Abstract
The electrochemical production of green and low-cost ammonia requests the development of high-performance electrocatalysts. In this work, the ampoule method was applied to modulate the surface of the zinc electrode by implanting defects and low-valent active sites. The N-doped ZnS electrocatalyst was thus generated by sulfurization with thiourea and applied for electrocatalytic nitrogen reduction reaction (ENRR). Given the rich sulfur vacancies and abundant Zn-N active sites on the surface, excellent catalytic activity and selectivity were obtained, with an NH3 yield rate of 2.42 × 10–10 mol s−1 cm−2 and a Faradaic efficiency of 7.92% at − 0.6 V vs. RHE in 0.1 M KOH solution. Moreover, the as-synthesized zinc electrode exhibits high stability after five recycling tests and a 24 h potentiostatic test. The comparison with Zn foil, non-doping ZnS/Zn and recent metal sulfide electrocatalysts further demonstrated advanced catalytic performance of N@ZnS/Zn for ENRR. By simple synthesis, S vacancies, and N-doping defects, this promising electrocatalyst would represent a good addition to the arena of transition-metal-based catalysts with superior performance in ENRR.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In decades, chemical nitrogen fixation has become a hotspot of chemistry research [1]. It is a sought-after process that converting atmospheric dinitrogen gas into the nitrogen-containing compounds acquired by modern civilization [2]. Nowadays, the utilization of dinitrogen gas is mainly focused on the synthesis of ammonia, a vital industrial raw material [3]. Emerged as one of the promising alternatives to the traditional Haber–Bosch process, electrocatalytic nitrogen reduction reaction (ENRR) has drawn increasing attention. Instead of the high consumption of fossil fuels and massive amounts of greenhouse gas emission, the ENRR provides a clean generation of NH3 in aqueous electrolytes under ambient conditions [4]. However, the reaction efficiency is restricted by the kinetic and thermodynamic features of dinitrogen gas under ambient conditions. And due to the inevitable electrocatalytic hydrogen emission reaction (HER) that took place in the aqueous electrolytes, the development of a practical ENRR technique is still challenging [5].
The past 5 years witnessed numerous metal-based materials that were fabricated and adopted as electrocatalysts for the ENRR process [6, 7]. Regarding the improvement of the catalytic efficiency and selectivity toward ENRR, defect engineering is widely used as an efficient protocol to tune the superficial morphology and band structure of the nano-electrocatalysts, which facilitates the adsorption and activation of the N2 molecule [8]. Therein, using sulfur-containing substances as synthetic precursors for producing metal-based electrocatalysts may originally generate a nitrogenases-mimicking active site for enhancing the ENRR process [9].
As an element in the VI group, sulfur has a relatively weak electronegativity, which determined its diversity of bonding form. For instance, the sulfur reacts with metal elements to form ionic compounds, and shares electrons to bond with itself to form a chain-shaped polysulfide ion Sn2−. Therefore, the structure of metal sulfides tends to be diversified and complicated, which make sulfur a fascinating and promising doping element for functional metal electrocatalysts construction. In addition, it is reasonable to suppose that sulfur defects and vacancies would exhibit great potential in improving the activity of ENRR due to the enhancement of ENRR performance presented by the oxygen vacancies. After theoretical study and experiments, the MoS2 catalysts provided a distinctive electrocatalytic effect in producing ammonia from N2 gas in an aqueous solution [10,11,12]. Furthermore, the N-doped MoS2 nanoflowers presented enhanced catalytic performance due to rich S vacancies and Mo–N active sites [13]. Besides, with the aid of abundance S vacancies, ZrS2 nanofibers were also proven as a stable and active catalyst for ambient electrochemical N2-to-NH3 conversion with excellent selectivity [14]. Apart from early stage transition metals, iron-series metals were adopted as robust alternatives in ENRR by diverse modifications. Through solvothermal method and plasma synthesis, the Fe3S4 nanosheet [15] and surface-sulfurized Fe foam [16] both exhibited outstanding catalytic performance due to the low valence metallic site and S vacancies with suitable Fe–Fe distance. Because of the various bonding modes and enhanced conductivity, cobalt-based composite materials, CoS2@NC [17], CoS2-N/S-C [18] and C@CoS@TiO2 [19], were certified as good candidates for ENRR with the gratifying NH3 yields [20, 21]. However, most of the sulfide electrodes were obtained in aqueous conditions, which displayed an inferior synthetic efficiency due to the insolubility issue of metal sulfides. And the complicated synthetic routes pose a significant barrier to the practical use of metal sulfides in ENRR.
On the other hand, the controllable synthesis of electrocatalysts for various electrochemical reactions is a state-of-art technological challenge in both electrochemistry and material science [22]. Among all of the existing synthetic processes, the ampoule method owns superpassingly versatility in preparing self-standing electrode materials, including alloys, nitrides, phosphides, chalcogenides, and halides. Made from silica, quartz, and corrosion-resistant niobium and tantalum, the ampoules can be simply self-designed according to the requirement of various materials synthetic processes. Meanwhile, the solid or liquid reactants can be loaded inside the ampoule under evacuated or inert gas conditions before sealing the open end. After that, the heat-up step is always implemented in a furnace with hetero- or homogeneous applying temperature over a wide range. By such manipulations, the reaction conditions and atmosphere are confined, sequentially eliminating the environmental impact and thus realizing the controllable synthesis. These days, the surface-modified transition-metal foil materials generated from the ampoule method have already been applied to various electrocatalytic reactions [23,24,25,26,27].
With our ongoing interests in the ampoule methods for synthesizing catalytic materials and exploration of electrocatalytic ENRR, [28,29,30] herein, we propose the N@ZnS/Zn materials fabricated through the facile ampoule method that enables the electrochemical synthesis of ammonia in ambient conditions. The S vacancies and Zn-N activate sites are speculated to facilitate the adsorption of the N2 and reduction process. The adopted alkaline electrolytes and potassium cations present enough HER inhibition, which allows efficient synthesis of NH3.
Experimental section
Chemicals
Zinc foil (99.95% trace metals basis, thickness 0.1 mm) was purchased from Sigma-Aldrich. Potassium sodium tartrate, thiourea (CS(NH2)2), sulfur power (re-sublimation), hydrochloric acid (HCl, 36–38%), and hydrogen peroxide (H2O2, 30%) were purchased from Tianjin Yongda Chemical Reagent Co., Ltd. Potassium hydroxide (KOH) and Nessler’s reagent were purchased from Tianjin Damao Chemical Reagent Co., Ltd. Nitrogen (N2, 99.999%) and argon (Ar, 99.999%) were purchased from Shenyang Zhaote Special Gas Co., Ltd. All the chemicals were used without further purification.
Zinc-based electrode synthesis
The zinc-based catalytic electrodes were synthesized through the ampoule method. In the beginning, the zinc foil was polished and cleaned with anhydrate ethanol, acetone, 1 M HCl, and Milli-Q water for 5 min in sequence. After cutting into pieces (180 mg, 2 cm × 0.4 cm × 0.01 cm, length × width × height), the pretreated zinc foils were sealed with sulfurization reagents ampoule under vacuum and calcinated at 400 °C for 5 h. The heating rate was 5 ºC/min and the cooling process took place naturally. During this process, the Zn atoms of the foil were sulfurized by sulfur atom vapor under pressure, leading to free-standing electrodes possessing ZnS species on the surface. The material that sulfurized with thiourea and sulfur power is named N@ZnS/Zn and ZnS/Zn, respectively.
Instruments and characterization
The X-ray diffraction (XRD) patterns were obtained on a Bruker (Germany) D8 Advance diffractometer with Cu Kα radiation in the range of 20°–80° (2θ). The scanning electron microscope (SEM) images and elemental mapping images were performed in a Hitachi SU8000. Transmission electron microscope (TEM) images were obtained from a JEOL JEM-2100 at an acceleration voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) measurement was carried out on an ESCALAB Mk II (Vacuum generators) spectrometer with an Al K X-ray source (240 W).
Electrochemical tests
In general, the ENRR experiments were conducted in a two-compartment electrolysis cell that separated by the Nafion 117 membrane under ambient condition. The membrane was protonated by first boiling in ultrapure water for 1 h and treating in H2O2 (5%) aqueous solution at 80 °C for another 1 h in sequence. The membrane was then treated in 0.5 m H2SO4 for 3 h at 80 °C and finally stored in ultrapure water overnight. The electrochemical measurements were conducted using CHI 760 E electrochemical workstation (CH Instruments, Inc., USA) with a three-electrode configuration. Before proceeding with electrocatalytic tests, the synthesized materials were ultrasonicated Milli-Q water for 30 min and activated by electrolysis with the setups of ENRR tests in Ar-saturated 0.1 M KOH electrolyte at − 0.7 V vs RHE for 48 h. The as-prepared and activated zinc sulfide materials were used as the working electrode, while the graphite rod electrode and Ag/AgCl electrode (3.5 M KCl electrolyte) were adopted as the counter and reference electrode, respectively. Unless otherwise specified, the 0.1 M KOH solution (pH = 13) was adopted as the electrolyte in the linear sweep voltammetry (LSV) and chronoamperometry tests. The measured potentials vs. Ag/AgCl were converted to a reversible hydrogen electrode (RHE) scale according to the Nernst equation (ERHE = EAg/AgCl + 0.059pH + 0.205 = EAg/AgCl + 0.972). Also, the outlet gas was introduced to an acid bottle with 5 mL of 2 M HCl for wet scrubbing to collect the possible leaking ammonia.
Determination of products
The concentrations of the synthesized NH3 are detected by ammonia-sensitive electrode measurement and colorimetric method using Nessler reagent.
For the ammonia-sensitive electrode measurement, 5 mL of a solution of electrolyte or five-time diluted tail gas absorber was mixed with 5 mL 1 M KOH solution and 0.2 mL low-level ammonia pH-adjusting ISA solution (Thermofisher). After stirring, the voltage was obtained using the ammonia-sensitive selecting electrode (Thermofisher) when stable. The standard curve is constructed by measuring a series of voltage for the reference solutions with 0.1 M KOH and different NH4Cl concentrations (0.8 μg mL−1, 0.4 μg mL−1, 0.2 μg mL−1, 0.1 μg mL−1).
For the colorimetric method, 25 mL solution of electrolyte and five-time diluted tail gas absorber was mixed with 0.5 mL 50% potassium sodium tartrate solution. Then, 0.75 mL Nessler’s reagent and the mixture were allowed still for 10 min. The absorbance was tested by UV–Vis spectrometer (Shimadzu UV-2600) under light at 420 nm wavelength with deionized water as a reference. The standard curve is constructed by measuring a series of absorbances for the reference solutions with 0.1 M KOH and different NH4Cl concentrations (2.0 μg mL−1, 1.6 μg mL−1, 1.2 μg mL−1, 0.8 μg mL−1, 0.4 μg mL−1, 0.2 μg mL−1, 0.1 μg mL−1, 0 μg mL−1). The background is corrected by a blank solution with 0.1 M KOH and Nessler’s reagent.
The concentration of hydrazine in the electrolyte was evaluated via Watt and Chrisp method. The chromogenic agent was produced by mixing para-(dimethylamino) benzaldehyde (2 g), concentrated HCl (10 mL), and ethanol (100 mL). After blending with the agent, the resulting electrolytes were detected by measuring the absorbance at 455 nm.
Results and discussion
Synthesis and characterization
The synthesis of N@ZnS/Zn was following the typical process of the ampoule method. At first, the electrode shape zinc foil and thiourea were sealed in an ampoule bottle under a vacuum line. Then the sulfurization took place when putting the sealed ampoule bottle in the tube furnace under 400 °C for 5 h, generating surface sulfurized N@ZnS/Zn material (Fig. 1a). Before characterizing and applying in the electrochemical tests, the activation of the as-prepared N@ZnS/Zn was conducted to get rid of the impurity adsorbed on the surface as well as the nitrogen-containing species.
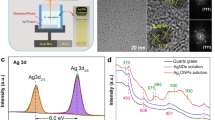
Synthesis and characterization of catalysts. a Synthetic route for zinc sulfide materials using ampoule method. b SEM image of N@ZnS/Zn. c TEM image of N@ZnS/Zn. d SEM image of N@ZnS/Zn; inset: SEM–EDS elemental mapping images of Zn, S and N. e HR-TEM image of N@ZnS/Zn with surface defects and cell spacing presented
The morphology and microstructure of as-prepared N@ZnS/Zn are examined by scanning electron microscopy (SEM). The rough surface of the materials is presented in Fig. 1b, with particle size between 50 and 100 nm (Fig. 1d). To obtain more evidence for the effectiveness of the material, the high-resolution transmitting electron microscopy (HRTEM) is adopted and the picture is shown in Fig. 1c. Moreover, plenty of crystal phases and abundant surface defects can be observed at 5 nm resolution (Fig. 1e), further proving the stability and robustness of the electrode material. Meanwhile, the energy dispersive spectroscopy (EDS) was applied to compare the element ratio of the non-activated N@ZnS/Zn with activated ones After the calculation and approximation, the Zn: S: N ratio is altered from 6: 3: 2.7 to 6: 4: 1 (Figure S1 and S2). The remaining N atoms on the surface indicate the formation of Zn-N bonds, which is one of the reasons for the electrode with extraordinary performance in ENRR (Figure S1). And the EDS-mapping exhibited a homogeneous distribution for the Zn, S, and N atoms (Fig. 1d).
The X-ray diffraction (XRD) patterns to the surface of as-prepared and activated N@ZnS/Zn are presented in Fig. 2. According to the standard PDF # 80-0200, the diffraction peaks at 28.5°, 47.5°, 56.3°, 69.4°, 76.6°, and 81.2° are the character peaks attributing to the (111), (220), (311), (400), (331), (420) planes of the zinc sulfide crystal, which implies the sphalerite ZnS species existing on the material surface. The relatively broad peaks and the rough baseline demonstrate the amorphous nature of the material surface. In addition, the XRD pattern of non-activated N@ZnS/Zn material is also demonstrated in Fig. 2. With more peaks of impurities presenting in the diagram, the activation process is therefore necessary. To obtain more details of the surface composition and structure, the ZnS/Zn with sulfur powder as sulfurizing agents by the ampoule method is also synthesized. The XRD pattern of ZnS/Zn shows the peaks at 28.5°, 47.5°, 56.3°, 76.6°, and 81.2° belong to the ZnS species, within which the lower peaks may ascribe to the moderated crystallinity.
The full-range X-ray photoelectron spectroscopy (XPS) spectra of activated N@ZnS/Zn is shown in Fig. 3a, which demonstrated the presence of the elements Zn, S, and N. The measured binding energies are in-line with those reported in the literature [31]. In particular, the binding energy of Zn 2p3/2 and Zn 2p1/2 is located at 1022.9 eV and 1045.9 eV, respectively (Fig. 3b). The difference between the two core level components of Zn 2p3/2 and Zn 2p1/2 is approximately 23 eV, indicating the bivalent state of Zn. Moreover, the fitting of the XPS spectrum sulfur S 2p peaks exhibits the main sulfur species on the surface of N@ZnS/Zn, which can be divided into two main characteristic peaks at 161.6 eV and 162.9 eV, ascribed to the S 2p3/2 and S 2p1/2 respectively, in a good agreement with the energy of the S-Zn bond (Fig. 3c) [31]. The existence of polysulfides, one of the primary reasons for emerged S vacancies, were speculated based on the 1.3 eV difference of the binding energies. Meanwhile, the presence of the N 1 s characteristic peak located at 395.38 eV and 396.43 eV reveals the existence of the Zn-N bond according to the literature (Fig. 3d) [32]. It is noteworthy that the residual nitrogen on the surface of N@ZnS/Zn after the activation would not be reduced into ammonia due to the binding.
The results from the above material characterizations demonstrate that the surface modification of Zn foil by sulfurization with thiourea through the ampoule method provides stable amorphous ZnS with abundant S vacancies and Zn-N binding active sites promising for electrocatalysis for ENRR.
Electrocatalytic ENRR performances
To evaluate the electrocatalytic ENRR efficiency of N@ZnS/Zn under ambient conditions, several electrochemical tests were performed in N2-saturated 0.1 M KOH electrolyte. The divided cells were used to carry out the experiments, and a proton conductive cation exchange membrane (Nafion 117) was adopted to separate the cathode and anode chamber. Ahead of the electrochemical tests, the 2 h electrolysis of N2-saturated electrolytes and Ar-saturated electrolytes with the working electrode was conducted at open circuit potential and − 0.7 V vs RHE, respectively. After that, the resulting solutions were examined by Nessler’s reagent with no obvious NH4+ detection, thus proving that there are no ammonia-like impurities in the feeding gas and reaction environment.
At first, we explored the performances of the N@ZnS/Zn electrodes in ENRR through the comparison of the linear sweep voltammetry (LSV) curves under Ar- and N2-saturated electrolytes (Fig. 4a). The increasing current density along with the increasing potentials is due to the competing hydrogen reduction reaction (HER) that is enhanced gradually in both LSV tests. A higher current density in N2-saturated electrolytes was then observed compare to the Ar-saturated ones when the applied potential was more negative than − 0.55 V vs RHE, which indicates the electrosynthesis of ammonia proceeding in the N2-saturated cell. This phenomenon further proves the effectiveness of the N@ZnS/Zn electrode in the electrocatalytic ENRR process.
Electrocatalytic performance of N@ZnS/Zn electrode for ENRR. a LSV in N2- and Ar-saturated electrolytes; b It-curves from chronoamperometry tests under different potentials; c Calculated FE and NH3 yield rate values at controlled potentials; d Comparison of the ammonia-sensitive selecting electrode and Nessler reagent-based colorimetric method for the quantitative analysis of FE values
After confirming the validity of the working electrode in ENRR, several chronoamperometry tests were conducted in N2-saturated 0.1 M KOH solution for 2 h each under controlled potentials (− 0.5 V, − 0.6 V, − 0.7 V, − 0.8 V, − 0.9 V vs RHE) to further evaluate the catalytic performance of N@ZnS/Zn material. The It-curves are shown in Fig. 4b. As the pH value of the electrolyte is higher than the pKa value of ammonia, an acidic gas absorber was also installed on the electrochemical cell, in the case of ammonia gas emitting.
After the chronoamperometry tests, the corresponding NH3 concentrations of the electrolytes, as well as solutions in the absorber, were all measured by spectroscopy using Nessler’s reagent and ammonia-sensitive selecting electrode methods (see more details in the Experimental section and Figures S3, S4 in supporting information, which shows the calibration curves for confirmation of ammonia concentration). Given that N2H4 is a possible by-product during the electrolysis, the colorimetric method was also employed to examine the existence of N2H4 for the electrolytes from the above-mentioned chronoamperometry tests. Since no N2H4 was detected in solutions, the good selectivity for the ENRR was thus verified. After the calculations, the Faradic efficiency (FE) and the yield rate of ammonia for all the chronoamperometry tests were obtained and listed in Fig. 4c. As shown, the highest FE, 7.92%, the highest yield rate of NH3, 2.42 × 10–10 mol s−1 cm−2, were obtained with onset potential at − 0.6 V vs RHE. Consistent with the observation in the LSV curves, a gradual decrease of both FE and yield rate can be observed with the more negative onset potentials, which might ascribe to the dominant role of competing for HER process at higher potentials. It is noteworthy that the yield rate of NH3 was remaining in the range of 1.3–2.3 mol s−1 cm−2, suggesting the steady catalytic efficiency of the electrode in alkaline solution under ambient conditions.
As a similar amount of ammonia generating at each potential, the higher and higher electricity consumed reduces the efficiency of this electrocatalytic N2 fixation. This also explains the increasing current density yet decreasing FE at high potentials observed in the It-curves. In the meantime, the validity and reliability of the NH3 concentrations that measured with the ammonia-sensitive selecting electrode as well as the colorimetric method using Nessler reagent were verified with close FE values (Fig. 4d).
With the evaluation of electrocatalytic performance for ENRR in hand, the long-term stability and robustness of the N@ZnS/Zn electrocatalyst were then examined. At first, a 24 h durability test was conducted at − 0.7 V vs RHE in alkaline electrolyte, and the It-curve was shown in Fig. 5a. The few fluctuations along the current density curve and a final 2.86% FE value illustrated the consistent durability of the N@ZnS/Zn electrocatalyst over a long time. Furthermore, to estimate the catalytic effectiveness of the N@ZnS/Zn electrode, five times parallel tests for 2 h each were also carried out at − 0.6 V vs RHE. After examining the ammonia concentration by the colorimetric method, the NH3 yield rates and FE values were calculated and presented in Fig. 5b. The long-acting of the N@ZnS/Zn electrocatalyst was thus exhibited with almost no decrease FE for the electrochemical generation of ammonia.
a Time-dependent current density curve for N@ZnS/Zn at − 0.7 V vs RHE for 24 h. b 5 times repeating ENRR at − 0.6 V vs RHE; c Comparison FE and yield rate values of Zn foil, ZnS/Zn, N@ZnS/Zn in alkaline electrolytes, and N@ZnS/Zn in neutral electrolytes for ENRR; d Comparison of the performance for ENRR with selected recently reported sulfide electrocatalysts
To gain more insight into the relationship between structure and function, the comparing of the catalytic performance in the electrocatalytic ENRR process was made through conducting chronoamperometry measurements using N@ZnS/Zn, ZnS/Zn, and Zn foil as the working electrode at − 0.6 V vs RHE in alkaline electrolytes. The related NH3 yield rates and FE values were listed and compared in Fig. 5c. As expected, the Zn foil exhibited no effect for electrocatalytic ENRR, while ZnS/Zn electrocatalyst mediated the NH3 synthesis with 4.11% FE and 0.24 × 10–10 mol s−1 cm−2 yield rate, almost one-tenth of the performance with N-doped. Considering the observation from XRD patterns, the attenuated electrocatalytic performance of ZnS/Zn material may attribute to the less formation of S vacancies and active sites on the surface caused by the lack of N dopant. In other words, the N doping not only plays a vital role in generating surface defects but also improve the band structure of the material, which would significantly improve the catalytic performance in the ENRR process.
Furthermore, another comparison was also held to evaluate the influence of the pH value of the electrolyte. As the Zn-based electrode is very reactive to an acidic solution, a neutral solution, 0.1 M K2SO4 electrolyte, was used in potentiostatistic tests at − 0.6 V vs RHE with N@ZnS/Zn as the working electrode with FE value and NH3 yield rate presented also in Fig. 5c. In contrast to alkaline electrolytes, neutral solution displayed a moderated effect in N2 gas fixation, which ascribe to less HER inhibition under the neutral conditions. Intriguingly, the appearance of these demonstrated that the possible vacancy filled by OH− rather than N2 from the alkaline electrolytes did not impede the electrosynthesis of NH3 by N@ZnS/Zn catalyzed.
Therefore, the formation of NH3 is plausibly triggered by the simultaneous adsorption and reduction of N2 and H2O molecules. The N–N bond is further weakened by the back-donation of zinc electrons to *π orbital with the aid of e-supply from heteroatoms, sulfur and nitrogen. Furthermore, an integrated reaction between the activated intermediates achieves the generation of N–H bonds and the cleavage of the N–N bond. After the transmitting of electrons and coupling with hydrogens, the final NH3 molecule desorbs and diffuses into the bulk electrolyte.
Additionally, regarding onset potentials and the FE values of electrochemical N2-to-NH3 transformation, several selected recent works using metal sulfide electrocatalysts were compared graphically with N@ZnS/Zn in Fig. 5d (more details in Table S1). In the diagram, the relatively superior electrocatalytic performance of N@ZnS/Zn was shown. In consideration of its low-cost and simple generation protocol, such a durable and robust electrocatalyst is very promising in the practical industrial use for green synthesis of NH3.
Conclusion
In conclusion, we have developed a novel N-doped zinc sulfide material through the facile ampoule method. The surface components and morphology of the material were examined through various characteristic techniques. Due to the rich S vacancies and abundant Zn-N activate sites, the as-fabricated N@ZnS/Zn enables the extraordinary electrocatalytic performance for the ENRR process in alkaline electrolytes under ambient conditions. As the solemn product, the NH3 product was obtained at a yield rate of 2.42 × 10–10 mol s−1 cm−2 and an FE of 7.92% at − 0.6 V vs RHE. The N-doping, surface defects, and the alkaline electrolyte are indispensable factors for the achievement of high electrocatalytic ENRR performance. In the end, the simple controllable synthesis of the electrocatalysts, as well as their long-term stability and catalytic activity, suggest a promising application in the practical industrial use of the functionalized zinc electrode for artificial N2 fixation.
References
Crabtree, W., Dresselhaus, M.S., Buchanan, M.V.: The hydrogen economy. Phys. Today 57, 39–44 (2004). https://doi.org/10.1063/1.1878333
Nocera, D.G.: The artificial leaf. Acc. Chem. Res. 45, 767–776 (2012). https://doi.org/10.1021/ar2003013
Canfield, D.E., Glazer, A.N., Falkowski, P.G.: The evolution and future of earth’s nitrogen cycle. Science 330, 192–196 (2010). https://doi.org/10.1126/science.1186120
Wang, L., Xia, M., Wang, H., Huang, K., Qian, C., Maravelias, C.T., Ozin, G.A.: Greening ammonia toward the solar ammonia refinery. Joule 2, 1055–1074 (2018). https://doi.org/10.1016/j.joule.2018.04.017
Li, Y., Wang, H., Priest, C., Li, S., Xu, P., Wu, G.: Advanced electrocatalysis for energy and environmental sustainability via water and nitrogen reactions. Adv. Mater. 32, 2000381 (2020). https://doi.org/10.1002/adma.202000381
Lv, X.-W., Weng, C.-C., Yuan, Z.-Y.: Ambient ammonia electrosynthesis: current status, challenges, and perspectives. Chemsuschem 13, 3061–3078 (2020). https://doi.org/10.1002/cssc.202000670
Cui, X., Tang, C., Zhang, Q.: A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv. Energy Mater. 8, 1800369 (2018). https://doi.org/10.1002/aenm.201800369
Yang, C., Zhu, Y., Liu, J., Qin, Y., Wang, H., Liu, H., Chen, Y., Zhang, Z., Hu, W.: Defect engineering for electrochemical nitrogen reduction reaction to ammonia. Nano Energy 77, 105126 (2020). https://doi.org/10.1016/j.nanoen.2020.105126
Wen, L., Ren, C., Zou, Y., Lin, W., Ding, K.: Why it is S-rich around Mo atom in the nitrogenase: a DFT investigation. Appl. Surf. Sci. 534, 147595 (2020). https://doi.org/10.1016/j.apsusc.2020.147595
Zhang, L., Ji, X., Ren, X., Ma, Y., Shi, X., Tian, Z., Asiri, A.M., Chen, L., Tang, B., Sun, X.: Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 Catalyst: theoretical and experimental studies. Adv. Mater. 30, 1800191 (2018). https://doi.org/10.1002/adma.201800191
Li, X., Li, T., Ma, Y., Wei, Q., Qiu, W., Guo, H., Shi, X., Zhang, P., Asiri, A.M., Chen, L., Tang, B., Sun, X.: Boosted electrocatalytic N2 reduction to NH3 by defect-rich MoS2 nanoflower. Adv. Energy Mater. 8, 1801357 (2018). https://doi.org/10.1002/aenm.201801357
Li, X., Ren, X., Liu, X., Zhao, J., Sun, X., Zhang, Y., Kuang, X., Yan, T., Wei, Q., Wu, D.: A MoS2 nanosheet-reduced graphene oxide hybrid: an efficient electrocatalyst for electrocatalytic N2 reduction to NH3 under ambient conditions. J. Mater. Chem. A 7, 2524–2528 (2019). https://doi.org/10.1039/C8TA10433F
Zeng, L., Chen, S., van der Zalm, J., Li, X., Chen, A.: Sulfur vacancy-rich N-doped MoS2 nanoflowers for highly boosting electrocatalytic N2 fixation to NH3 under ambient conditions. Chem. Commun. 55, 7386–7389 (2019). https://doi.org/10.1039/c9cc02607
Xu, T., Ma, D., Li, T., Yue, L., Luo, Y., Lu, S., Shi, X., Asiri, A.M., Yang, C., Sun, X.: Enhanced electrocatalytic N2-to-NH3 fixation by ZrS2 nanofibers with a sulfur vacancy. Chem. Commun. 56, 14031–14034 (2020). https://doi.org/10.1039/d0cc05917
Zhao, X., Lan, X., Yu, D., Fu, H., Liu, Z., Mu, T.: Deep eutectic-solvothermal synthesis of nanostructured Fe3S4 for electrochemical N2 fixation under ambient conditions. Chem. Commun. 54, 13010–13013 (2018). https://doi.org/10.1039/C8CC08045C
Xiong, W., Guo, Z., Zhao, S., Wang, Q., Xu, Q., Wang, X.: Facile, cost-effective plasma synthesis of self-supportive FeSx on Fe foam for efficient electrochemical reduction of N2 under ambient conditions. J. Mater. Chem. A 7, 19977–19983 (2019). https://doi.org/10.1039/C9TA07790A
Wei, P., Xie, H., Zhu, X., Zhao, R., Ji, L., Tong, X., Luo, Y., Cui, G., Wang, Z., Sun, X.: CoS2 nanoparticles-embedded N-doped carbon nanobox derived from ZIF-67 for electrocatalytic N2-to-NH3 fixation under ambient conditions. ACS Sustain. Chem. Eng. 8, 29–33 (2020). https://doi.org/10.1021/acssuschemeng.9b06272
Chen, P., Zhang, N., Wang, S., Zhou, T., Tong, Y., Ao, C., Yan, W., Zhang, L., Chu, W., Wu, C., Xie, Y.: Interfacial engineering of cobalt sulfide/graphene hybrids for highly efficient ammonia electrosynthesis. Proc. Natl. Acad. Sci. USA 116, 6635–6640 (2019). https://doi.org/10.1073/pnas.1817881116
Liu, Y., Chen, X., Yu, J., Ding, B.: Carbon-nanoplated CoS@TiO2 nanofibrous membrane: an interface-engineered heterojunction for high-efficiency electrocatalytic nitrogen reduction. Angew. Chem. Int. Ed. 131, 19079–19083 (2019). https://doi.org/10.1002/ange.201912733
Huang, H., Li, F., Xue, Q., Zhang, Y., Yin, S., Chen, Y.: Salt-templated construction of ultrathin cobalt doped iron thiophosphite nanosheets toward electrochemical ammonia synthesis. Small 15, 1903500 (2019). https://doi.org/10.1002/smll.201903500
Wu, X., Wang, Z., Han, Y., Zhang, D., Wang, M., Li, H., Zhao, H., Pan, Y., Lai, J., Wang, L.: Chemically coupled NiCoS/C nanocages as efficient electrocatalysts for nitrogen reduction reactions. J. Mater. Chem. A 8, 543–547 (2020). https://doi.org/10.1039/C9TA10142J
Zhang, D., Gokce, B., Barcikowski, S.: Laser synthesis and processing of colloids: fundamentals and applications. Chem. Rev. 117, 3990–4103 (2017). https://doi.org/10.1021/acs.chemrev.6b00468
Zhao, Y., Jin, B., Vasileff, A., Shi, B., Jiao, Y., Qiao, S.-Z.: The ampoule method: a pathway towards controllable synthesis of electrocatalysts for water electrolysis. Chem. Eur. J. 26, 3898–3905 (2020). https://doi.org/10.1002/chem.201903060
Zhao, Y., Jin, B., Zheng, Y., Jin, H., Jiao, Y., Qiao, S.Z.: Charge state manipulation of cobalt selenide catalyst for overall seawater electrolysis. Adv. Energy Mater. 8, 1801926 (2018). https://doi.org/10.1002/aenm.201801926
Zhao, Y., Jin, B., Vasileff, A., Jiao, Y., Qiao, S.Z.: Interfacial nickel nitride/sulfide as a bifunctional electrode for highly efficient overall water/seawater electrolysis. J. Mater. Chem. A 7, 8117–8121 (2019). https://doi.org/10.1039/C9TA01903K
Zhao, Y., Ling, T., Chen, S., Jin, B., Vasileff, A., Jiao, Y., Song, L., Luo, J., Qiao, S.Z.: Non-metal single-iodine-atom electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 58, 12252–12257 (2019). https://doi.org/10.1002/anie.201905554
Zhao, Y., Jin, B., Vasileff, A., Jiao, Y., Qiao, S.Z.: Contemporaneous oxidation state manipulation to accelerate intermediate desorption for overall water electrolysis. Chem. Commun. 55, 8313–8316 (2019). https://doi.org/10.1039/C9CC04231H
Feng, D., Zhang, X., Sun, Y., Ma, T.: Surface-defective FeS2 for electrochemical NH3 production under ambient conditions. Nano Mater. Sci. 2, 132–139 (2020). https://doi.org/10.1016/j.nanoms.2019.07.002
Yu, B., Li, H., White, J., Donne, S., Yi, J., Xi, S., Fu, Y., Henkelman, G., Yu, H., Chen, Z., Ma, T.: Tuning the catalytic preference of ruthenium catalysts for nitrogen reduction by atomic dispersion. Adv. Funct. Mater. 30(6), 1905665 (2019). https://doi.org/10.1002/adfm.201905665
Sun, Y., Deng, Z., Song, X., Li, H., Huang, Z., Zhao, Q., Feng, D., Zhang, W., Liu, Z., Ma, T.: Bismuth-based free-standing electrodes for ambient-condition ammonia production in neutral media. Nano-Micro Lett. 12, 133 (2020). https://doi.org/10.1007/s40820-020-00444-y
Barreca, D., Casparotto, A., Maragno, C., Tondello, E., Spalding, T.R.: Analysis of nanocrystalline ZnS thin films by XPS. Surf. Sci. Spectra 9, 54–61 (2003). https://doi.org/10.1116/11.20030117
Tabet, N., Faiz, M., Al-Oterbi, A.: XPS study of nitrogen-implanted ZnO thin films obtained by DC-Magnetron reactive plasma. J. Electron Spectrosc. Relat. Phenom. 163, 15–18 (2008). https://doi.org/10.1016/j.elspec.2007.11.003
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 52071171), Liaoning Revitalization Talents Program—Pan Deng Scholars (XLYC1802005), Liaoning BaiQianWan Talents Program (LNBQW2018B0048), Natural Science Fund of Liaoning Province for Excellent Young Scholars (2019-YQ-04), Key Project of Scientific Research of the Education Department of Liaoning Province (LZD201902), Foundation for Young Scholars of Liaoning University (LDQN2019007), and Natural Science Foundation of Liaoning Province of China (2020-MS-137).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical statement
The authors consciously assure that this work is the authors’ own original work, which has not been previously published elsewhere, the paper is not currently being considered for publication elsewhere, the paper reflects the authors’ own research and analysis in a truthful and complete manner, the paper properly credits the meaningful contributions of co-authors and co-researchers, the results are appropriately placed in the context of prior and existing research, all sources used are properly disclosed (correct citation), and all authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, DM., Sun, Y., Yuan, ZY. et al. Ampoule method fabricated sulfur vacancy-rich N-doped ZnS electrodes for ammonia production in alkaline media. Mater Renew Sustain Energy 10, 8 (2021). https://doi.org/10.1007/s40243-021-00193-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-021-00193-x