Abstract
Efficient and cheap dye-sensitized solar cells (DSSCs) were fabricated using natural dyes from Pastinaca sativa and Beta vulgaris. Natural dyes are environmentally and economically superior to ruthenium-based dyes because they are nontoxic and cheap. However, the conversion efficiency of dye-sensitized solar cells based on natural dyes is low. One way to improve the DSSC performance is to enhance the absorptivity of extracted dyes. We investigated the influence of various factors in the extraction process, such as utilization of different extraction approaches, the acidity of extraction solvent, and different compounds of solvents on the optical absorption spectra. It was found that we could considerably enhance the optical absorptivity of dye and consequently the performance of DSSC by choosing a proper mixture of ethanol and water for extracting solvent and also the acidity of dye solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
One of the greatest challenges in the next 50 years is the production of clean and renewable energy [1]. Our current energy consumption predominately relies on fossil fuels that generate greenhouse gases, most notably carbon dioxide. Greenhouse gases are directly implicated in the rise of average global temperature over the last century, which has widespread effects on ocean levels, biodiversity, crop production, natural disasters, and other aspects of the ecosystem [2]. Among the renewable energy technologies, solar cells utilizing solar energy are considered as the most promising ones. First-generation solar cells are based on silicon materials [3]. Second-generation solar cells rely on thin films such as cadmium telluride and copper indium selenide. However, indium, tellurium, and selenium are relatively scarce. Cadmium is a highly toxic heavy metal, and mining of these metals causes various environmental hazards [4]. Because of the high cost of solar cells, we must look for other technologies if solar cells become environmentally and economically competitive with fossil fuels.
Dye-sensitized solar cells (DSSCs) are third-generation solar cells that exhibit many advantages over the previous generations of solar cells [5, 6]. In an environmental comparison of electricity generation from a DSSC system and a natural gas combined cycle power plant, the gas power plan would result in 450 g CO2/kW h and DSSCs would result in between 19 and 47 g CO2/kW h [7–9]. One of the key materials in DSSCs is the sensitizer. Ruthenium complex dyes are capable of delivering DSSCs with high conversion efficiencies [10]. However, ruthenium dyes are not suitable for environmentally friendly photovoltaic systems. Ruthenium is expensive and environmentally hazardous. Ruthenium compounds are treated as highly toxic and carcinogenic. When ruthenium compounds are heated in the presence of air, they form ruthenium tetroxide, which is a highly volatile and toxic compound that damages the eyes and upper respiratory system [4].
Natural dyes such as pigments used in food coloring are easily and safely extracted from plants [11]. It means that they do not require complex synthesis or toxicity test [12] and can be used in DSSCs. Since natural dyes have low cost of synthesis and are environmentally friendly, they are considered as a viable option for dye-sensitized solar cells in future research [13]. Natural dyes in DSSCs have shown overall conversion efficiencies below 1%. Until now, several natural dyes such as betalains [14, 15], anthocyanins [16, 17], and carotenes [18] have been used as sensitizers in DSSCs. Betalains are water-soluble pigments that can be found in roots, fruits, and flowers. Betalain definition embraces all compounds with structures based on the general formula shown in Figure 1. Betalains have the requisite functional groups (-COOH) to bind better to the TiO2 nanostructure [19]. In this work, we developed an effective approach for extraction of dyes from Beta vulgaris and Pastinaca sativa in such a way that we could modify the optical absorption spectra of the extracted dye. To this end, we examined the influence of several parameters such as extraction solvents, acidity of solutions, and mixture of the extraction solvents on the absorptivity of the extracted dyes. Then, we used these dyes to fabricate DSSC under analogous conditions.
General formula of betalain [[19]].
Mechanism of dye-sensitized solar cell
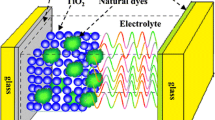
DSSC is composed of five elements: two transparent conductive substrates, nanostructured titanium dioxide layer, Pt layer, dye molecules, and electrolyte. Transparent conductive substrates are coated with a thin layer of TiO2 and Pt nanostructures, respectively. The mechanism of this type of solar cell is similar to the photosynthesis process in plants [20]. In photosynthesis, light is converted into chemical energy. Photons with different energies in sunlight strike on the cell and penetrate into the dye layer since both the fluorine-doped tin oxide (FTO) layer with glass substrate and the TiO2 nanocrystals are transparent to visible light. If photon energy is close to the energy gap of the dye molecule, namely the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), it will be absorbed by the dye, promoting one electron from HOMO to LUMO [21, 22]. The excited electron will then be injected into the conduction band of TiO2 through the interfacial bonds between the dye and the TiO2, then be transported, and finally collected by the FTO (working electrode). The hole which was generated by photon excitation remains on the molecule during the process since the HOMO of dye is separated from all other energy levels. The hole eventually will be filled up by electrons from electrolyte ions. At the same time, reduction of oxidized dye by iodide produces triiodide. The triiodide diffuses to a counter electrode and accepts electrons from external load, regenerating the iodides. The overall process will provide electron flow from the working electrode to the outer circuit [23, 24]. The dye would be regenerated by the electrolyte solution and would get ready for the next photon [24]. The general structure of a DSSC is illustrated in Figure 2.
Experimental details
Preparation of natural dye as sensitizer
The extracts of P. sativa and B. vulgaris were obtained from fresh materials. The solvents extracted from P. sativa (ethanol and water extract) were added over the small pieces of clean P. sativa in a solvent of 0.1 and 1 N HCl. The dyes extracted from B. vulgaris were obtained by immersing the B. vulgaris slice in HCl (0.1 and 1 N) solution (ethanol extract). The mixtures were kept overnight, and the resulting extracts were filtered to remove any solid residue and were used for sensitization. All solutions were protected from direct light and stored in the refrigerator at about +5°C; the dye solutions were stable for more than 12 months. The features of solutions extracted from P. sativa and B. vulgaris are listed in Table 1.
Fabrication of dye-sensitized solar cell
For the fabrication of dye-sensitized solar cells, the FTO conductive glass with a resistance of 8 Ω was used. TiO2 paste with nanoparticle sizes ranging from 15 to 20 nm was deposited on the FTO conductive glass with doctor blade technique [25], and then TiO2 paste with particle sizes ranging from 100 to 200 nm as a scatterer layer was used. The TiO2 film was sintered at 500°C for 30 min. After cooling, the TiO2 electrode was immersed in a solution of dye for a time period varying from 6 to 24 h; TiO2 film and substrates were rinsed in ethanol. The dye-covered TiO2 electrode and Pt counter electrode was assembled into a sandwich type cell and sealed with a sealing spacer. The electrolyte solution was prepared by dissolving 2.07 g of KI and 0.19 g of I2 in 25 ml ethylene glycol. After injection of the electrolyte, the hole in the counter electrode was sealed using a sealing spacer.
Result and discussion
Sensitizers for DSSCs need to fulfill important requirements such as absorption in the visible and near-infrared regions of the solar spectrum and should be strongly chelated to the semiconductor oxide surface. Moreover, the LUMO of the dye should lie at a higher energy level than the conduction band of the semiconductor so that, upon excitation, the dye injects electrons into the conduction band of the TiO2. The HOMO energy level of the dye needs to be more positive than the redox potential of the couple (I-/I3 -) [5]. In this study, sensitizers such as dye extracted from B. vulgaris, P. sativa were used to fabricate DSSCs.
Betalains in acidic environments have strong absorption in the 400- to 600-nm range due to the color combination of yellow-orange betaxanthins and red-pink betacyanins [26]. Since the destruction of the environment and nonacidic betalains occurs very quickly, the acidic environment was an important factor for the extracted betalain dyes as previously observed by Zhang et al. [27]. The acid concentration in aqueous extract was changed from its initial value of 0.1 to 1 N for extracted dye from P. sativa and B. vulgaris. In addition to the significant increase of absorption intensity, this change has caused variation in the electrical current of the cells. Figure 3 shows the absorption spectra of four dyes extracted from P. sativa in which two of them were diluted in water and the others were diluted in ethanol.
The intensity of absorptions is approximately equal for water and ethanol extracts. An explanation for these results may come from the difference between compositions of the extracts. In the water extract, the concentration of dyes is expected to be higher than in ethanolic extracts, probably because of a higher solubility. Figure 4 shows the absorption spectrum of natural dyes extracted from P. sativa diluted in water and ethanol.
Consequently, although the UV-vis spectra of the extracts indicated the most suitable dye of the ethanolic extract, the performance of the DSSCs suggested that the proper sensitizer is the one obtained from the water extract. The acidity of the extract likely influences the solubility of various dyes, leading to extracts with different compositions. Although the absorption intensity of dye E, that is the mixture of A and C, is low, it indicates better performance in DSSCs. Figure 5 shows the absorption spectrum of dyes E, C, and A.
Changes which were made in the synthesis of natural dyes cause increase in absorption intensity which directly affects the rate of increase in some other parameters such as excited state lifetime of the dye molecules. We have shown that changing the acidity of the solution to extracted dye from B. vulgaris had an extensive effect on the absorption spectra. Figure 6 shows the effects of acidic solution in the absorption spectra of dye extracted from B. vulgaris.
Under equal preparation and irradiation conditions, the performance of DSSCs with eight different extracts was examined. The experimental setup for measuring the performance of DSSCs using natural dyes was built using a xenon lamp as solar simulator with full spectrum in the visible light. The first four ones correspond to extracts obtained from P. sativa, the other two ones were extracted from B. vulgaris, and the last one was obtained from P. sativa in acidic solution diluted in ethanol and water mixture. Table 2 summarizes the results for the extracts. In this table, the short-circuit photocurrent density (JSC), the open-circuit voltage (VOC) and the fill factor (FF) are reported.
We achieved a better sensitization activity using the dye extracted from P. sativa compared to the dye extracted from B. vulgaris. It was found that the best results were recorded for P. sativa. This is because these extracts both have betaxanthins and betacyanins, each with an absorption at different wavelengths, helping the cell to capture photons of two different energies. The best FF was obtained by the P. sativa extract (dye B), showing VOC and JSC values of 0.39 V and 7 mA/cm2, respectively, with an active area = 0.9 cm2. Dye extracted from P. sativa (dye D) displays a promising photoelectrochemical performance showing a JSC = 7.2 mA/cm2, a VOC = 0.42 V, a fill factor = 0.9, and an active area = 0.9 cm2. Despite a maximum FF value of 0.95, the DSSC equipped with B. vulgaris extract (dye G) shows a JSC = 3.45 mA/cm2, a VOC = 0.46 V, an FF = 0.9, and an active area = 0.9 cm2. These values are in agreement with those obtained in [14–16]. The current-voltage curve obtained with solar cells using P. sativa (dye B) as sensitizer is presented in Figure 7. Fill factor values from 0.4 to 0.95 were obtained with the natural dye extracted. The best values of FF for DSSCs using betalain dyes are less than 0.7 [28], significantly smaller than our best FF value of 0.95. Calogero et al. reported that a maximum FF of 0.57 was obtained using betalain dye extracted from Bougainvillea as sensitizer. They also reported that using B. vulgaris rubra as sensitizer for DSSC with maximum conversion efficiency has an FF of 0.37 [15], while we achieved a maximum FF of 0.9 (B solution). Betalains extracts from Sicilian prickly pear are employed as sensitizer for DSSCs with the highest performance reported by Calogero et al. at 0.62 [16]. In particular, the use of betalains gives a promising result with an FF of 0.95 which we know to be among the highest one so far that is reported with natural dyes. From the above results, it is seen that most of the natural dyes based DSSCs extracted from B. vulgaris and P. sativa, with the fill factor is enhanced with the increase of extract acidity. We have found that the dye extracted from P. sativa shows the highest solar energy conversion efficiency. Enhancement of performance of DSSCs has been successfully accomplished using extracts of the B. vulgaris and P. sativa plants as natural sensitizers. The dye extracts present good light harvesting properties and sensitize the charge transfer of TiO2. The use of natural sources for the sensitizer simplifies the points involved in the simple and environmentally friendly production of DSSCs, providing an interesting alternative to commonly used organic and inorganic dyes.
Conclusions
We studied the use of dye extracted from B. vulgaris and P. sativa for DSSCs considering the improvement of natural pigment use. In our study, we tried to enhance the performance of DSSCs using natural dyes. The increase of acidity of dyes extracted from B. vulgaris and P. sativa leads to an increase of the DSSC performance. The simple extraction procedure, low cost, and environmentally friendly nature make natural dyes promising sources of sensitizers for DSSCs. The efficiency increases after the acidity of the extracts increased. The open-circuit voltage has small differences, but the current density varies significantly. Our experimental results show that the short-circuit current density and the efficiency of the DSSCs increased by the acidification of dye extracted from B. vulgaris and P. sativa. We observed that the nature of solvent has an effect on the performance of DSSCs using the solvent as sensitizer. Although the spectrum of the extracts is considered as the most suitable dye of the ethanolic extract, the performance of the cells suggested that the proper solvent as sensitizer was obtained from the water extract in acidic solution. We stress that these results are achieved by optimization of the extraction dyes and enhance the optical absorption of natural dyes. The results are encouraging and may boost additional studies to search for new natural dyes and to optimize DSSCs with natural dyes.
Authors' information
RH is an MSc student of Physics at Persian Gulf University. AM is an assistant professor of Physics at Persian Gulf University.
References
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H: Dye-sensitized solar cells. Chem Rev 2010, 110: 6595–6663. 10.1021/cr900356p
Krauter SCW: Total energy balance. In Solar Electric Power Generation–Photovoltaic Energy Systems. Edited by: Krauter SCW. New York: Springer; 2006:177–186.
Szlufcik J, Agostinelli G, Duerinckx F, Van Kerschaver E, Beaucarne G: Low cost industrial technologies of crystalline silicon solar cells. In Solar Cells: Materials, Manufacture and Operation. Edited by: Castaner L, Markavart T. Oxford: Elsevier Ltd; 2006:89–102.
Gratzel M: Photovoltaic and photoelectrochemical conversion of solar energy. Phil Trans R Soc A 2007, 365: 993–1005. 10.1098/rsta.2006.1963
Gratzel M: Solar energy conversion by dye-sensitized photovoltaic cells. Inorg Chem 2005, 44: 6841–6851. 10.1021/ic0508371
O’regan B, Gratzel M: A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO 2 films. Nature 1991, 353: 737–740. 10.1038/353737a0
Greijer H, Karlson L, Lindquist SE, Hagfeldt A: Environmental aspects of electricity generation from a nanocrystalline dye sensitized solar cell system. Renew Energy 2001, 23: 27–39. 10.1016/S0960-1481(00)00111-7
Dubois DL: Development of molecular electrocatalysts for CO 2 reduction and production/oxidation. Acc Chem Res 2009, 42: 1974–1982. 10.1021/ar900110c
Morris AJ, Meyer GJ, Fujita E: Molecular approaches to the photocatalytic reduction of carbon dioxide for solar fuels. Acc Chem Res 2009, 42: 1983–1994. 10.1021/ar9001679
Chiba Y, Islam A, Watanab Y, Komiya R, Koide N, Han L: Dye-sensitized solar cells with conversion efficiency of 11.1%. Jpn J Appl Phys 2006, 45: l638-l640. 10.1143/JJAP.45.L638
Smestad GP, Gratzel M: Demonstrating electron transfer and nanotechnology: a natural dye-sensitized nanocrystalline energy converter. J Chem Educ 1998, 75: 752–756. 10.1021/ed075p752
Patrocinio AOT, Iha NYM: Toward sustainability, solar cells sensitized by natural extracts. Quim Nova 2010, 33: 574–578. 10.1590/S0100-40422010000300016
Davies KM: Plant Pigments and Their Manipulation. Palmerston North: Blackwell Publishing Ltd; 2004.
Calogero G, Di Marco G, Cazzanti S, Caramori S, Argazzi R, Carlo AD, Bignozzi CT: Efficient dye-sensitized solar cells using red turnip and purple wild Sicilian prickly pear fruits. Int J Mol Sci 2010, 11: 254–267. 10.3390/ijms11010254
Martinez ARH, Esteves M, Vargas S, Quintanilla F, Rodriguez R: New dye-sensitized solar cells obtained from extracted bracts of Bougainvillea glabra and spectabilis betalain pigments by different purification processes. Int J Mol Sci 2012, 12: 5565–5576.
Calogero G, Yum HJ, Sinopoli A, Di Marco G, Gratzel M, Nazeeruddin MK: Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol Energy 2012, 86: 1563–1575. 10.1016/j.solener.2012.02.018
Garcia CG, Polo AS, Iha NYM: Fruit extracts and ruthenium polypridinic dyes for sensitization of TiO 2 in photoelectrochemical solar cells. J Photochem Photobiol A Chem 2003, 160: 87–91. 10.1016/S1010-6030(03)00225-9
Yamazaki E, Murayama M, Nishikawa N, Hashimoto N, Shoyama M, Kurita O: Utilization of natural carotenoids as photosensitizers for dye-sensitized solar cells. Sol Energy 2007, 81: 512–516. 10.1016/j.solener.2006.08.003
Azeredo HMC: Betalains: properties, sources, applications, and stability—a review. Int J Food Sci Technol 2009, 44: 2365–2376. 10.1111/j.1365-2621.2007.01668.x
Mayer TJ: Chemical approaches to artificial photosynthesis. Acc Chem Res 1989, 22: 163–170. 10.1021/ar00161a001
Gratzel M: Dye-sensitized solar cells. J Photochem Photobiol C Photochem Rev 2003, 4: 145–153. 10.1016/S1389-5567(03)00026-1
Chang H, Wu HM, Chen TL, Huang KD, Jwo CS, Lo YJ: Dye-sensitized solar cell using natural dyes extracted from spinach and ipomoea. J Alloys Compd 2010, 495: 606–610. 10.1016/j.jallcom.2009.10.057
Gratzel M, Hagfeldt A: Molecular photovoltaics. Acc Chem Res 2000, 33: 269–277. 10.1021/ar980112j
Ito S, Saitou T, Imahori H, Uehara H, Hasegawa N: Fabrication of dye-sensitized solar cells using natural dye for food pigment: monascus yellow. Energy Environ Sci 2010, 3: 905–909. 10.1039/c000869a
Ito S, Murakami TN, Comte P, Liska P, Gratzel C, Nazeeruddin MK, Gratzel M: Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%. Thin Solid Films 2008, 516: 4613–4619. 10.1016/j.tsf.2007.05.090
Quin C, Clark AE: DFT characterization of the optical and redox properties of natural pigments relevant to dye-sensitized solar cells. Chem Phys Lett 2007, 438: 26–30. 10.1016/j.cplett.2007.02.063
Zhang D, Lanier SM, Downing JA, Avent JL, McHale JL: Betalains pigments for dye-sensitized solar cells. J Photochem Photobiol A Chem 2008, 195: 72–80. 10.1016/j.jphotochem.2007.07.038
Oprea CI, Dumbrava A, Enache I, Georgescu A, Girtu MA: A combined experimental and theoretical study of natural betalain pigments used in dye-sensitized solar cells. J Photochem Photobiol A Chem 2012, 240: 5–13.
Acknowledgements
The first author expresses his gratitude to A. Vaziri and A. Jamali for providing valuable comments on the preparation of solvents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that have no competing interests.
Authors' contributions
AM participated in the design of the study and drafted the manuscript. Both authors, AM and RH, had the same participation in any section of the article. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hemmatzadeh, R., Mohammadi, A. Improving optical absorptivity of natural dyes for fabrication of efficient dye-sensitized solar cells. J Theor Appl Phys 7, 57 (2013). https://doi.org/10.1186/2251-7235-7-57
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-7235-7-57