Abstract
We demonstrated the basil sensitized hybrid photoelectrodes for photocurrents and fuel generation. Hybrid photoelectrochemical cells (PECs) were proposed for direct solar energy conversion. The biohybrid device allows tunable control of energy conversion through the chemically stable photoelectrode. Biohybrid PEC was prepared by integrating organic and inorganic layers on fluorine doped tin oxide substrate. This integrated assembly produces electricity upon the illumination of visible light and drives overall water splitting reaction to generate solar fuel. The basil layer enhances the overall absorption with wide spectrum range and hence, a strong increment in generation of photocurrent is observed in the biohybrid PEC device. This hybrid PEC device can also be used to generate solar fuels and solar power.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solar energy is the most abundant and clean energy source. It can be used to generate clean energy in the form of electricity as well as solar fuels (hydrogen and oxygen) [1,2,3]. Plants produce their food using solar energy through photosynthesis [3, 4]. Artificial photosynthesis is promising to produce solar fuels. Currently, solar photovoltaic [4] and solar thermal [5] are main energy conversion technologies in market [6]. Light-matter interactions are fundamental reactions for theoretically [7] and practical applications, for example energy conversion devices [8, 9].
Dye-sensitized photoelectrochemical cells (PECs) offer a promising method to generate solar fuel and solar power. This technology has the potential for zero greenhouse gas emissions [10,11,12,13]. A PEC device is made up of a photoanode, photocathode and an electrolyte, connected in an external circuit [14, 15]. Light-absorbing semiconductor electrode coated with green pigment is a key component in PEC device [16, 17]. During the operation of PEC device, photons are absorbed by the dye molecule and create exciton that is quickly split at the surface of semiconducting film with injecting electrons into film and leaving holes at the opposite side with electrons injected into the thin film [18, 19]. The understandings of the semiconductor–dye and dye-electrolyte interfaces are important to optimize the PEC devices [20,21,22]. The performance of PEC devices can be improved through enhanced absorption of sunlight, novel materials and protective surface coatings [23, 24].
Nanotechnology research and development has created the engineered nanomaterials those have achieved better performance and multifunctional nanohybrids [25, 26]. The surface of nanocrystalline films adsorbs more dye molecule because of higher surface to volume ratio [27]. Metal oxides, such as ZnO and TiO2, are used widely as photoelectrodes in PECs due to their band edge alignments relative to the redox potentials [28, 29]. ZnO has direct wide bandgap of about 3.37 eV [30]. ZnO absorbs mostly ultraviolet light of solar spectrum. The high electron mobility of n-type ZnO layer has increased its applications as electron collector in solar cells [31,32,33]. There is a need to understand semiconductor-dye interface to improve the device performance.
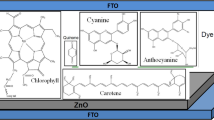
Natural dyes are cheap and environment friendly. They can be extracted from plant leaves, flowers, seeds, and fruits, etc. [34, 35]. The dye absorbs a larger fraction of the visible light from solar spectrum. The lowest unoccupied molecular orbit (LUMO) and highest occupied molecular orbit (HOMO) of the dye plays a important role in photo excitation process of PEC. The pigments of natural dyes such as chlorophyll, quinine, and carotene, etc. have been used with semiconductors for photo conversion applications [36, 37].
Here, we have fabricated and utilized basil sensitized biohybrid photoelectrodes for solar photovoltaic and photoelectrochemical energy conversion applications.
Experimental details
Preparation of biohybrid photoelectrodes
The basil (oscimum) leaves were washed, crushed into fine powder, and immersed in distilled water to extract basil dye at room temperature for 24 h. The extraction was prepared from the solution using filter paper [38, 39].
ZnO thin films were deposited on FTO ubstrate (size of 1 × 1 cm2, resistivity 7 Ωcm, Sigma Aldrich) using magnetron sputtering method with thickness about 500 nm. The commercially purchased ZnO bulk target was used for deposition. A basil natural dye layer was prepared on ZnO using drop casting technique [40]. These biohybrid electrodes were used for preparing PV and PEC devices.
Preparation of photoelectrochemical (PEC) device
PEC device configuration was prepared in a three electrode system. The hybrid electrodes (ZnO/FTO and Basil/ZnO/FTO), platinum (Pt) wire and saturated calomel electrode (SCE) were used as working, counter and reference electrode, respectively. The aqueous solution of 0.1 M Na2SO4 was used as an electrolyte [41].
Characterization methods
The optical properties of photoelectrodes were studied using UV–Visible spectrometer (Shimadzu, model 6210) in the wavelength range from 200–1100 nm. An X-ray diffractometer (Rigaku, D8) was used to record X-ray diffraction (XRD) pattern of ZnO thin film with CuKα radiation (λ = 0.17890 nm). Photoelectrochemical (PEC) properties were studied using Scanning electrochemical microscopy techniques (CH Instrument, CHI920D) in three electrode testing configuration under white LED light (56 mW/cm2) and dark as shown in Fig. 1. The linear sweep voltammetry and photoamperometry techniques were used for measuring current–voltage (I-V) and time dependent current flow behavior, respectively [42,43,44].
Result and discussion
Structural properties of ZnO thin film
XRD pattern of ZnO thin film was recorded with θ-2θ configuration, which is shown in Fig. 2. This pattern was used to identify the crystal phase and plane orientation in the sample. The main peak at 2θ = 33.58° corresponds to ZnO (002) according to JCPDS (No. 036–1451). This pattern confirms the growth of ZnO thin film with high crystalline structure. The crystallite size was determined about 7 nm using Debye-Scherer’s Eq. (1) [45, 46].
where D, K, λ, β, θ are crystal size, dimensionless shape factor (0.94), wavelength of X-ray, FWHM (full width at half maximum intensity of the peak, and the Braggs angle, respectively.
Optical properties of photoelectrodes
The absorption spectra of Basil/ZnO/FTO electrode, ZnO/FTO photoelectrodes and basil dye are shown in Fig. 3a, b and c, respectively. The absorption peak for ZnO and basil dye are observed at 349 nm 675 nm those are in ultraviolet and visible range, respectively. The observed peak in basil dye at 675 nm and 286 nm corresponds to chlorophyll and quinine, respectively [47, 48]. The band gap energy were determined at 3.98 eV for ZnO and 5 eV for basil dye using Eq. (2), which is expressed as Tauc plot in inset of Fig. 3.
where, m = 1/2, 3/2 for direct and or indirect semiconductor, respectively. α, h, v, Eg are absorption co-efficient, plank constant, frequency of light, band gap.
Figure 4a and b shows the Urbach plot for ZnO film and basil dye, respectively. Urbach relation is expressed in Eq. (3). The Urbach energy (Eu) is determined from Urbach plot and calculated about 0.0854 eV for ZnO film and 0.6751 for basil dye [49].
The optical parameters are listed in Table 1.
where α0, Eu, ʋ, and ℎ are for a constant, Urbach energy, frequency, and Planck’s constant.
Solar energy conversion properties
Photo electrochemical photocurrents generation
The energy diagram of prepared device is shown in Fig. 5 that gives the explanation of charge transfer mechanism and energy conversion in PEC [50]. The basil-ZnO interface plays important role in operation and characteristics of PEC. The surface to volume ratio is large in nanostructured ZnO particles and hence dye molecules adsorbed in large numbers on the surface [51]. The dye molecules excite upon the illumination of light on basil layer. This photo excitation happens because of electron charge transfer between HOMO to LUMO in basil. The photo excited electron enters into the area of ZnO film and diffuses at backside contact of device. Hence, the photocurrents are generated as a result and can be collected through complete a circuit. The separation energy of HOMO–LUMO and interfaces controls PEC response in device. More interactions between dye molecule and ZnO particle can improve the charge transfer characteristics and performance of the device [52].
The photoelectrochemical current–voltage characteristics of basil/ZnO/FTO and ZnO/FTO are shown in Fig. 6. The transient photocurrent behaviors are shown in 6c, and 6d. The basil sensitized ZnO photoelectrode has shown enhanced PEC properties. The open circuit potential (Voc) was increased from -0.46 V to 0.6 V whereas short circuit current (Isc) was increased from -0.05 mA to 0.14 mA due to usage of basil layer, respectively. The basil sensitized photoelectrode has shown fast switching properties and higher photoresponsivity compared to bare ZnO photoelectrode. The transient photocurrent characteristics of basil sensitized device show the slow degrading nature of natural basil dye due to the reason that photo voltage is decreasing with time. The photoelectrochemical parameters are listed in Table 2.
Tafel curve is shown in Fig. 7 that consists of three different zones as the polarization, Tafel and diffusion zone at low, middle (with a sharp decrease), and high potential (horizontal region) [53, 54]. The current density decides the electrocatalytic ability of photoelectrode, which can be determined by extrapolating the intercept of cathodic and anodic regions in Tafel zone. A sharp slope can be seen in Tafel zone for ZnO/FTO and basil/ZnO/FTO device. A larger exchange current density (J0) in basil/ZnO/FTO than ZnO/FTO device shows the lower charge transfer resistance (Rct) and higher electrocatalytic activity at the interface of photoelectrode and electrolyte. J0 is inversely related with Rct according to Eq. (4). Basil sensitized photo electrode has shown positively shifted equilibrium corrosion potential (Ecorr) compared to that of bare ZnO device, which is indicating an improvement in the corrosion protection.
where R is gas constant, T is temperature, n is number of electrons, F is faraday constant and Rct is charge transfer resistance.
Mott-Schottky plots for bare and hybrid electrodes are shown in Fig. 8. A representation of the reciprocal square capacitance is a linear function of the potential, which is known as Mott-Schottky plot. These plots give information on doping density and flatband potential (Vfb). The intercept to the x-axis determines the value of Vfb. The positive slop confirms the n-type behaviour of semiconductor. The Mott-Schottky plot is sensitive to the surface of the electrode and electrode–electrolyte interface. Vfb shifts about 0.99 V (vs. SCE) toward cathodic potential in basil sensitized ZnO that attributes for higher Voc value. The decline in potential show the higher stability in basil/ZnO/FTO than ZnO/FTO [55,56,57].
Photoelectrochemical fuel generation
Photoelectrochemical water splitting process gives hydrogen and oxygen using light energy [58,59,60]. We have demonstrated the PEC device in which a ZnO electrode is modified with basil layer and connected with a platinum counter electrode through a circuit. The route of the photocurrent tells the reduction (hydrogen evolution) at counter electrode and the oxidation (oxygen evolution) at working electrode. Many gas bubbles could be observed at the basil/ZnO/FTO photoelectrode surface that continuously evolved from the surface as shown in Fig. 9 [61,62,63]. In PEC water splitting, the photo excited electron leaves a hole in semiconductor and leaves positive charge carriers (H+ ions or protons) in the solution. This unites with other proton and two electrons to make hydrogen (H2) gas. The generation of solar fuel using visible light is expressed in below reactions.
Conclusion
The photoelectrochemical cells were demonstrated for solar energy conversion applications. PECs were prepared using ZnO, and basil dye. Natural dyes play a significance role to improve the light absorption in device. The ultra-small sized particles and thin layers of hybrid nanostructures at interface in PEC device can enhance the values of photovoltage and photocurrent with a significant percentage. We achieved Voc of 0.6 V and Isc of 0.14 mA. The basil coated photoelectrode has shown better photo switching and photo responsive properties. The shifting in flat band potential is also an important reason behind higher Voc in hybrid PEC. The hybrid PEC also has been studied for production solar fuel as hydrogen and oxygen through water splitting. The hybrid PEC can solve the challenging problems in renewable energy and solar fuel in future.
References
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37 (1972)
Parkinson, B.: An overview of the progress in photoelectrochemical energy conversion. J. Chem. Educ. 60(4), 338 (1983). https://doi.org/10.1021/ed060p338
Gratzel, M.: Photovoltaic and photoelectrochemical conversion of solar energy. Philos. Trans. R. Soc. A 365, 993 (2007)
Ragauskas, A.J., et al.: The path forward for biofuels and biomaterials. Science 311, 484–489 (2006)
Hoffmann, M.R., Martin, S.T., Choi, W.Y., Bahnemann, D.W.: Environmental applications of semiconductor photocatalysis. Chem. Rev. 95(1), 69–96 (1995)
Chu, S., Majumdar, A.: Opportunities and challenges for a sustainable energy future. Nature 488(7411), 294–303 (2012)
Shiyani, T., Bagchi, T.: Hybrid nanostructures for solar energy conversion applications. Nanomater. Energy 9(1), 1–7 (2020)
Blankenship, R.E., et al.: Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011). https://doi.org/10.1126/science.1200165
Semonin, O.E., et al.: Peak external photocurrent quantum efficiency exceeding 100% via MEG in a quantum dot solar cell. Science 334, 1530 (2011)
O’Regan, B., Grätzel, M.: A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737 (1991)
Günes, S., Sariciftci, N.S.: Hybrid solar cells. Inorg. Chim. Acta. 361(3), 581–588 (2008)
Brentner, L.B., Peccia, J., Zimmerman, J.B.: Challenges in developing biohydrogen as a sustainable energy source: implications for a research agenda. Environ. Sci. Technol. 44(7), 2243–2254 (2010)
Taesoo, D., Ebong, A.U.: A review of thin film solar cell technologies and challenges. Renew. Sustain. Energy Rev. 70, 1286–1297 (2017)
Cates, E.L., Chinnapongse, S.L., Kim, J.-H., Kim, J.-H., Light, E.: Advances in wavelength conversion materials for energy and environmental technologies. Environ. Sci. Technol. 46(22), 12316–12328 (2012)
Crabtree, G.W., Dresselhaus, M.S., Buchanan, M.V.: The hydrogen economy. Phys. Today 57(12), 39 (2004)
Wang, D., Saleh, N.B., Sun, W., Park, C.M., Shen, C., Aich, N., Peijnenburg, W.J., Zhang, W., Jin, Y., Su, C.: Next-generation multifunctional carbon–metal nanohybrids for energy and environmental applications. Environ. Sci. Technol. 53(13), 7265–7287 (2019). https://doi.org/10.1021/acs.est.9b01453
Lu, Lu., Williams, N.B., Turner, J.A., Maness, P.-C., Jing, G., Ren, Z.J.: Microbial photoelectrosynthesis for self-sustaining hydrogen generation. Environ. Sci. Technol. 51(22), 13494–13501 (2017)
Licht, S.: Multiple band gap semiconductor/electrolyte solar energy conversion. J. Phys. Chem. B 105(27), 6281–6294 (2001). https://doi.org/10.1021/jp010552j
Kumaravel, V., Abdel-Wahab, A.: A short review on hydrogen, biofuel, and electricity production using seawater as a medium. Energy Fuels 32(6), 6423–6437 (2018). https://doi.org/10.1021/acs.energyfuels.8b00995
Mark, S.W.: Photoelectrochemical conversion of optical energy to electricity and fuels. Acc. Chem. Res. 12(9), 303–310 (1979)
Kumar, M.P., Jagannathan, R., Ravichandran, S.: Photoelectrochemical system for unassisted high-efficiency water-splitting reactions using N-doped TiO2 nanotubes. Energy Fuels 34(7), 9030–9036 (2020)
Rocheleau, R.E., Miller, E.L., Misra, A.: High-efficiency photoelectrochemical hydrogen production using multijunction amorphous silicon photoelectrodes. Energy Fuels. 12(1), 3–10 (1998). https://doi.org/10.1021/ef9701347
Bak, T., Nowotny, J., Rekas, M., Sorrell, C.C.: Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int. J. Hydrogen Energy 27, 991 (2002)
Gupta, M., Sharma, V., Shrivastava, J., Solanki, A., Singh, A.P., Satsangi, V.R., Dass, S., Shrivastav, R.: Preparation and characterization of nanostructured ZnO thin films for photoelectrochemical splitting of water. Bull. Mater. Sci. 32(1), 23–30 (2009)
Xianzhi, Fu., Clark, L.A., Yang, Q., Anderson, M.A.: Enhanced photocatalytic performance of titania-based binary metal oxides: TiO2/SiO2 and TiO2/ZrO2. Environ. Sci. Technol. 30(2), 647–653 (1996)
Greijer, H., Karlson, L., Lindquist, S.E., Hagfeldt, A.: Environmental aspects of electricity generation from a nanocrystalline dye-sensitized solar cell system. Renew. Energy 23, 27 (2001)
Hisatomi, T., Kubota, J., Domen, K.: Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7520 (2014)
Dai, W., Tao, Y., Zou, H., Xiao, S., Li, G., Zhang, D., Li, H.: Gas-phase photoelectrocatalytic oxidation of NO via TiO2 nanorod array/FTO photoanodes. Environ. Sci. Technol. 54(9), 5902–5912 (2020)
Cui, W., Shan, W., Chen, F., Xia, Z., Li, Y., Zhang, X.-H., Song, T., Lee, S.-T., Sun, B.: Silicon/organic heterojunction for photoelectrochemical energy conversion photoanode with a record photovoltage. ACS Nano 10(10), 9411–9419 (2016). https://doi.org/10.1021/acsnano.6b04385
Gratzel, M.: Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 44, 6841 (2005)
Paracchino, A., Laporte, V., Sivula, K., Grätzel, M., Thimsen, E.: Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 10(6), 456–461 (2011)
Hardin, B.E., Hoke, E.T., Amstrong, P.B., Yum, J.H., Comte, P., Torres, T., Frechet, J.M.J., Nazeeruddin, M.K., Gratzel, M., McGehee, M.D.: Increased light harvesting in dye-sensitized solar cells with energy relay dyes. Nat. Photonics 3, 406 (2009)
Dubois, D.L.: Development of molecular electrocatalysts for CO2 reduction and H2 production/oxidation. Acc. Chem. Res. 2009, 42 (1974)
Hemmatzadeh, R., Mohammadi, A.: Improving optical absorptivity of natural dyes for the fabrication of efficient dye sensitized solar cells. J. Theor. Appl. Phys. 7, 1 (2013)
Vargas, F.D., Jimenez, A.R., Lopez, O.P.: Natural pigments: carotenoids, anthocyanins, and betalains–characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 40, 173 (2000)
Snaith, H.J.: Estimating the maximum attainable efficiency in dye-sensitized solar cells. Adv. Funct. Mater. 20, 13 (2010)
Hamann, T.W., Jensene, R.A., Martinson, A.B.F., Ryswyk, H.V., Hupp, J.T.: Advancing beyond current generation dye-sensitized solar cells. Energy Environ. Sci. 1, 66 (2008)
Fountaine, K.T., Lewerenz, H.J., Atwater, H.A.: Efficiency limits for photoelectrochemical water-splitting. Nat. Commun. 7, 13706 (2016)
Dresp, S., Dionigi, F., Klingenhof, M., Strasser, P.: Direct electrolytic splitting of seawater: opportunities and challenges. ACS Energy Lett. 4, 933–942 (2019)
Shiyani, T., Agrawal, S., Banerjee, I.: Natural dye sensitized photoelectrochemical cells for solar energy conversion. Nanomater. Energy 9(2), 1–12 (2020)
Kumaravel, V., Bartlett, J., Pillai, S.C.: Photoelectrochemical conversion of carbon dioxide (CO2) into fuels and value-added products. ACS Energy Lett. 5, 486 (2020)
Li, S., Li, Z.-J., Yu, H., Sytu, M.R., Wang, Y., Beeri, D., Zheng, W., Sherman, B.D., Yoo, C.G., Leem, G.: Solar-driven lignin oxidation via hydrogen atom transfer with a dye-sensitized TiO2 photoanode. ACS Energy Lett. 5, 777 (2020)
Sayama, K.: Production of high-value-added chemicals on oxide semiconductor photoanodes under visible light for solar chemical-conversion processes. ACS Energy Lett. 3, 1093–1101 (2018)
Agrawal, S., et al.: ZnO thin film deposition for TCO application in solar cell. Hindawi Conf. Pap. Energy 2013, 718692 (2013)
Jakub, Z., Kraushofer, F., Bichler, M., Balajka, J., Hulva, J., Pavelec, J., Sokolovic, I., Mullner, M., Setvin, M., Schmid, M., Diebold, U.: Partially dissociated water dimers at the water–hematite interface. ACS Energy Lett. 4(2), 390–396 (2019)
Jin, S.: “What else can photoelectrochemical solar energy conversion do besides water splitting and CO2 reduction? ACS Energy Lett. 3, 2610–2612 (2018)
Zhang, H., Cheng, C.: Three-dimensional FTO/TiO2/BiVO4 composite inverse opals photoanode with excellent photoelectrochemical performance. ACS Energy Lett. 2, 813–821 (2017)
Debajeet, K.B., Braun, A.: Solution processed transparent nanoparticulate ZnO thin film electrode for photoelectrochemical water oxidation. RSC Adv. 4, 23562 (2014)
Fan, W., Li, M., Bai, H., Xu, D., Chen, C., Li, C., Ge, Y., Shi, W.: Fabrication of MgFe2O4/MoS2 heterostructure nanowires for photoelectrochemical catalysis. Langmuir 32, 1629–1636 (2016)
Kenney, M.J., et al.: High-performance silicon photoanodes passivated with ultrathin nickel films for water oxidation. Science 342, 836 (2013)
Alexander, B.D., et al.: Metal oxide photoanodes for solar hydrogen production. J. Mater. Chem. 18, 2298 (2008)
Walter, M.G., et al.: Solar water splitting cells. Chem. Rev. 110, 6446 (2010)
Dom, R., Govindarajan, S., Joshi, S.V., Borse, P.H.: A solar-responsive zinc oxide photoanode for solar-photon-harvester photoelectrochemical (PEC) cells. Nanoscale Adv. 2, 3350 (2020)
Sheng-Jun, L., Yuan, L., Wei-Wei, T., Jing-Bo, Z., Xiao-Wen, Z., Jin-Mao, C., Zeng, C.: Preparation and performance of dye-sensitized solar cells based on ZnO-modified TiO2 electrodes. Int. J. Miner. Metall. Mater. 17(1), 92 (2010)
Syed, A.A.S., Muhammad, H.S., Nazia, N., Ramshah, A.T., Sarah, S., Hytham, E., Qiquan, Q.: Photovoltaic performance and impedance spectroscopy of a purely organic dye and most common metallic dye based dyesensitized solar cells. J. Mater. Sci. 28, 6552–6559 (2017)
Fleischmann, S., Mamontov, E., Jiang, D., Augustyn, V.: Interlayer separation in hydrogen titanates enables electrochemical proton intercalation. J. Mater. Chem. A. 8, 412 (2020)
Banerjee, S., Pillai, S.C., Falaras, P., O’Shea, K.E., Byrne, J.A., Dionysiou, D.D.: New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 5(15), 2543–2554 (2014)
Chen, X.B., Liu, L., Yu, P.Y., Mao, S.S.: Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331(6018), 746–750 (2011)
Yang, W., Prabhakar, R.R., Tan, J., Tilley, S.D., Moon, J.: Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 48(19), 4979–5015 (2019)
Xin, R., Abhijeet, S., Siyuan, Z., Shuai, Y., Yin, Z., Liyi, S., Robert, L.Z.H., Seungho, C.: Dongdong L and Judith LM-D, Photoelectrochemical water splitting strongly enhanced in fast-grown ZnO nanotree and nanocluster structures. J. Mater. Chem. A 4, 10203 (2016)
Li, J., Xingming, W., Shirolkar, M.M., Li, M., Chunye, Xu., Wang, H.: A high performance ZnO based photoelectrochemical cell type UV photodetector with [Co(bpy)3]3+/2+ electrolyte and PEDOT/ITO counter electrode. RSC Adv. 7, 18987 (2017)
Shrok, A., Zainab, D.A.A., Ying, L., Hayder, H., Basher, H.J., Li, L., Tianshu, L.: Photoelectrochemical performance of N-doped ZnO branched nanowire photoanodes. Heliyon 3, e00423 (2017). https://doi.org/10.1016/j.heliyon.2017
Inguanta, R., Garlisi, C., Spano, T., Piazza, S., Sunseri, C.: Growth and photoelectrochemical behaviour of electrodeposited ZnO thin films for solar cells. J. Appl. Electrochem. 43, 199–208 (2013)
Acknowledgements
Authors are thankful for UGC Non-NET fellowship to TS. Authors are also thankful to FCIPT-IPR for providing sputtering facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shiyani, T., Dube, C.L. Photoelectrochemical energy conversion using hybrid photoelectrodes. Mater Renew Sustain Energy 11, 251–258 (2022). https://doi.org/10.1007/s40243-022-00221-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-022-00221-4