidene fluoride polymer blend electrolytes using dimethyl phthalate (DMP) and LiCF2SO3 as plasticizers and a lithium salt, respectively, through solution casting techniques. The synthesized PBEs (i.e. plasticized) exhibited a conductivity of 9.14 × 10−4 S/cm at 30 °C. The casting approach has the benefit of not requiring any special instruments, but the drawback is that the porosity and film thickness cannot be precisely adjusted. Some of the PBEs synthesized through the solution-casting approach are tabulated in Table 1.
Phase inversion or separation
Phase inversion is a simple and versatile technique that produces more porous structures than that membranes synthesized through solution casting methods, hence improved conductivity. The initial stage is similar to that of the solution casting method, involving dissolving the polymers in a solvent, incorporating the salts, fillers, and plasticizers under stirring, and then casting the solution on a substrate. In the next step, the membrane is immersed in a flowing non-solvent liquid, allowing the solvent to diffuse to the non-solvent liquid and the non-solvent liquid oppositely penetrates the synthesized membrane. The resulting homogeneous film is then precipitated, due to the exchange of solvent and non-solvent, and finger-like, as well as sponge-like structures, are formed. Finally, the porous PBEs are obtained after drying at elevated temperatures. The solvent—non-solvent mutual diffusion is significantly important to enhance the porosity of the system. For example, Xi J groups [38] synthesized plasticized Polyvinylidene fluoride (PVdF)/ Polyethylene oxide (PEO) blend electrolytes via the phase inversion method, delivering an ionic conductivity of 2 × 10–3 S/cm with porosity greater than 84%. The addition of PEO in PVdF enhances the pore configuration, including porosity, pore size, pore connectivity, and improves room temperature ionic conductivity. By adjusting the experimental conditions, the structure of the membranes may be changed using this approach, and the membranes have fewer flaws and a continuous production process. However, the technique generates a significant volume of organic effluent, which is easily a source of environmental damage. The successful casting of phase inversion membranes is hampered by the inability to forecast the best solvent systems. Some of the PBEs synthesized through the phase inversion approach are tabulated in Table 2.
Electrospinning
Electrospinning is an advanced method used for the synthesis of nanofibers and microfibers with definite porous structures that could effectively be employed as electrolytes with better electrochemical performances. In this method, the polymer precursors are dissolved in a volatile solvent and then the polymer solution is forced through a spinneret under a high voltage to form polymeric fibers with nanometer diameters. The required components for producing the PBEs are mixed before electrospinning. It helps to develop polymer matrices with manageable porosity, pore size, and thickness. The factors influencing the morphology of the synthesized fibers are the voltage, the concentration of the solution, the ambient temperature and humidity, and others. For example, Mahant YP groups synthesized an electrospun plasticized nanofibrous Polyvinylidene fluoride (PVdF)/ polymethyl methacrylate (PMMA) blend electrolytes with a fiber diameter of 325 nm at high spinning voltage, exhibiting an electrolytes uptake of 275% and a porosity value of 85%. The ionic conductivity of the membrane was 2.95 × 10–3 S/cm [43].
The electrospinning technique can be used to produce nanofiber membranes with excellent liquid electrolyte absorption, consistent pore size distribution, suitable specific surface area, and safe compatibility. It is important to note, however, that electrospinning has some drawbacks, including difficult-to-control variables like temperature and humidity. Some of the PBEs synthesized through the electrospinning approach are tabulated in Table 3.
Main parameters of polymer blend electrolytes
Polymer electrolytes display poor ionic conductivity despite having better thermal, mechanical, and electrochemical stabilities because of a confluence of slow dynamics and poor ionic solvation [49]. To improve the ionic conductivity, different methods such as polymer blending and the incorporation of additives (i.e. fillers and plasticizers) into the electrolyte systems are proposed and emerged as the most feasible approaches [50,51,52]. Two or more different polymers could be blended to form a uniform polymer blend mixture that will be employed to fabricate polymer blend electrolytes with improved properties. To synthesize efficient polymer blend electrolytes, polymers having complementary properties, such as one having good ion solvation power and the other with good mechanical performance, should be selected and blended with the appropriate proportions and conditions. In energy storage devices, PBEs conduct ions between electrodes and also separate electrodes. Hence, for real applications, appropriate PBEs are chosen based on the various criteria outlined below.
Conductivity
In batteries, the primary function of PBEs is serving as an ion-conducting medium between the electrodes, and hence their ionic conductivity is one of the most important parameters to be considered. PBEs with better ionic conductivity are required for providing fast ion mobility between electrodes during the charging/discharging operations. The ionic conductivity in PBEs depends on the amorphousness, the porosity, the proportion of better ion-conducting components, and the temperature of the system [9, 53]. The amorphousness, porosity, and temperature dependence of conductivity are related to the segmental mobility and creation of free volume in the system whereas the dependence of conductivity on the proportions of better ion-conducting component is related to the salt solvating power of the polymers, depicting that improving amorphousness might not guarantee the enhancement of conductivity in PBEs but works in homopolymer based electrolytes [53]. The temperature dependency of the ionic conductivity in PBEs could be explained by the Arrhenius theory or Vogel–Tammann–Fulcher (VTF) theory [9]. The Arrhenius expression can be expressed by Eq. (1)
where σ ionic conductivity, and \({\sigma }_{0}\) ionic conductivity at the unlimited temperature, T temperature in Kelvin, K and \({E}_{a}\) rate constant and activation energy for the conductivity, respectively. On the other hand, VTF theory can deduce ionic conductivity through Eq. (2), which can be expressed as follows:
where B pseudoactivation energy, \({T}_{0}\) reference temperature. Moreover, \({T}_{0}\) 10—50 K lower than Tg (i.e. the experimental (kinetic) glass transition and a vital parameter)
Ionic mobility in PBEs is determined by the translational and rotational motion (i.e. segmental mobility) of the polymer blend chains [9, 52], and these motions depend on the amorphousness of the system, amorphous membrane promotes chain segmental mobility, and thus ionic mobility along the amorphous regions is fast [50]. Additionally, the ionic conductivity of PBEs depends on the interactions between the polymer blend polar groups and the salt. Besides, the glass transition temperature of polymer blend membranes governs the mobility of ionic species at lower temperatures, as Tg determines membrane flexibility [50]. The lower the Tg, the greater the local motion of chains at lower temperatures, and thus the greater the conductivity. When plasticizers are added to the PBEs, ionic mobility mainly occurs along the plasticizer-rich phase rather than the local motion of the polymer blend chains, and the polymeric hosts primarily provide mechanical integrity to the system. Hence, in plasticized PBEs, ions primarily interact with the incorporated plasticizers and, to a lesser extent, with the polar groups of the polymers, improving the ionic conductivity of the system. In PBEs, both the cations and anions migrate between the electrodes, and lithium ions interact with the electron-pair donating sites of polymer polar groups. The mobility of anions reduces the lithium-ion transference number in the electrolyte, which is usually less than the anion transference number, indicating that the energy density and ionic conductivity are affected as it causes the poor utilization of active materials in the electrodes (i.e. the majority of ion migration is irrelevant to energy generation) and polarization (i.e. increasing the internal resistance [9, 54]. To address this limitation, various approaches such as designing single-ion conducting polymer hosts, polymer blend membranes (composed of electron donating and electron accepting polymer components), and incorporating additives acting as proton donors have been proposed to fix anions in the membranes, resulting in a cation transference number close to one as more free cations are facilitated to be released. The ionic conductivity of the PBEs could be computed by Eq. (3)
where t thickness of PBEs, A area of PBEs, R bulk resistance obtained from the impedance spectra
Mechanical strength
The mechanical characteristics of PBEs are the other important parameter to be considered in selecting suitable electrolytes for real applications, guaranteeing the free standing of PBEs, suppression of dendrite growth and resistance to volume changes of electrodes during the battery operation [55, 56]. Hence, high robust and elastic mechanical strength is required for PBEs. PBEs are supposed to have a mechanical strength of at least 30 MPa to withstand the stresses caused by the volumetric change of the electrodes that will be generated during battery operations [55, 56], resulting in the reduction of internal resistance and side reactions in the battery. Although PBEs have good stretching properties, the majority of them have significant residual deformation after being elongated, leading to failure mechanically after a few stretching events. The mechanical strength of PBEs could be enhanced through different methods including the addition of fillers, crosslinking of the polymer chains either using a cross-linker agent or via crosslinking chemical reactions, and others. Importantly, a thorough understanding of the interactions between PBE functional groups is required as they could play a significant role in boosting the mechanical properties of the system. Modifying the chemistry of polymer chains is a common approach to enhancing the mechanical strength of PEs. Bending and stretching tests can be used to analyze the mechanical properties of PEs. All in all, the mechanical strength of PBEs strongly depends on the inherent properties of the polymers including their configuration, and also the content of salts, the content of fillers, and also the degree of cross-linking. Shi et al. [57], for example, synthesized thermoplastic polyurethane (TPU) and polyimide (PI) blend host-based PBEs, and they investigated the effect of polymer blending on the mechanical strength of the system. As shown in Fig. 3, the tensile strength of the TPU/PI system was higher than that of the parental components due to the stretching of the parental polymers into fiber during the electrospinning electrolyte preparation technique, and hence TPU and PI fiber were in a symmetric distribution and interconnected by an interface transition layer formed by the inter-diffusion of the TPU and PI solution but the elasticity of the system has been decreased.
Influence of polymer blending on the mechanical properties of PBEs [57]
Thermal and chemical stability
A thorough understanding of polymer blend thermal stability and kinetic characteristics are critical in designing materials for specific applications such as PBEs being operated at higher/lower temperatures. The intrinsic properties of the hosts such as decomposition temperature, the phase transition temperatures (i.e. glass transition temperate, crystallization temperature, and melting temperature), and the degree of crystallinity are important criteria for the complete analysis of the thermal stabilities of PBEs. Glass transition temperature is linked to amorphous regions that allow polymer chains to move locally, facilitating ionic mobility and illustrating the ionic conductive nature of PBEs. When the system temperature reaches its melting point, PBEs soften, allowing direct contact between the cathode and anode which leads to short-circuit and heat generation in the system. The decomposition temperature, on the other hand, reveals that the polymer membranes decompose into oligomers and monomers (Fig. 4), which causes thermal runaway and the decomposed products to burn, even more, resulting in battery failure [58]. The most frequent characterization techniques for analyzing the thermal stability of PBEs are differential scanning calorimetry and Thermogravimetry. Polymer blending is critical for improving the thermal stability of the polymer blend electrolytes [59]. The thermal stability of polymer blends is related to bond energies in high molecular weight structures, type of intra/intermolecular forces, degree of unsaturation and branching, length of polymerization segments, composition, and degree of crystallization of blends [59, 60]. Under equivalent conditions including their molecular weight, polytetrafluoroethylene (PTFE, –(CF2–CF2)n–) polymer, for example, is more thermally stable than polyethylene (PE –(CH2–CH2)n–) polymer due to the difference in bond energies of C–F and C–H (116 and 97 kcal/mol, respectively) [59]. Amorphous polymers are more prone to thermal breakdown than crystalline polymers, and the existence of branches diminishes the interaction forces between polymer chains, reducing crystallinity and consequently polymer thermal stability. As illustrated in Fig. 4, the thermal disintegration of pure PEO is primarily linked to the breakage of two types of bonds (C–C and C-O) since it is a heteroatoms polymer, and the decomposition is categorized into two scission types [58]. In LIBs, the thermal profile of PBEs is influenced by the electrolyte preparation methods, the molecular weight, degree of crystallinity and branching, length of polymerization segments, the integrated additives such as salts, fillers, and plasticizers, and others. Abid et al. [61]., for example, reported that adding KI salt to the polymer blend membrane decreases the Tg of the blends while increasing the melting and degradation temperatures of the system (Fig. 4b, c) due to the plasticizing and cross-linking actions of salts. Moreover, nanofillers intentionally incorporated for improving the ionic conductivity and reducing the crystallinity and Tg of PBEs improve the thermal stability of the system significantly, hence reinforced PBEs have better thermal stability than the unreinforced PBEs. Morsi et al. [62], for example, found that the thermal degradation of polymer blend electrolytes could be significantly enhanced with the addition of nanofillers, serving as a heat sink in the system.
In battery applications as an electrolyte, if the employed PBE is thermally and chemically unstable, it would potentially damage the performance and safety of the LIBs. Furthermore, when exposed to different potentials, side reactions occur at the electrode/PBEs interfaces. Indeed, PBEs are more thermally and chemically stable than liquid electrolytes, which is advantageous for LIB applications. There would be no liquid decomposition reaction in solid PBEs, but in plasticized PBEs, liquid decomposition could occur, affecting battery performance significantly. When the liquid component is volatilized, the incorporated salts precipitate, causing the battery systems to fail catastrophically. Moreover, the liquid component would freeze at some lower temperatures which potentially impacts the electrochemical performances of batteries. As a result, plasticized polymer blend electrolytes with special functions such as antifreeze should be synthesized. These problems can also be addressed using other techniques, such as interpenetrating polymeric networks and crosslinking with an agent.
Electrochemical stability and PBEs-electrode interface
The interactions between PBEs and the electrodes, leading to the formation of a solid electrolyte interphase (SEI) at the interphases, significantly influence the electrochemical performance of the battery. The irreversible reduction and oxidation reactions of PBEs at the electrodes should be prevented or minimized to avoid the lowering of Coulombic efficiency and cycle life, and charge/discharge process hindrance. Hence, the PBEs should have wider electrochemical window potential to improve Coulombic efficiency and capacity retention. PBEs have better electrochemical stability than homopolymer-based electrolytes [63]. The chemical/electrochemical stability of the electrolytes indicates the absence or the minimal of side reactions, and dendrites at SEI. High internal resistance and serious dendrites would result from side reactions at the interface. The electrochemical stability of the PBEs can be evaluated using cyclic voltammetry (CV) or linear sweep voltammetry (LSV) characterizing instruments via Li/PBEs/LFP or Li/PBEs/Stainless steel configurations.
The SEI layer should be chemically, electrochemically, and mechanically stable to kinetically hamper the decomposition of electrolytes at the electrode, and to prevent chemical reactions between the electrolyte and the electrode, providing good electrochemical performance. As anion accumulation at the anode produces issues like concentration polarization, low Coulombic efficiency and voltage efficiency, high internal impedance, and others, it is important to control the anion transit through the polymer blend that results in anion accumulation at the anode. The anion mobility can be managed by blending electron-donating and electron-accepting polymers or by modifying the polymers with some positively charged functional groups or by incorporating some anion-trapping additives. Moreover, the mechanical stability of SEI supports the layer to withstand the stresses raised by the expansion and shrinkage of the electrodes and PBEs during the charge/discharge process. Commonly, the PBEs have poor contact at the electrode/electrolyte interface after a long time of cycling due to a lack of elastic properties. The crystallinity of the polymer blend matrix also affects the interfacial contact between the electrolyte and the electrode: amorphous phases have better adhesions with the electrodes than the crystalline parts. Poor physical contact at the interphase can result in overpotential and heterogeneous electric current, causing batteries to have poor electrochemical performance. The lower Tg value improves the conductivity and the adhesion strength of the PBEs due to improving the flexibility of the system. The electrochemical, mechanical, and chemical stability and also interfacial resistance can be improved by incorporating additives such as fillers.
Recent advancements in polymer blend electrolytes
PBEs have received a lot of attention because of their advantages like lack of leakage, low flammability, good processability and flexibility, a wide electrochemical stability window, and good thermal stability. However, at room temperature, the crystallinity and structural rigidity of the polymer blend membranes are high, limiting segmental motion and resulting in very low ionic conductivity. Significant advancements have been made in increasing the conductivity of PBEs by decreasing the crystallinity of the system using various methods, including blending of different polymers (i.e. homopolymer/homopolymer blends, homopolymer/copolymer blends), adding nanofillers, introducing liquid plasticizers, immobilizing anions with a cationic metal–organic framework filler, complexing with binary salt systems, and others.
Pristine polymer blend electrolytes
Even though homopolymer matrix provides valuable properties for use as an electrolyte, their ionic conductivity and electrochemical properties are inferior to those of liquid electrolytes because of their inherent properties such as structural rigidity, high Tg value, the high tendency of crystallinity, lack of appropriate functional groups for interacting and also solvating the incorporated salts efficiently, physical entanglement of polymeric chains, the existence of limited/lack of segmental mobility and others. So, different modifications can be adapted to the polymer matrix, in which polymer blending is considered to be one of the simplest methods that can be used for the improvement of ionic conductivity and electrochemical properties of the PEs by making use of the synergy between the characteristics of each of the individual components. Polymer blending improves the electrochemical performances of electrolytes by (1) enhancing the functional groups of the systems (2) enhancing the mechanical integrity of the system (3) decreasing the Tg of the systems (4) boosting the amorphous contents of the system (5) improving the interface between the electrodes/electrolytes (6) reducing the activation energy (7) reducing the bulk and interface resistance and others. The electrolytes made from polymer blends have improved electrochemical characteristics compared to the individual homopolymer matrix [64]. Polymer blends can be synthesized by combining either homopolymers with homopolymers, homopolymers with copolymers or copolymers with copolymers. For electrolytic applications, the homopolymer/homopolymer blend membranes have got great attentions while homopolymer/copolymer and copolymer/copolymer blend-based electrolytes have very few considerations. Hence, research work on homopolymer/copolymer and copolymer/copolymer blend-based PBEs is required to be conducted. It is worth noting that copolymerizing reaction is one of the most important approaches to enhance the miscibility of polymer blends, yielding better physiochemical and electrochemical properties. For example, instead of blending two immiscible polymers directly, the polymers can be copolymerized together, and then the copolymerized component can be blended with the two polymers, yielding better miscibility. Miscibility in polymer blends avoids phase separation, hence preventing the existence of cracks in the system and improving performance. For electrolytic applications, the miscibility of polymers will be affected not only by the nature of polymers and the processing conditions but also by the additives that will be incorporated into the system such as salts and others, see Fig. 2.
Mallaiah et al. [13] synthesized polymer blends comprising PEO/PVdF with varying PVdF contents and investigated their miscibility as well as the electrochemical performances of the blend systems. It was found that PEO/PVdF blend system exhibits a rough surface and the crystallinity nature dominates over the amorphous due to a lack of strong connectivity or functionality between the polymers (i.e. PEO and PVdF are immiscible). However, the polymer blend structure depicts a somewhat smooth surface when blended with salt due to the ion–dipole interactions. This indicates that the incorporated salt for polymer blend electrolyte preparation could serve as both a source of ionic species and also a compatibilizer between immiscible polymer components. In this study, the effect of salt on making a relatively smooth surface of the blended membrane sounds better at 20 wt% of PVdF and the electrolytes of the blended polymers coordinated with NaNO3 salt have the highest ionic conductivity at this composition due to the increased amorphousness. For synthesizing effective PBEs, the participating polymers are expected to be miscible for providing an effective path for cation transportation. To provide an ideal channel for the cation between the electrodes and enhance ionic transport, immiscible blends must have good interphases (interconnectivity) between each polymer phase [65]. Additionally, the blending of polymers having complementary properties such as semi-crystalline polymers with amorphous polymers improves the electrochemical performances of the system. Zhu et al. [53] fabricated PBEs composed of PEO (semi-crystalline) and polypropylene carbonate (PPC) (amorphous) polymers and investigated the effect of blending the amorphous and semi-crystalline polymers on the electrochemical performances and amorphousness of the system. According to XRD analysis (Fig. 5a), the addition of PPC considerably reduced the crystallinity of PEO, and the decrease in crystallinity increased with PPC content and reaches a maximum when the content of PPC exceeds 50%. Additionally, the inclusion of PPC decreases the interaction between lithium ions and PEO chains, promoting the migration of Li+ in the electrolytes—a factor that is very important practically for improving ion conductivity. The Tg values and crystallinity of the system diminished from -35.2 to—41.3 and 48.07% to 22.10%, respectively (refer Figs. 5b and c). As shown in Figs. 5d and e, the impedance of the electrolyte decreases and the Li+ conductivity increases with the increase in PPC content at different temperatures, respectively. Additionally, PPC increases the amount of salts dissociated in the system by increasing the dielectric constant of the PBEs due to the presence of its polar carbonate group (–o–[c=o]–o–). The PBEs revealed curved conductivity graphs between the low and high-temperature zones (i.e. 25–50 ℃ and 50–80 ℃ respectively, see Fig. 5e) due to the recrystallization of PEO at the low-temperature zone. The LSV analysis showed that the electrochemical stability window of the PBEs increased from 4.25 to 4.9 V with the increase in PPC (see Fig. 5f) due to the wide electrochemical window of PPC itself. Figure 5g also depicts that the PBE has a higher specific discharge capacity than that of the neat PEO-based electrolyte (112 mAh/g vs 93 mAh/g) after 100 cycles due to the enhancement in amorphousness resulting in smaller bulk and interface impedance. As seen in Fig. 5h, the specific capacity of the assembled cell significantly decreased when the rate is enhanced from 0.1 to 1 °C due to the increase of overpotential. Additionally, because of the lower interface resistance and electrolyte buck resistance at 0.1 °C, the polarization voltage is relatively low and the charge and discharge voltage platform is comparatively flat. After discharging PBE at 0.1 °C to 1 °C and restoring the operation to 0.1 °C again, the discharge capacity was almost completely recovered, showing the stability and reversibility of the PBEs as shown in Fig. 5i. Besides, the cycling performance of the cell assembled with PBE containing 60% PPC was lower than that of the cell assembled with PBE containing 50% PPC, indicating that the electrochemical performance is best at 50% PPC. It is interesting to note that when the PPC content is too high, the crystallinity of the PBE is reduced even more as the PEO content is lower. However, in contrast to being more amorphous, the electrochemical performance of the PBE deteriorates as the PPC's dense structure is less favorable for Li+ migration than that of PEO. This result answers the question of “could increasing the amorphousness of the PBEs to 100% improve its the electrochemical performances?” to some extent by showing that decreasing the crystallinity and Tg of the PBEs could not guarantee the increase in electrochemical performances of the PBEs. Instead, the amorphousness and Tg of the system should be balanced with the presence of the appropriate conductive membranes. The ionic conductivity, ion migration number, and interface stability have all been significantly enhanced by combining PEO with PPC. Babu et al. [66] synthesized polyvinyl chloride (PVC)/poly (butyl methacrylate) (PBMA)/LiClO4 electrolytes, and investigated the effect of PBMA on the characteristics of the PBEs. The XRD analysis exhibited that the amorphousness of the PBEs increased with increasing PBMA concentration up to 50%, however, beyond 50% concentration, the XRD peak corresponding to the PBMA system is observed to dominate. The conductivity of PBEs increased with increasing in PBMA concentration and reached a maximum of 6.27 × 10–5 S/cm at 100% PBMA but its mechanical strength is significantly reduced from 7.29 to 0.48 MPa due to the dipole–dipole interaction between chlorine and hydrogen atom in PVC weakened by the addition of flexible and amorphous PBMA. Hence, balancing the conductivity and mechanical characteristics of the PBEs is necessary. When compared to other electrolytes, PBEs synthesized from a 50:50 blend of PVC and PBMA polymers offered reasonable mechanical stability (4.23 MPa tensile strength) and noticeable ionic conductivity of 1.11 × 10–5 S/cm, and the optimized PBEs are thermally stable up to 250 ℃.
Illustration of a amorphous/crystallinity arrangement b XRD analysis c DSC analysis d Nyquist plots e conductivity at different temperatures f LSV plots g specific capacity and columbic efficiency vs cycle number h the charge–discharge platform i rate capacity of neat PEO electrolyte and the blended electrolytes [53]
The presence of polar chains having lone pair electrons to form co-ordination bonds with cations and less barrier to bond rotations influences the choice of polymers as a host for PEs preparation, and polymer blending plays an important role in achieving good mechanical, thermal, and electrical properties [13]. Despite the fact that polymer blending is proposed to enhance the conductivity of homopolymer electrolytes, their conductivity remains below the required level at lower temperatures. As a result, improving the conductivity of PBEs through various approaches remains an open question. In addition to polar groups and barrier-to-bond rotation, the molecular weight of polymers, tacticity of polymers, and blend preparation methods should be taken into account when synthesizing PBEs, as these parameters can influence the morphology, amorphous domains, Tg, and other properties of the electrolytes. In addition to these parameters, the chain length of the grafted component should be considered when synthesizing homopolymer/copolymer or copolymer/copolymer blend electrolytes. The molecular weight of polymers influences the compatibility and miscibility of the amorphous phases of both blend components. For example, the molecular weight of PMMA polymer doped on PEO-alkali electrolytes was observed to potentially affect the conductivity of electrolytes inversely at a lower temperature (i.e. below the melting temperature of PEO), conductivity increased with decreasing the molecular weight of PMMA [67]. This could be because shorter molecules of PMMA can penetrate the PEO chains more easily, increasing distances between helices and thus lowering the crosslinking effect of the added salt. Such interactions may result in a lower Tg value for the complex's amorphous phase, increasing its ambient temperature conductivity. Furthermore, because tacticity determines the morphology of the system, the tacticity of polymers may affect the conductivity of the polymer blend electrolytes. It was discovered that PEO-PMMA blend electrolytes based on isotactic PMMA polymer have conductivities that are about one order of magnitude higher than PEO-PMMA blend electrolytes containing syndiotactic and atactic PMMA polymer across the entire range of temperatures due to the lower Tg value of isotactic PMMA and the morphology of the PEO-isotactic PMMA system, which creates fast ionic transport channels [67]. The PBE preparation methods have a strong influence on the conductivity of the electrolytes by determining the porosity, pore size, and pore distribution, as well as the amorphousness and electrolyte uptake of the system. Furthermore, the electrolyte/polymer blend preparation techniques could potentially affect the conductivity of PBEs. Overall, the molecular weight, tacticity, polar groups, the ratio of participating polymers, barriers to bond rotation, preparation methods, and side chain size should be taken into account when synthesizing PBEs as these parameters influence the morphology, amorphousness, viscosity, Tg, mechanical, electrochemical performances, and other physical properties of the system. Furthermore, the role, size, and structure (tacticity) of the grafted copolymer in homopolymer/copolymer and copolymer/copolymer blend electrolytes should be thoroughly investigated.
Polymer blend-composite electrolytes
Composite polymer blend electrolytes are similar to PBEs but with the inclusion of different nanofillers dispersed in the polymer blend matrix to improve the interface with the electrodes, thermal, mechanical, and electrochemical performances of the PBEs [68]. Moreover, fillers play a significant role to increase (1) the amorphousity of the system (2) the amounts of salts dissociated (3) the adhesion force between the immiscible blends, and the wettability of the electrolytes. The incorporated nanofillers improve the ionic conductivity of PBEs though reducing the crystallinity of polymeric hosts and also the Lewis acid–base type interactions between the fillers and the electrolyte solution, improving the porosity and pore morphology of the PBEs, increasing the electrolyte uptake and Tg of the system [14, 69]. In addition to weakening the inter and intrachain interactions in the polymer blend hosts, the nanofillers weaken the polymer-cation associations and hence leading to an enhancement in ionic conductivity. Generally, fillers are broadly classified as active and passive fillers [68]. Active fillers directly contribute to ion transport by enhancing free Li+ concentrations, Li+ surface conduction, anion attraction, or acting as a Li+ source in lithium batteries. Active ceramic fillers for lithium batteries include -LiAlO2, LiAl2O3, Li3N, LiN2O3, and others, whereas passive fillers influence ion transport mechanisms in polymer blends in a variety of indirect ways, including acting as "solid plasticizers," inhibiting polymer crystallization, increasing free volume, and speeding up segmental dynamics. Inert oxides (such as SiO2, TiO2, Al2O3, and others), ferroelectric materials, clays, carbonaceous and molecular sieves, and others have all been used as passive fillers in various polymer blend matrices. Composite PBEs composed of Carboxymethyl cellulose (CMC)/PEO matrices and SWCNTs/TiO2 nanohybrid fillers were synthesized [70]. With the inclusion of the nanohybrid, the ionic conductivity, thermal, dielectric, and optical characteristics of the CMC/PEO blend-based electrolytes were improved, and the crystalline phase was significantly reduced, resulting in increased ionic conductivity (increasing from 4.77 × 10− 6 S/cm to 9.23 10− 4 S/cm). The increase in dielectric property improved the content of salts to be dissociated. Li et al. [71] also investigated the effect of SiO2 nanofillers and PMHS polymer on the properties of the PEO/LiTFSI electrolyte system. The composite PBEs were created by combining SiO2, LiTFSI, Polymethylhydrosiloxane (PMHS), and PEO ingredients in an anhydrous acetonitrile solvent, and the incorporation of PHMS and SiO2 nanoparticles improved the conductivity and mechanical stability of the electrolyte. The peak of PEO totally vanished when the PMHS content reached 60%, proving that the PMHS addition prevented PEO from crystallizing. Nevertheless, the composite PBEs with 40% PMHS displayed a maximum conductivity of 2 × 10–2 S/cm at 80 °C, broad electrochemical windows (5.2 V), excellent flexibility, and thermal stability, and the cell assembled with Li/PEO-PMHS-SiO2-LiTFSI/LiFePO4 system revealed a capacity close to 140 mAh/g (0.1C) at 60 °C. This work showed that increasing the amorphousness of composite PBEs might not result in the maximum ionic conductivity since the fraction of more effective ion conductive membranes, such PEO, also affects ionic transportation. The effectiveness of the incorporated fillers in boosting the electrochemical performances of the PBEs also depends on the nature, size, distribution and concentration of the fillers[69]. The methods used to incorporate the nanofillers into the PBEs (e.g. mixing the nanofillers with the PBE solution as opposed to polymerizing one of the polymers or copolymerizing polymers on the surface of the nanofillers and then blending the polymerized/copolymerized' system with the remaining ingredients) may also have an impact on the properties of the composite PBEs. Polymerizing one of the participating polymers on the surface of the nanofillers and then blending the polymerized system with the remaining components to create composite PBEs improves electrochemical performances, amorphousness, electrode–electrolyte interfacial stability, and electrochemical window stability by improving the uniform distribution of the incorporated fillers. For example, Li et al. [72] prepared composite PBEs and investigated the effect of filler incorporation techniques on the characteristics of the composite PBEs. The composite PBEs were prepared by mixing SiO2 with the PBE solutions and also by polymerizing the Methyl methacrylate (MMA) monomer on the surface of SiO2 and then blending the SiO2-PMMA polymerized system with the remaining components. The SiO2-PMMA polymerized system-based composite PBEs exhibit better amorphousness, electrochemical window stability, electrode–electrolyte interfacial stability, and cyclability but lower thermal stability, refer to Fig. 6, due to the uniform distribution of the nanofillers in the electrolyte system, indicating that the SiO2 particles are well dispersed and embedded in the polymer matrix, yielding the most amorphous electrolyte system as shown in Fig. 6a. As shown in Fig. 6c, the SiO2—PMMA polymerized (i.e. SiO2 @PMMA) system has better stability (4.7 V) due to the interaction of SiO2 nanoparticles with the polymer matrix and TFSI anion to prevent their decomposition, and the homogenously dispersed SiO2-PMMA polymerized nanoparticles have functional sites for crosslinking with polymer segments, which could stabilize polymer-filler interfaces. Furthermore, due to the evenly distributed nano-SiO2 particles, the cells with the SiO2-PMMA polymerized system revealed better cycling performances, with approximately 81.7% capacity after 100 cycles at 0.5 °C (Fig. 6d). Figures 6e and f showed that SiO2-PMMA polymerized system-based cells displayed exceptional cyclic performances and rate capability due to the efficient Lewis- acid–base interaction between SiO2-PMMA polymerized system and the polymer matrix, which promoted salt dissociation, provided additional sites for transporting Li+ in the system, modified the polymer conformation, prevented polymer chain recombination, and maintained the low crystallinity state of the polymer matrix.
a XRD image b TGA image c electrochemical window stability (LSV curves) d cycling performance at 0.5 C discharge rate e rate performance at different current densities f evolution of normalized capacity as a function of discharge rate for three different composite membranes (CPE–PBE without filler, CPE-SiO2–composite PBE synthesized by directly adding the SiO2 filler into the PBE system and CPE-(SiO2@PMMA—composite PBE synthesized by blending the SiO2-PMMA polymerized system with the other ingredients) [72]
In composite PBEs preparation, synthesizing effective particles size with appropriate morphology, and getting the even distribution and optimal concentration of the nanofillers are some of the factors to be considered critically. Optimizing the filler content in the PBEs is critical as fillers beyond the optimum level cause a crystalline effect in the system, reducing the effective surface area between the electrode–electrolyte interface and ionic conductivity [35]. Although ceramic fillers can boost the ionic conductivity of PBEs, it is still far from meeting the demands of practical application. The electrochemical performances of some of the PBEs are presented in Table 4.
Polymer blend-plasticized electrolytes
Plasticizers, which are typically lower molecular weight compounds, have the potential to reduce the brittleness and increase the flexibility of polymers by reducing the cohesive forces that exist between polymer chains and thus decreasing the glass transition temperature of the host [73]. To effectively permeate into the polymer matrix and produce superior results, lower molecular weight plasticizers are preferred and the plasticizers should establish reasonable polymer-plasticizer interactions to reduce the cohesive forces of attraction between polymer chains. Plasticization is one of the most commonly used methods for reducing crystallinity and increasing the amorphous phase of PBEs to boost ion mobility and conductivity [19, 35, 74]. Plasticized PBEs are created by incorporating a certain amount of plasticizers into PBE systems. The addition of plasticizers to the polymer matrix increases deformability, elasticity, abrasion resistance, and elastic recovery in the electrolyte system [11]. Additionally, it can encourage ion-pair dissociation, which in turn increases the amount of charge carriers available for charge transfer and improves ionic conductivity [35] and the polymer blend host, on the other hand, provides the essential stability to the lithium anode-electrolyte interface, reducing the likelihood of dendrite formation on the lithium anode [75]. In contrast to their exceptional conductivity, plasticized PBEs suffer from drawbacks such as solvent volatility, poor mechanical properties due to a high degree of plasticization, and the reactivity of a polar plasticizer with the lithium electrode [69, 75, 76]. From the perspective of practical use, the plasticized PBEs should have the following qualities: strong ionic conductivity, high mechanical strength, and electrochemical stability toward the electrodes.
Xi et al. [77] prepared plasticized PBEs composed of PVdF/PEO/LiClO4/PC systems and investigated the effect of PEO concentration on the properties of the plasticized PBEs via a simple phase inversion method. It was found that the porosity and pore connectivity of the system was enhanced with the addition of PEO. The modification in porosity improved the conductivity of the plasticized PBEs greatly and the ionic conductivity was increased with the increase in the PEO content in the blend. However, as the PEO level increased in the blend, the membrane's mechanical strength declined from 85 to 30 MPa. Furthermore, at 0.1 C, the cell assembled with this plasticized PBE had an initial discharge capacity of 150 mAh/g. Thayumanasundaram et al. [78] also synthesized plasticized PBEs using polyvinyl alcohol (PVA)/ Polyacrylic acid (PAA) blend films and Pyrrolidinium-based Ionic Liquid for Lithium-Ion Batteries via solution casting techniques and investigated the effect of PAA on the performances of the PBEs. The addition of PAA reduced the crystallinity of the PVA polymer due to the formation of interpenetrating polymer chains and strong hydrogen bonding between the polymers. The glass transition and melting temperatures of the PBEs reduced with the increase in the ionic liquid contents due to the plasticization effect of the IL, decreasing the interactions between the polymer chains and, thus, increasing the free volume. The synthesized PBE exhibited a maximum ionic conductivity of 1 × 10–3 S/cm at 90 °C with 70 mol % ionic liquid.
The effectiveness of plasticizers on the segmental mobility of polymeric hosts, ion mobility, and ionic conductivity of PBEs is determined by factors such as the characteristics of plasticizers (i.e. molecular weight, dielectric constant, and viscosity as well as their amount), polymer-plasticizer interaction, ion-plasticizer interaction, and plasticizer concentration [19, 79, 80]. Plasticizers' high dielectric constant aids in salt dissociation by increasing the relative permittivity of the system, whereas their low viscosity leads to high mobility and thus higher conductivity. Rajendran et al. [80] synthesized plasticized PBEs composed of PVA/PMMA/LiBF3 systems that were plasticized with various plasticizers and investigated the effect of the nature of plasticizers on the conductivity of the electrolytes. It was found that the ethylene carbonate-based plasticized PBEs exhibited the maximum ionic conductivity due to the higher dielectric constant of EC compared to other plasticizers. Similar results were obtained in other works also [19]. To improve the conductivity of plasticized PBEs, the synergy of dielectric constant and viscosity properties needs to be considered by combining two or more plasticizers having high dielectric constant and lower viscosity values without dwarfing the effect of dielectric constant [19] as dielectric constant is critical in dissociating the incorporated salts. Moreover, the solidifying issues of the plasticizers at ambient temperature should be considered for better conducting plasticized PBEs at lower temperatures.
Overall, plasticized PBEs have improved ionic conductivity and can operate at lower temperatures. However, the mechanical, thermal, and chemical stability of the electrolytes to withstand dendrite growth resulting in short circuit, unwanted chemical reactions with the electrodes resulting in the loss of active components, the decomposition of electrolytes, and other issues become serious drawbacks, necessitating an effort to address these issues. Combining plasticizers and inorganic fillers, which trade off the conductivity and mechanical integrity of PBEs, is one potential remedy for the drawbacks of plasticized PBEs. For example, Rajendran et al. [81] synthesized a PVC/PAN/LiClO4/EC plasticized polymer blend electrolytes, and the electrolytes exhibited a conductivity of 7.57 × 10−5 S/cm) at room temperature. The ionic conductivity of the plasticized PBEs was boosted to 4.46 × 10−3 S/cm upon dispersing TiO2 fillers due to the increase in amorphousness of the system. Simari et al. [82] also prepared a plasticized and reinforced polymer blend electrolytes and investigated the effect of additives on the electrochemical performances of the PBEs. The SEM study revealed that the plasticized and reinforced PBEs are homogenous, demonstrating a positive interaction between the system's constituent parts. The amorphousness and the mechanical property (i.e. Modulus of elasticity) of the plasticized PBEs increased with the inclusion of clay fillers. Additionally, the ionic conductivity of the system has been improved with the inclusion of fillers due to the decrease in amorphousness and the increase in free volume. The electrochemical performances of some of the plasticized and reinforced composite PBEs are tabulated in Table 4.
Opportunities and challenges of polymer blend electrolytes
Based on the preceding analysis and discussion, PBEs, as opposed to liquid electrolytes, can effectively relieve electrode material dissolution and provide stable electrochemical performance while avoiding the leakage and electrolyte evaporation issues associated with liquid electrolytes. Furthermore, using polymer membranes, the mechanical properties of the electrolytes could be improved. However, the undeniable challenges of PBEs prevent them from being used in real-world applications, particularly in solid electrolyte form. To develop high-performance batteries using PBEs, it is critical to understand how to mitigate the following challenges:
-
1.
Low ionic conductivity at room temperature: even though the conductivity of homopolymers has been improved by 1–2 orders when blended with other polymers, the conductivity of PBEs is still inadequate for real applications at room temperature (RT) due to improper management of membrane crystallinity or losing the contents of better ion conductive component during amorphousizing process. In addition to polymer blending, the conductivity of PBEs could be improved at least by one order when doped with nanofillers by reducing crystallization of the membranes, and also by increasing the dielectric constant of the system and supplying more free ions in low-dissociation environments, increasing the cation transference number, thereby improving ionic conduction. Moreover, the conductivity of PBEs could be significantly enhanced by adding plasticizers and ionic liquids into the system, which improves ionic mobility by weakening polymer chain interactions as well as cation-polymer chain interactions but at the cost of mechanical and thermal stability. In this regard, developing new additives that can enhance conductivity with minimum destruction of the dimensional and thermal stability of plasticized PBEs is very appealing. Additionally, the cation ion transfer number can be improved using single ion conducting PBEs, synthesized through blending electron-donating and electron-accepting polymers or by modifying the surface of one of the participating polymers using positively charged sulfonic acid and other groups or by adding some additives that can trap the anion species. Furthermore, polymerizing alkali salt monomers and blending them with other polymers can be a possible technique to fabricate single-ion conducting PBEs. However, it is still under investigation.
-
2.
Mechanical instability. The ionic conductivity of PBEs is inversely related to their mechanical strength. It is well recognized that the enhancements in ionic conductivity of PBEs come true at the cost of their mechanical strength, indicating that the dimensional stability PBEs should be addressed without damaging the conductivity of the system. Cross-linking of the polymer blend hosts/or chains of the polymers and also incorporating the nanofillers into the polymer blend systems are the two most important mechanisms to improve the mechanical stability of the system, highly porous 3D architecture with high mechanical stability could be created through crosslinking reactions. However, high loading of the crosslinking agents and nanofillers impedes the segmental mobility of PBEs and also decreases electrolyte uptake, declining conductivity.
-
3.
Uneven formation SEI. The rate of ion flow in battery systems is significantly influenced by SEI, and a properly generated SEI avoids electrolyte decomposition, anode dendrite formation, and cathode pulverization. However, SEI is frequently unevenly formed and destroyed by anode volume changes, leading to dendrite formation. Therefore, employing a 2D or 3D phase anode can produce a bigger specific active surface area and a greater cation transference number, assisting in the reduction of concentration polarization and the inhibition of dendrite formation. An artificial SEI could prevent cathode pulverization, and a 3D skeleton could improve electrode–electrolyte wettability. Both of them could also improve the electrode's electronic conductivity and ion diffusion, thereby improving cathode-PBE compatibility. Many efforts have been made to stabilize SEI. However, additional efforts are still required.
Conclusion and future outlook
This paper shows a general overview of PBEs for battery applications. PBEs play a significant role in the electrochemical performances of battery systems and improve the poor qualities of homopolymer-based electrolytes. To achieve the desired electrochemical and physiochemical performances, the selection of materials and PBE synthesizing methods are critical. In this regard, this review presents the different synthesizing methods and analyzes the most important recent works in this field. The improvement of porous structure, electrolyte uptake, and ionic conductivity while maintaining the mechanical, thermal, and electrochemical stability of the PBEs is, however, a challenging issue that could be achieved by the combination of blending, crosslinking, reinforcing, and plasticizing of the appropriate polymer hosts. Regarding polymer blend membranes, the inherent properties of the participating polymers such as their tacticity, functional groups, molecular weight, amorphousity, and other issues should be considered, and combining polymers having complementary properties provides better results. Additionally, the polymer blending techniques or PBEs synthesizing techniques should be considered seriously as the synthesizing techniques control the size, content, connectivity, distribution of porosity, and pore structure (i.e. determining the mechanical and electrochemical properties) and also electrode compatibility. A promising approach being explored is the use of PBEs with nanofillers to enhance the conductivity and mechanical integrity of the system, revealing an improvement in ionic conductivity by 1–2 orders through suppressing the crystallinity of the system, increasing the content of salts dissociated by competing for cations though Lewis acid–base interactions and also increasing the dielectric constant of the system. However, the effectiveness of nanofillers depends on their size, concentration, nature, distribution, morphology, and also preparation methods. Surface functionalizing of fillers could be carried out to improve the compatibility between fillers and the polymer blend host for better conductivity and stability of the system. Additionally, the composite PBEs preparation techniques should be considered depending on the characteristics to be achieved. Moreover, incorporating plasticizers or ionic liquids into PBEs with the appropriate amount of nanofillers or crosslinking the polymer blend host could produce impressive PBEs having better electrochemical performances. All in all, combining different materials with the appropriate amount and preparation techniques can boost the porosity and pore size, and stability of the system. While combining various materials can produce positive results, they can pose some challenges, especially regarding filler dispersion in polymeric matrices and unexpected effects at the interfaces between them, hindering the scalability of these materials for large-scale applications.
Despite these challenges, it can be concluded that PBEs can provide impressive performances and have significant potentials to be used in battery systems through the incorporation of nanofillers, and other advanced additive materials with tailored properties, to enable more efficiency.
Data availability
The papers used to write this review article are openly available in google scholar.
References
Yi, J., Liang, P., Liu, X., Wu, K., Liu, Y., Wang, Y., et al.: Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc-air batteries. Energy Environ. Sci. 11(11), 3075–3095 (2018)
Yi, J., Liu, X., Liang, P., Wu, K., Xu, J., Liu, Y., et al.: Non-noble Iron group (Fe Co, Ni)-based oxide electrocatalysts for aqueous zinc-air batteries: recent progress, challenges, and perspectives. Organometallics 38(6), 1186–1199 (2019)
Zhang, H., Zhao, H., Khan, M.A., Zou, W., Xu, J., Zhang, L., et al.: Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. J. Mater. Chem. A. 6(42), 20564–20620 (2018)
Dou, F., Shi, L., Chen, G., Zhang, D.: Silicon/carbon composite anode materials for lithium-ion batteries [Internet]. Elect. Energy Rev. 2, 149–198 (2019). https://doi.org/10.1007/s41918-018-00028-w
Wang, T., Su, D., Shanmukaraj, D., Rojo, T., Armand, M., Wang, G.: Electrode materials for sodium-ion batteries: considerations on crystal structures and sodium storage mechanisms [Internet]. Elect. Energy. Rev. 1, 200–237 (2018). https://doi.org/10.1007/s41918-018-0009-9
Chen, X., Wu, Y., Huang, Z., Yang, X., Li, W., Yu, L.C., et al.: C10H4O2S2/graphene composite as a cathode material for sodium-ion batteries. J. Mater. Chem. A. 4(47), 18409–18415 (2016)
Amogne, N.Y., Ayele, D.W., Habtu, N.G., Thothadri, G., Dagnew, M.: Insights into the use of polyepichlorohydrin polymer in lithium battery energy storage/conversion devices: review. SN Appl. Sci. (2023). https://doi.org/10.1007/s42452-022-05234-2
Dhatarwal, P., Sengwa, R.J.: Synergistic effects of salt concentration and polymer blend composition on the crystal phases, dielectric relaxation, and ion conduction in PVDF/PEO/LiCF 3 SO 3 solid polymer electrolytes. Ionics 26, 2259–2275 (2019)
Bocharova, V., Sokolov, A.P.: Perspectives for polymer electrolytes: a view from fundamentals of ionic conductivity. Macromolecules 53(11), 4141–4157 (2020)
Li, Z., Mindemark, J., Brandell, D., Tominaga, Y.: A concentrated poly ( ethylene carbonate )/ poly (trimethylene carbonate) blend electrolyte for all-solid-state Li battery. Polym. J. [Internet] 51, 753–760 (2019). https://doi.org/10.1038/s41428-019-0184-5
Amiri, H., Mohsennia, M.: Impedance study of PVA/PEG/LiClO 4/TiO 2 nanocomposite solid polymer blend electrolyte. Mater. Electron. 28, 4586–4592 (2017)
Li, J., Zhu, K., Wang, J., Yan, K., Liu, J., Yao, Z.: Impact of polymer blending on ionic conduction mechanism and dielectric properties of sodium based PEO-PVdF solid polymer electrolyte systems. Mater Technol (2020). https://doi.org/10.1080/10667857.2020.1827873
Mallaiah, Y., Ramana, V., Swarnalatha, R., Raju, A., Reddy, S.N., Chary, A.S.: Impact of polymer blending on ionic conduction mechanism and dielectric properties of sodium based PEO-PVdF solid polymer electrolyte systems. J. Phys. Chem. Solids (2021). https://doi.org/10.1016/j.jpcs.2021.110096
Tran, H.K., Wu, Y., Chien, W., Wu, S., Jose, R., Lue, S.J., et al.: Composite polymer electrolytes based on PVA/PAN for all-solid- state lithium metal batteries operated at room temperature. Appl Energy Mater. 3, 11204–11235 (2020)
Gao, K.W., Loo, W.S., Snyder, R.L., Abel, B.A., Choo, Y., Lee, A., et al.: Miscible polyether/poly(ether−acetal) electrolyte blends. Macronolecules 53, 5728–5739 (2020)
Aziz, S.B., Brza, M.A., Saed, S.R., Hamsan, M.H., Kadir, M.F.Z.: Ion association as a main shortcoming in polymer blend electrolytes based on CS : PS incorporated with various amounts of ammonium tetrafluoroborate. Integr. Med. Res. 9(3), 5410–5421 (2020). https://doi.org/10.1016/j.jmrt.2020.03.067
Rajendran, S., Kalaiappan, K.: Effect of CeO2 on conductivity of PMMA/PEO polymer blend electrolytes effect of CeO 2 on conductivity of PMMA/PEO polymer blend electrolytes. J. New Mater. Electrochem. Sys. 6, 25–28 (2016)
Ramana, V., Laxmi, J.E., Mallaiah, N., Swarnalatha, Y.R., Reddy, S.N.: Structural and electrical studies of PMMA and PVdF based blend polymer electrolyte. SN Appl. Sci. (2020). https://doi.org/10.1007/s42452-020-03868-8
Prabakaran, P., Manimuthu, R.P., Gurusamy, S., Sebasthiyan, E.: Plasticized polymer electrolyte membranes based on PEO/PVdF-HFP for use as an effective electrolyte in lithium-ion batteries. Chinese J. Polym. Sci. 35(3), 407–421 (2017)
El Kharbachi, A., Zavorotynska, O., Latroche, M., Cuevas, F., Yartys, V., Fichtner, M.: Exploits, advances and challenges benefiting beyond Li-ion battery technologies. J Alloys Compd (2020). https://doi.org/10.1016/j.jallcom.2019.153261
Ren, W., Ding, C., Fu, X., Huang, Y.: Advanced gel polymer electrolytes for safe and durable lithium metal batteries: challenges, strategies, and perspectives. Energy Storage Mater 34, 515–535 (2021). https://doi.org/10.1016/j.ensm.2020.10.018
Tang, S., Guo, W., Fu, Y.: Advances in composite polymer electrolytes for lithium batteries and beyond. Adv Energy Mater. 11(2), 1–29 (2021)
Nair, R., Imholt, L., Brunklaus, G., Winter, M.: Lithium metal polymer electrolyte batteries: opportunities and challenges. Electrochem. Soc. Inter. 28, 55–61 (2019)
Utracki LA. Polymer blends : fundamentals. (1).
Nyamweya, N.N.: Applications of polymer blends in drug delivery. Futur J Pharm Sci. 7(1), 1–5 (2021)
Stephan, A.M., Kumar, S.G., Renganathan, N.G., Kulandainathan, M.A.: Characterization of poly (vinylidene fluoride-hexafluoropropylene) (PVdF-HFP) electrolytes complexed with different lithium salts. Eur Polym J. 41(1), 15–21 (2005)
Pu, W., He, X., Wang, L., Jiang, C., Wan, C.: Preparation of PVDF-HFP microporous membrane for Li-ion batteries by phase inversion. J Memb Sci. 272(1–2), 11–14 (2006)
Tong, Y., Que, M., Su, S., Chen, L.: Design of amphiphilic poly(vinylidene fluoride-co-hexafluoropropylene)-based gel electrolytes for high-performance lithium-ion batteries. Ionics (Kiel). 22(8), 1311–1318 (2016)
Rajendran, S., Kannan, R., Mahendran, O.: An electrochemical investigation on PMMA/PVdF blend-based polymer electrolytes. Mater Lett. 49(3–4), 172–179 (2001)
Mazuki, N.F., Majeed, A.P.P.A., Nagao, Y., Samsudin, A.S.: Studies on ionics conduction properties of modification CMC-PVA based polymer blend electrolytes via impedance approach. Polym Test 81, 106234 (2020). https://doi.org/10.1016/j.polymertesting.2019.106234
Li, J., Zhu, K., Wang, J., Yan, K., Liu, J., Yao, Z.: Optimisation of conductivity of PEO/PVDF-based solid polymer electrolytes in all-solid-state Li-ion batteries. Mater Technol (2020). https://doi.org/10.1080/10667857.2020.1827873
Yap, Y.L., You, A.H., Teo, L.L.: Preparation and characterization studies of PMMA–PEO-blend solid polymer electrolytes with SiO2 filler and plasticizer for lithium ion battery. Ionics (Kiel). 25(7), 3087–3098 (2019)
Sundaramahalingam, K., Muthuvinayagam, M., Nallamuthu, N., Vanitha, D., Vahini, M.: Investigations on lithium acetate-doped PVA/PVP solid polymer blend electrolytes. Polym Bull 76(11), 5577–5602 (2019). https://doi.org/10.1007/s00289-018-02670-2
Aravindan, V., Vickraman, P., Kumar, T.P.: ZrO 2 nanofiller incorporated PVC/PVdF blend-based composite polymer electrolytes ( CPE ) complexed with LiBOB. J. Memb. Sci. 305, 146–151 (2007)
Singh, P., Saroj, A.L.: Effect of SiO2 nano-particles on plasticized polymer blend electrolytes: vibrational, thermal, and ionic conductivity study. Polym. Technol. Mater. 60, 298–305 (2020)
Rajendran, S., Babu, R.S., Rani, M.U.: Effect of complexing salt on conductivity of PVC/PEO polymer blend. Bulletin of Mater. Sci. 34(7), 1525–1530 (2011)
Vanitha, D., Asath, S., Nallaperumal, B.: Electrical impedance studies on sodium ion conducting composite blend polymer electrolyte. J Inorg Organomet Polym Mater. 27(1), 257–265 (2017)
Xi, J., Qiu, X., Li, J., Tang, X., Zhu, W., Chen, L.: PVDF – PEO blends based microporous polymer electrolyte : Effect of PEO on pore configurations and ionic conductivity. J. Power Sources 157, 501–506 (2006)
Jamalpour, S., Ghahramani, M., Reza, S., Javanbakht, M.: The effect of poly ( hydroxyl ethyl methacrylate ) on the performance of PVDF / P ( MMA-co-HEMA ) hybrid gel polymer electrolytes for lithium ion battery application. Polymer (Guildf). 195, 122427 (2020). https://doi.org/10.1016/j.polymer.2020.122427
Vc-vac, S.P.P., Zhao, M., Zuo, X., Ma, X.: Self-supported PVdF/P(VC-VAc) blended polymer electrolytes for LiNi0.5Mn1.5O4/Li batteries. J. Memb. Sci. (2017). https://doi.org/10.1016/j.memsci.2017.03.009
Amaral, F.A., Sousa, R.M., Morais, L.C.T., Rocha, R.G., Campos, I.O., Fagundes, W.S., et al.: Preparation and characterization of the porous solid polymer electrolyte of PAN/PVA by phase inversion. J. Appl. Electrochem. 45, 809–820 (2015)
Deng, F., Wang, X., He, D., Hu, J., Gong, C., Ye, Y.S., et al.: Microporous polymer electrolyte based on PVDF/PEO star polymer blends for lithium ion batteries. J. Memb. Sci. 491, 82–89 (2015)
Mahant, Y.P., Kondawar, S.B., Bhute, M., Nandanwar, D.V.: Electrospun poly ( vinylidene fluoride )/poly (methyl methacrylate ) composite nanofibers polymer electrolyte for batteries. Procedia Mater. Sci. 10, 595–602 (2015). https://doi.org/10.1016/j.mspro.2015.06.011
Padmaraj, O., Venkateswarlu, M., Satyanarayana, N.: Effect of PMMA blend and ZnAl 2 O 4 fillers on ionic conductivity and electrochemical performance of electrospun nanocomposite polymer blend fibrous electrolyte membrane for lithium battery. RSC. Advances. (2015)
Mahant, Y.P., Kondawar, S.B., Nandanwar, D.V., Koinkar, P.: Poly (methyl methacrylate) reinforced poly ( vinylidene fluoride) composites electrospun nanofibrous polymer electrolytes as potential separator for lithium ion batteries. Mater Renew Sustain Energy 7(2), 1–9 (2018). https://doi.org/10.1007/s40243-018-0115-y
Zhong, Z., Cao, Q., Wang, X., Wu, N., Wang, Y.: PVC – PMMA composite electrospun membranes as polymer electrolytes for polymer lithium-ion batteries. Ionics. 18, 47–53 (2012). https://doi.org/10.1007/s11581-011-0615-6
Liu, Y., Peng, X., Cao, Q., Jing, B., Wang, X., Deng, Y.: Gel Polymer Electrolyte Based on Poly ( vinylidene fluoride )/Thermoplastic Polyurethane/Polyacrylonitrile by Electrospinning Technique Gel Polymer Electrolyte Based on Poly (vinylidene fluoride )/Thermoplastic Polyurethane / Polyacrylonitrile by Electrospinning Technique. J. Phys. Chem. C. (2017). https://doi.org/10.1021/acs.jpcc.7b03411
Wu, N., Cao, Q., Wang, X., Li, X., Deng, H.: A novel high-performance gel polymer electrolyte membrane basing on electrospinning technique for lithium rechargeable batteries. J. Power Sources 196, 8638–8643 (2011)
Wheatle, B.K., Lynd, N.A., Ganesan, V.: Effect of Host Incompatibility and Polarity Contrast on Ion Transport in Ternary Polymer-Polymer-Salt Blend Electrolytes. Macromolecules. (2020). https://doi.org/10.1021/acs.macromol.9b02510
Leš, K., Jordan, C.S.: Ionic conductivity enhancement in solid polymer electrolytes by electrochemical: In situ formation of an interpenetrating network. RSC Adv. 10(68), 41296–41304 (2020)
Zhao, B., Lu, X., Wang, Q., Yang, J., Zhao, J., Zhou, H.: Enhancing the ionic conductivity in a composite polymer electrolyte with ceramic nanoparticles anchored to charged polymer brushes. Chinese Chem Lett. 31(3), 831–835 (2020)
Yao, P., Yu, H., Ding, Z., Liu, Y., Lu, J., Lavorgna, M., et al.: Review on polymer-based composite electrolytes for lithium batteries. Front Chem. 7(August), 1–17 (2019)
Zhu L. Toward high performance solid-state lithium-ion battery with a promising PEO / PPC blend solid polymer electrolyte. 2020;(May):1–11.
Macklin C.: UC Berkeley UC Berkeley Electronic Theses and Dissertations. DNA Mediat Assem Protein Heterodimers Membr Surfaces [Internet] (2020). Available from: https://escholarship.org/uc/item/98384265
Sudiarti, T., Wahyuningrum, D., Bundjali, B., Made, Arcana I.: Mechanical strength and ionic conductivity of polymer electrolyte membranes prepared from cellulose acetate-lithium perchlorate. IOP Conf Ser Mater Sci Eng. (2017). https://doi.org/10.1088/1757-899X/223/1/012052
Yue, L., Ma, J., Zhang, J., Zhao, J., Dong, S., Liu, Z., et al.: All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 1(5), 139–164 (2016)
Cai, M., Zhu, J., Yang, C., Gao, R., Shi, C., Zhao, J.: A parallel bicomponent TPU/PI membrane with mechanical strength enhanced isotropic interfaces used as polymer electrolyte for lithium-ion battery. Polymers (Basel). 11(1), 185 (2019)
Wu, Y., Wang, S., Li, H., Chen, L., Wu, F.: Progress in thermal stability of all-solid-state-Li-ion-batteries. InfoMat. 3(8), 827–853 (2021)
Tomić, N.Z.: Thermal studies of compatibilized polymer blends compat polym blends micro nano scale phase morphol interphase charact prop, pp. 489–510. Elsevier, Amsterdam (2019)
Stivala, S.S., Reich, L.: Structure vs stability in polymer degradation. Polym Eng Sci. 20(10), 654–661 (1980)
Abid, M.K., Abbas, M.M., Abid, N.K., Jwad, M.K.: Investigate the thermal analysis properties of polymer electrolyte. J. Phys. Conf. Ser. 2114(1), 012048 (2021)
Morsi, M.A., El-Khodary, S.A., Rajeh, A.: Enhancement of the optical, thermal and electrical properties of PEO/PAM: Li polymer electrolyte films doped with Ag nanoparticles. Phys B Condens Matter. 15(539), 88–96 (2018)
Li, J., Zhu, L., Shen, X.: Boosting the performance of poly(ethylene oxide)-based solid polymer electrolytes by blending with poly (vinylidene fluoride-co-hexafluoropropylene) for solid-state lithium-ion batteries. Int J Ene Res. (2020). https://doi.org/10.1002/er.5476
Ngai, K.S., Ramesh, S., Ramesh, K., Juan, J.C.: A review of polymer electrolytes: fundamental, approaches and applications. Ionics (Kiel). 22(8), 1259–1279 (2016). https://doi.org/10.1007/s11581-016-1756-4
Lepage, D., Nicolle, P., Pre, A., Ayme, D.: E ff ect of Li + A ffi nity on Ionic Conductivities in Melt-Blended Nitrile Rubber/Polyether. ACS Appl. Polym. Mater. (2020). https://doi.org/10.1021/acsapm.0c00827
Arunkumar, R., Babu, R.S., Usha Rani, M., Kalainathan, S.: Effect of PBMA on PVC-based polymer blend electrolytes. J Appl Polym Sci. 134(27), 1–12 (2017)
Przytuski, J., Floriaficzyk, Z.: Blend-based and composite polymer solid electrolytes. Syn Metals 45, 373–383 (1991)
Costa, C.M.: Polymer composites and blends for battery separators : State of the art, challenges and future trends. J Power Sources 281, 378–398 (2015)
Ali, T.M., Padmanathan, N., Selladurai, S.: Structural, conductivity, and dielectric characterization of PEO–PEG blend composite polymer electrolyte dispersed with TiO2 nanoparticles. Ionics 19, 1115–1123 (2013)
Alsulami, Q.A., Rajeh, A.: Synthesis of the SWCNTs/TiO2 nanostructure and its effect study on the thermal, optical, and conductivity properties of the CMC/PEO blend. Results Phys. 1(28), 104675 (2021)
Li, Y.J., Fan, C.Y., Zhang, J.P., Wu, X.L.: A promising PMHS/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Dalt Trans. 47(42), 14932–14937 (2018)
Li, J., Hu, R., Zhou, H., Tao, S., Wang, Y.: Nano-SiO 2 @ PMMA doped composite polymer PVDF–HFP/PMMA/PEO electrolyte for lithium metal batteries. J. Mater. Sci. Mater. Electron. 31(3), 2708–2719 (2020). https://doi.org/10.1007/s10854-019-02811-x
Victor, M., Chandra, L., Karthikeyan, S.: Study of PVAc-PMMA-LiCl polymer blend electrolyte and the effect of plasticizer ethylene carbonate and nanofiller titania on PVAc-PMMA- LiCl polymer blend electrolyte. J Polym Eng. (2016). https://doi.org/10.1515/polyeng-2016-0145
Gohel, K., Kanchan, D.K.: Ionic conductivity and relaxation studies in PVDF-HFP: PMMA-based gel polymer blend electrolyte with LiClO4 salt. J. Adv. Dielectr. 8(1), 1–13 (2018)
Rajendran, S., Uma, T., Mahalingam, T.: Conductivity studies on PVC ± PMMA ± LiAsF 6 ± DBP polymer blend electrolyte. Mater Lett 36, 2617–2620 (2000)
Hou, X., Siow, K.S.: Mechanical properties and ionic conductivities of plasticized polymer electrolytes based on ABS/PMMA blends. Polymer (Guildf). 41(24), 8689–8696 (2000)
Xi, J., Qiu, X., Li, J., Tang, X., Zhu, W., Chen, L.: PVDF-PEO blends based microporous polymer electrolyte: effect of PEO on pore configurations and ionic conductivity. J Power Sources. 157(1), 501–506 (2006)
Thayumanasundaram, S., Shankar Rangasamy, V., De Greef, N.: Hybrid polymer electrolytes based on a poly(vinyl alcohol)/poly(acrylic acid) blend and a pyrrolidinium-based ionic liquid for lithium-ion batteries. Eur. J. Inorg. Chem (2015). https://doi.org/10.1002/ejic.201402603
Rajendran, S., Sivakumar, P.: An investigation of PVdF/PVC-based blend electrolytes with EC/PC as plasticizers in lithium battery applications. Phys. B Condens. Matter. 403(4), 509–516 (2008)
Rajendran, S., Sivakumar, M., Subadevi, R.: Investigations on the effect of various plasticizers in PVA–PMMA solid polymer blend electrolytes. Mater. Lett. 58, 641–649 (2004)
Rajendran, S., Babu, R., Shanker Sivakumar., P.: Investigations on PVC/PAN composite polymer electrolytes. J. Memb. Sci. 315(1–2), 67–73 (2008)
Simari, C., Lufrano, E., Coppola, L., Nicotera, I.: Composite gel polymer electrolytes based on organo-modified nanoclays: investigation on lithium-ion transport and mechanical properties. Membranes (Basel). 8(3), 69 (2018)
Ulaganathan, M., Nithya, R., Rajendran, S., Raghu, S.: Li-ion conduction on nanofiller incorporated PVdF-co-HFP based composite polymer blend electrolytes for flexible battery applications. Solid State Ionics 218, 7–12 (2012). https://doi.org/10.1016/j.ssi.2012.04.029
Barkoula, N.M., Alcock, B., Cabrera, N.O., Peijs, T.: Influence of barium titanate on poly(vinyl pyrrolidone)-based composite polymer blend electrolytes for lithium battery applications K. Polym Polym Compos. 16(2), 101–113 (2008)
Kuo, C.W., Huang, C.W., Chen, B.K., Bin, L.W., Chen, P.R., Ho, T.H., et al.: Enhanced ionic conductivity in pan-pegme-liclo4-PC composite polymer electrolytes. Int. J. Electrochem. Sci. 8(3), 3834–3850 (2013)
Mohamed Ali, T., Padmanathan, N., Selladurai, S.: Structural, conductivity, and dielectric characterization of PEO-PEG blend composite polymer electrolyte dispersed with TiO2 nanoparticles. Ionics (Kiel). 19(8), 1115–1123 (2013)
Sadiq, N.M., Aziz, S.B., Kadir, M.F.Z.: Development of flexible plasticized ion conducting polymer blend electrolytes based on polyvinyl alcohol (PVA): chitosan (CS) with high ion transport parameters close to gel based electrolytes. Gels. 8(3), 1–23 (2022)
Sengwa, R.J., Dhatarwal, P., Choudhary, S.: Effects of plasticizer and nanofiller on the dielectric dispersion and relaxation behaviour of polymer blend based solid polymer electrolytes. Curr. Appl. Phys. [Internet]. 15(2), 135–143 (2015). https://doi.org/10.1016/j.cap.2014.12.003
Pradeepa, P., Edwinraj, S., Ramesh, P.M.: Effects of ceramic filler in poly(vinyl chloride)/poly(ethyl methacrylate) based polymer blend electrolytes. Chinese Chem. Lett. 26(9), 1191–1196 (2015). https://doi.org/10.1016/j.cclet.2015.05.007
Subramania, A., Sundaram, N.T.K., Kumar, G.V.: Structural and electrochemical properties of micro-porous polymer blend electrolytes based on PVdF-co-HFP-PAN for Li-ion battery applications. J. Power Sources. 153(1), 177–182 (2006)
Hou, X., Siow, K.S.: Electrochemical characterization of plasticized polymer electrolytes based on ABS/PMMA blends. J. Solid State Electrochem. 5(4), 293–299 (2001)
Dhatarwal, P., Sengwa, R.J.: Dielectric relaxation, Li-ion transport, electrochemical, and structural behaviour of PEO/PVDF/LiClO4/TiO2/PC-based plasticized nanocomposite solid polymer electrolyte films. Compos Commun. [Internet]. 17, 182–191 (2020). https://doi.org/10.1016/j.coco.2019.12.006
Kesavan, K., Mathew, C.M., Rajendran, S.: Lithium ion conduction and ion-polymer interaction in poly(vinyl pyrrolidone) based electrolytes blended with different plasticizers. Chinese Chem. Lett. [Internet]. 25(11), 1428–1434 (2014). https://doi.org/10.1016/j.cclet.2014.06.005
Prasanth, R., Aravindan, V., Srinivasan, M.: Novel polymer electrolyte based on cob-web electrospun multi component polymer blend of polyacrylonitrile/poly(methyl methacrylate)/polystyrene for lithium ion batteries - Preparation and electrochemical characterization. J. Power Sources [Internet]. 202, 299–307 (2012). https://doi.org/10.1016/j.jpowsour.2011.11.057
Zhu, Y., Xiao, S., Shi, Y., Yang, Y., Hou, Y., Wu, Y.: A composite gel polymer electrolyte with high performance based on poly(vinylidene fluoride) and polyborate for lithium ion batteries. Adv. Energy Mater. 4(1), 1–9 (2014)
Hariyawati, N.K., Putu, L., Saraswati, A., Arcana, M.: Properties of polymer electrolytes based on chitosan/poly(vinyl alcohol) for lithium battery application. Am J. Eng. Res. 8, 135–143 (2019)
Liu, T.M., Saikia, D., Ho, S.Y., Chen, M.C., Kao, H.M.: High ion-conducting solid polymer electrolytes based on blending hybrids derived from monoamine and diamine polyethers for lithium solid-state batteries. RSC Adv. 7(33), 20373–20383 (2017)
Xu, L., Wei, K., Cao, Y., Ma, S., Li, J., Zhao, Y., et al.: The synergistic effect of the PEO-PVA-PESf composite polymer electrolyte for all-solid-state lithium-ion batteries. RSC Adv. 10(9), 5462–5467 (2020)
Zhang, J., Huang, X., Fu, J., Huang, Y., Liu, W., Tang, X.: Novel PEO-based composite solid polymer electrolytes incorporated with active inorganic-organic hybrid polyphosphazene microspheres. Mater. Chem. Phys. [Internet]. 121(3), 511–518 (2010). https://doi.org/10.1016/j.matchemphys.2010.02.016
Prabakaran, P., Manimuthu, R.P., Gurusamy, S.: Influence of barium titanate nanofiller on PEO/PVdF-HFP blend-based polymer electrolyte membrane for Li-battery applications. J Solid State Electrochem. 21(5), 1273–1285 (2017)
Lee, L., Park, S.J., Kim, S.: Effect of nano-sized barium titanate addition on PEO/PVDF blend-based composite polymer electrolytes. Solid State Ionics [Internet]. 234, 19–24 (2013). https://doi.org/10.1016/j.ssi.2012.12.011
Beshahwured, S.L., Wu, Y.S., Huang, Wu.S., Chien, W.C., Jose, R., Lue, S.J., et al.: Flexible hybrid solid electrolyte incorporating ligament-shaped Li6.25Al0.25La3Zr2O12 filler for all-solid-state lithium-metal batteries. Electrochim Acta [Internet]. 366, 137348 (2021). https://doi.org/10.1016/j.electacta.2020.137348
Sasikumar, M., Raja, M., Krishna, R.H., Jagadeesan, A., Sivakumar, P., Rajendran, S.: Influence of Hydrothermally Synthesized Cubic-Structured BaTiO3 ceramic fillers on ionic conductivity, mechanical integrity, and thermal behavior of P(VDF-HFP)/PVAc-based composite solid polymer electrolytes for lithium-ion batteries. J Phys Chem C. 122(45), 25741–25752 (2018)
Author information
Authors and Affiliations
Contributions
All authors listed have significantly contributed to the development and the writing of this article.
Corresponding author
Ethics declarations
Conflict of interest
This study was not funded by any organization and the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yazie, N., Worku, D., Gabbiye, N. et al. Development of polymer blend electrolytes for battery systems: recent progress, challenges, and future outlook. Mater Renew Sustain Energy 12, 73–94 (2023). https://doi.org/10.1007/s40243-023-00231-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-023-00231-w