Abstract
The primary goal of the current study is to improve the specific capacitance of electric double-layer (EDLC) device using biomass (Tribulus Terrestris) derived activated carbon electrodes synthesized by chemical activation method. Furthermore, high surface area carbon electrodes are characterized using X-ray diffraction (XRD), RAMAN spectroscopy, and scanning electron microscopy (SEM) to confirm the morphological structure. Finally, the electrochemical performance of fabricated EDLC proves a good agreement data using Cyclic Voltammetry (CV), Low Impedance Spectroscopy (LIS), and Galvanostatic Charge–Discharge (GCD) analysis showing the high specific capacitance of 115 Fg−1 for the optimized 1:2 activated carbon material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the whole world, rise in the energy demand is a critical factor which is currently affected by the growth in industries and human population, since everything in our surrounding, in some form and another, requires energy. Moreover, we must be able to store energy obtained from our surrounding or chemical reactions to use it when the main source is unavailable. Energy can be stored in different ways and form, for example, there are electrochemical devices like batteries, supercapacitors, fuel cells, etc., or more mechanical methods like flywheels, hydraulic accumulators, or thermal methods like molten salts, steam, bodies of water, and many more for staring the energy [1].
In case of energy storage devices (ESD), the focus is on storing the energy in different configurations, such as electrochemical, kinetic energy, potential energy, electromagnetic, chemical energy, and thermal energy using different devices like batteries, capacitors, supercapacitors, etc. There are salient features by which these ESD are chosen to be a promising need for meeting the human resource needs; it includes: specific power, specific capacity, self-charge/discharge cycle, specific energy, high efficiency, environment friendliness, and low cost. Supercapacitor has made a significant entry in the world between batteries and capacitor-based devices after the analysis of their energy and power densities. Furthermore, these devices are successfully been used in various commercialized business applications, such as aerospace, automotive, heavy transportation, or electronics [2, 3].
Strong electrochemically conducting, mechanically stable, and free-standing supercapacitors are attractive development directions for sustainable and biomass-derived materials. The two methods proposed for fabricating high conducting and flexible carbon materials are deposition of carbon nanotubes onto a substrate, and the fabrication of free-standing carbon materials by simple one-step carbonization process and activation of carbon. The electrode materials are available in different carbon derivative forms, such as porous activated carbon (PAC) materials, carbon nanofibers (CNfs), carbon nanotubes (CNTs), and graphene or reduced graphene oxide (r-GO), which are basically the exotic carbonaceous materials having dominant properties like high surface area, high ion adsorption capability, high conductivity, high dimensional stability, low weight, and physical strength [4,5,6]. There are several ways to choose and prepare these derivatives, out of which pyrolysis of particular carbon precursor using physical activation, chemical activation, and hydrothermal activation are widely known. These activation processes are performed at very high temperature (9000C) under inert environment to avoid oxidation reaction and achieving highly conducting porous carbon material [7]. Lab-scale carbon derivatives are inexpensive and abundantly available electrode material with large surface area. Natural resources have become increasingly important in optimizing current structures and developing new energy sources. Biomass-derived materials and biopolymers have shown tremendous promise as sustainable alternatives to typical synthetic organics in the preparation of commercial products that are inexpensive and easy to distribute. Various studies at national and international level have been done in analyzing the actual effect of using green electrodes over the electrochemical performance of supercapacitor. There have been numerous initiatives to create flexible energy storage systems with idealized performance. However, numerous contemporary hurdles continue to obstruct their basic research and large-scale commercial applications. Some biomass electrodes that have been researched so far (including human hair, peanut shell, pistachio shell, cow dung, sewage sludge, watermelon, bamboo waste, etc.) show that the average specific capacitance reaches up to average value of 250 Fg−1, but pore size is below 2 nm. Therefore, it is reported in the literature that poor rate performance is due to the absence of mesopores and macropores in these electrode materials [5, 8, 9].
Biomass naturally has a far more organized structure that come down in the range of nano size, unlike standard materials, which have structural control. Biomass materials with such exact properties can perform unique activities far more effectively than other options presented by researchers [10]. It is believed to change the performance of the material electrochemically and modify them to various types of electrolytic medium and system arrangement by modifying these parameters. In current research work, the main focus is on the Electric Double Layer Capacitor (EDLC) device because of its attractive characteristics like long life cycle, quick charge/discharge cycle rate, and most importantly being eco-friendly in nature. The following paragraphs give a brief description about capacitors, batteries, and supercapacitors.
Experimental methods
Synthesis of activated carbon material
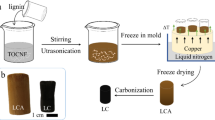
Chemical activation method was chosen to synthesize the activated carbon (AC) as reported earlier [11]. The host material Tribulus Terrestris (60 g) was put into the two ceramic boats and sealed into tubular furnace after rigorously washing with double deionized water (DD water) to remove the external impurities. The furnace was programmed for pyrolysis at maximum temperature of 900 ℃ with different temperature ramp rate (200°, 600°, and 900° with hold time of 30 min in between). After the pre-carbonization, material was obtained which was basically a charcoal material. Thereafter, this material was mixed in different ratio using activating agent ZnCl2 in ethanol solvent in the ratio of 1:2 and 1:4, as shown in Fig. 3.2. These samples are named as G1 and G2. These two activated materials were stirred for 8 h and kept in vacuum oven at 80 ℃ for 12 h. After the complete removal of solvent, G1 and G2 samples were kept in separate ceramic boats for post-carbonization at 900 ℃ using previous programmed protocol in tubular furnace. After carbonization, the samples were taken out and washed rigorously with Hydrochloric acid (HCl) and DD water to remove the traces of zinc in the samples. The pH value was checked regularly to maintain the basic nature after washing process. Finally, G1 and G2 samples were characterized using various techniques and used for EDLC device fabrication (Figs. 1, 2).
Fabrication of EDLC device
The electrical double-layer capacitor (EDLC) was fabricated using 1 × 1 cm2 graphite sheets as a current collector (cc), as synthesized unactivated carbon (UA Carbon), G1 and G2 derive porous carbon were used as porous carbon electrode material which was synthesized using electrochemical method, and PVDF-HFP used as a binding material. 1 mgcm−1 of electrode material was coated onto the cc (the homogenous mixture of binder-to-AC ratio was fixed at 90:10) as reported in the literature [12]. After coating, highest conducting solid polymer electrolyte film was sandwiched between the current collectors, and finally, the EDLC performance was measured.
Results and discussion
X-Ray diffraction (XRD)
Figure 3 shows the XRD patterns of UA carbon, G1 and G2 activated carbon. From Fig. 3, it can be seen that the unactivated carbon shows numerous sharp peaks which proclaim the crystalline nature of the carbon, but with activation, most of the sharp peaks vanished which indicates the decrease in crystallinity. All the three samples show hump like peaks in between 23°–26° and 42°–44° which are corresponding to (002) and (100) planes of carbon which peaks confirm the graphitic like microcrystalline composition of all the samples. Some crystalline peaks are also present in both activated carbon which shows the presence of crystalline graphitic domains in both of the carbons [5]
RAMAN spectroscopy
Figure 4 shows the Raman spectrum of the UA carbon, G1 and G2 activated carbon. The comparative plot of the Raman spectroscopy shows the D and G peak of all the three materials at approximately 1340 cm−1 and 1595 cm−1, respectively, depicting the Raman shift in the X-axis region. The blue curve for the UA carbon shows very ideal D and G peak in the X- axis region which confirms that the material is ideally suitable for energy applications. The D band depicts the presence of the sp3 hybridized carbon in the samples. We can see more steep D peak for the G2 activated carbon (red curve) in compared to the UA pure carbon sample as because during the chemical activation, many of the sp2 hybridized carbon atoms transformed into sp3 hybridized structure which resembles the presence of other functional groups in the sample and so we can say that the D band depicts the distorted geometry or defects present in the sp2 hybridized carbon atoms7. Due to low functionalization in the G1 carbon, the D band is not very steep and we can say that less sp3-to-sp2 transformation took place during the activation process. On the other hand, the degree of graphitization of the sp2 hybridized carbon atoms can be known from the G band of the Raman spectrum and almost all the three samples show very high degree of graphitization, but to make a comparative statement, the G2 activated carbon has the highest degree of graphitization above all which is a very good factor for the material to be used in energy application.
Scanning electron microscopy (SEM)
The scanning electron micrographs of unactivated carbon (UA carbon), G1 and G2 activated carbon are shown in Fig. 5a, b, c, respectively, at 10 µm. The micrograph for pure carbon shows a plane surface with no pores, while G1 is flaky and G2 is granular in appearance. Figure 5b and 4c show that after activation, the outer surface of the carbon is become rough and ruptured and showing somehow porous structure. It can be concluded from SEM studies that the volatiles evolved in the reaction are responsible for the rupturing, cracking, and pore formation on the surface of the activated carbon.
Electrochemical performance of EDLC
The performance of EDLC device fabricated using porous carbon derived from ‘Tribulus Terrestris” and optimized 8 wt% IL doped in PEO + 5 wt% NaI polymer electrolytes were studied using Cyclic Voltammetry (CV), Low Frequency Impedance Spectroscopy (LIS), and Galvanostatic Charge–Discharge (GCD) techniques.
The CV curves of EDLC devices focused on UA Carbon, G1 and G2 carbon materials are shown in Fig. 6. From the CV analysis, the specific capacitance of UA carbon, G1 and G2 were found to be 29 F/g, 115 F/g, and 53F/g at 10 mV/s, in voltage range 0–1 V, as shown in Fig. 6.
From the LIS technique, the specific capacitances of G1 and G2 were found to be 13 F/g, 95 F/g, and 30F/g found to be F/g at 10 m Hz frequency, as shown in Fig. 7.
Furthermore, using GCD technique, the specific capacitance of high capacitive G2 sample was found to be 72 F/g, as shown in Fig. 8.
Further, the specific energy, power density, and coulombic efficiency of highest capacitive carbon of G1 sample were also calculated which was found to be 12.2 Wh/kg, 2200 W/kg and 69%, respectively. From all above techniques, the performance of EDLC was good agreement to each other which shows the well-stabilized nature of fabricated EDLC using developed IL doped in polymer/salt complex and porous carbon derived from “Tribulus Terrestris” as an electrode material.
Conclusion
The synthesized porous carbon from “Tribulus Terrestris” has been studied thoroughly and we can conclude that it has the potential to be used as an electrode material in supercapacitor. However, the current study is just a preliminary work to develop the porous material and check the compatibility to use as an electrode material in supercapacitor. We are trying to enhance the material properties by tuning the extrinsic properties of the material. We hope that the material will be highly compatible to use as an active material for electrode material in batteries and supercapacitor.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ahuja, H., Dhapola, P.S., Rahul, Sahoo, N.G., Singh, V., Singh, P.K.: Ionic liquid (1-hexyl-3-methylimidazolium iodide)-incorporated biopolymer electrolyte for efficient supercapacitor. High Perform. Polym. 32, 220–225 (2020). https://doi.org/10.1177/0954008319897763
Hashmi, S.: Supercapacitor: An emerging power source. Natl. Acad. Sci. Lett. 27, 27–46 (2004)
Kumar, Y., Rawal, S., Joshi, B., Hashmi, S.A.: Background , fundamental understanding and progress in electrochemical capacitors. (2019)
Zakariya’u, I., Gultekin, B., Singh, V., Singh, P.K.: Electrochemical double-layer supercapacitor using poly(methyl methacrylate) solid polymer electrolyte. High Perform. Polym. 32, 201–207 (2020). https://doi.org/10.1177/0954008319895556
Farma, R.: Synthesis of highly porous activated carbon nanofibers derived from bamboo waste materials for application in supercapacitor. J. Mater. Sci. Mater. Electron. 32, 7681–7691 (2021). https://doi.org/10.1007/s10854-021-05486-5
Surana, K., Konwar, S., Singh, P.K., Bhattacharya, B.: Utilizing reduced graphene oxide for achieving better efficient dye sensitized solar cells. J. Alloys Compd. 788, 672–676 (2019). https://doi.org/10.1016/j.jallcom.2019.02.287
Nath, G., Singh, P.K., Dhapola, P.S., Dohare, S., Noor, I.M., Sharma, T., Singh, A.: Fabrication of cornstarch biopolymer-derived nano porous carbon as electrode material for supercapacitor application Biomass Convers. Biorefinery. (2022). https://doi.org/10.1007/s13399-022-02656-1
Gao, Y., Yue, Q., Gao, B.: High surface area and oxygen-enriched activated carbon synthesized from animal cellulose and evaluated in electric double-layer capacitors. RSC Adv. 5, 31375–31383 (2015). https://doi.org/10.1039/c4ra16965d
Dawood, S., Sen, T.K., Phan, C.: Synthesis and characterisation of novel-activated carbon from waste biomass pine cone and its application in the removal of congo red dye from aqueous solution by adsorption. Water. Air. Soil Pollut. 225, (2014). https://doi.org/10.1007/s11270-013-1818-4
Wang, Q., Lian, P., Wang, B., Tang, Y., Liu, H., Mei, Y.: Red phosphorus encapsulated in porous carbon derived from cigarette filter solid waste as a promising anode material for lithium-ion batteries. Ionics (Kiel). 24, 3393–3403 (2018). https://doi.org/10.1007/s11581-018-2487-5
Dhapola, P.S., Sahoo, N.G., Bhattacharya, B., Kumar, Y., Singh, P.K., Gupta, M.: Elaborative studies on non-porous carbon material for super capacitor application. Macromol. Symp. 388, 1–6 (2019). https://doi.org/10.1002/masy.201900035
Pandey, S., Karakoti, M., Surana, K., Dhapola, P.S., SanthiBhushan, B., Ganguly, S., Singh, P.K., Abbas, A., Srivastava, A., Sahoo, N.G.: Graphene nanosheets derived from plastic waste for the application of DSSCs and supercapacitors. Sci. Rep. 11, 1–17 (2021). https://doi.org/10.1038/s41598-021-83483-8
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally for this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, A., Nath, G., Dhapola, P.S. et al. Biomass stemmed activated carbon electrodes toward a significant electric double-layer capacitor. Mater Renew Sustain Energy 12, 39–45 (2023). https://doi.org/10.1007/s40243-023-00227-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-023-00227-6