Abstract
TiO2, ZrO2 and layer-by-layer TiO2–ZrO2 films were prepared by doctor blade method followed by sensitization with natural dye for dye-sensitized solar cell application. The structural and optical, morphological and compositional properties were investigated by X-ray diffraction and UV–Vis spectroscopy, scanning electron microscopy, BET, surface profilometer and energy-dispersive X-ray analyzer, respectively. Dye-sensitized solar cells were fabricated using the prepared electrodes of TiO2, ZrO2 and layer-by-layer TiO2–ZrO2 films sensitized with natural dye extracted from Pandan leaves (Pandanus amaryllifolius). The J–V characteristics were recorded to measure photo-response of fabricated devices and electrochemical impedance spectroscopy to study the electron behavior, series resistance and lifetime. Photovoltaic parameter like short-circuit current, open-circuit voltage, fill factor, and power conversion efficiency were evaluated for fabricated solar cell under artificial light supplied from white LED (15 mW·cm−2). The average values of power conversion efficiency of DSSCs fabricated with TiO2, ZrO2 and layer-by-layer TiO2–ZrO2 photoanodes were found to be 0.65%, 3.04% and 3.13%, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, due to the increasing global warming and energy crisis worldwide, it is important to find renewable and green energy source, which are cost effective and reliable. Solar energy is one of the most gifted sources for producing environmentally friendly energy. Compared to first- and second-generation solar cells, dye-sensitized solar cells (DSSCs) can be considered as one of the most favorable photovoltaic devices because of the low-cost, simplicity in fabrication along with moderated efficiency. In general, TiO2 and/or ZnO are used as a wide band gap semiconductor material in DSSCs [1,2,3]. Still there is a need to search for alternative materials towards the enhancement in device efficiency. In this direction, one can look for alternative semiconductor such as ZrO2 and its bilayered structure with routine TiO2. ZrO2 is a wide band gap semiconductor with direct band gap values ranging from 2.5 to 3.8 eV [4], which may be an attractive candidate for dye-sensitized solar cell application [5].
Doping in TiO2 can modified optical and electrical properties leads to increase the performance by providing more active sites on the surface that can play a significant role for adsorption of dye molecules, improvement in light absorption, and also enhance the electron collection in the conduction band [6, 7]. On the contrary, one can enhance the performance of DSSC using multi-layered or composite photoanodes [8, 9]. Recently, Mohamed et al. reported the improvement in efficiency from 1.61 to 4.51% of DSSC fabricated with doping of ZrO2 in TiO2 nanofibers photoanode sensitized using N719 dye [7]. Jin et al. observed that the efficiency of DSSCs with a TiO2–ZrO2 composite electrode was 6.2%, an increase of 26.5% than pure TiO2 electrode (4.9%) for N719 dye as sensitizer. This is because, the addition of ZrO2 in TiO2 increased dye loading, thereby improving the electron recombination times than for a pure TiO2 electrode results in the decreased electron recombination at the TiO2/electrolyte interface, increasing electron transfer [10]. The highest efficiency of 6.5% was achieved for N719 dye-sensitized ZrO2 nanofiber-doped TiO2 photoelectrode prepared by squeeze printing [11]. Furthermore, the significant enhancement in power conversion efficiency can be ascribed for TiCl4-treated TiO2 nanosheets film coated with ZrO2 and the highest efficiency of 7.33% is achieved for N719 dye [12].
Usually, Ru-metal complexes and metal-free organic dyes are used as sensitizer due their good stability and light harvesting property with high molar extinction coefficient [13, 14]. However, application of these types of sensitizers also faced problems regarding complicated synthetic routes and most importantly use of environmentally non-benign chemicals [15]. Hence, dyes obtained from nature having similar characteristic were used with the advantages such as availability, low-cost, environmental friendliness and as a green alternative to commercially available dyes [16,17,18,19,20]. To the best of our knowledge; it is observed that not many reports are available on natural dye-sensitized TiO2–ZrO2 bilayer electrode for DSSC application. Win et al. reported sensitization of Rose extract over TiO2–ZrO2 film prepared by rolling method of DSSC and observed 0.23% efficiency [21]. DSSC fabricated using nanocrystalline ZrO2–TiO2 film prepared by doctor blade method and sensitized with the bioorganic dye, chlorophyll extracted from green leaves of Chromolaena odorata shows 0.1% efficiency. Recently, Tomar et al. reported improvement in efficiency of DSSC using Zn-doped TiO2–ZrO2 prepared by hydrothermal method with juice of pomegranate as natural sensitizer. The efficiency of DSSC fabricated with TiO2–ZrO2 is 1.97% and that of the Zn-doped TiO2–ZrO2 nanocomposites were found 4.58%. The enhancement in efficiency is because the Zn doping has introduced new energy levels and lowered the recombination [22].
From literature survey including recent reports, very few reports are available on sensitization of chlorophyll dye with different metal oxides for dye-sensitized solar cell. The comparison of photovoltaic performance of chlorophyll dye-sensitized photoanodes for DSSC application under different light intensities with reported literature is summarized in Table 1 [21, 23,24,25,26,27,28,29,30,31,32,33,34,35,36].
In this article, we promote to use natural dyes as sensitizer towards the development of attractive, colorful and low-cost DSSCs. Hence, attempt has been focused in this direction to develop the low cost and eco-friendly source of green energy by using inexpensive and naturally available dye extracted from Pandan leaves as sensitizer over TiO2, ZrO2 and layer-by-layer TiO2–ZrO2 photoelectrodes towards the fabrication of DSSCs.
Experimental
Material and chemicals
Fluorine-doped tin oxide (FTO)-coated glass with a sheet resistance of ~ 15 Ω cm−2 was used as substrate. Nanocrystalline powder of TiO2 (particle size ~ 7 nm with anatase phase), zirconium oxide powder (particle size ~ 45 nm from SRL Chem. India), ethyl cellulose (SDFCL, India), ethanol (AR, 99.9%, Changshu Yangyuan Chemical, China), α-terpineol (LR, 98%, HPLC, India), acetyl acetone (98%, Merck, India), potassium iodide (99.8%, SRL, India), iodine (99.5%, Fisher Scientific, UK), t-butyl pyridine (96%, Acros organics, USA), acetonitrile (AR, 99.5%, SDFCL, India) were used as it is without any further purification.
Substrate cleaning
Prior to deposition FTO substrates were cleaned by using soap solution followed by double distilled water and then with acetone in ultrasonic bath for 10–15 min.
Preparation of TiO2 and ZrO2 paste
In detail, 0.5 g TiO2 nanoparticles powder was ground in mortar pestle with addition of 7 ml ethanol and kept in ultrasonic bath for 15–20 min. Afterwards 0.3 g ethyl cellulose was ground in same mortar pestle in 2 ml ethanol. Then the sonicated paste of TiO2 was subsequently added and ground with ethyl cellulose in mortar pestle for uniform mixing. Then the mix solution was transferred in the borosilicate glass bottle for sonication with the addition of 1.3 g α-terpineol for 3 h followed by addition of 3 drops of acetyl acetone to the above mixture and allowed to sonicate for another 1 h. Finally, for the evaporation of ethanol; the prepared solution was placed in incubator at 60 °C till it becomes more viscous. Similar procedure was repeated for the preparation of ZrO2 paste.
Preparation of photoelectrodes
Pre-cleaned FTO substrate was covered at the edges by using scotch tape of ~ 30 μm thickness and layer of prepared TiO2 paste was then coated on FTO substrates using doctor blade method at room temperature. The as-prepared films of pristine TiO2 were dried at 60 °C in incubator for 10–15 min. The above procedure was repeated four times to get optimized thickness of ~ 12 μm measured by using surface profilometer. Similar route is employed for the preparation of ZrO2 films followed by drying at 60 °C for 10–15 min.
Layer-by-layer approach was utilized for the preparation of TiO2–ZrO2 bilayered films. Briefly, pre-cleaned FTO substrate was covered at the edges by using scotch tape and first layer of prepared TiO2 paste was coated on FTO using doctor blade method. Then the as-prepared film was dried at 60 °C for 15 min followed by the coating of second layer of ZrO2 on putting scotch tape over TiO2 in similar way. Again the as-prepared bilayered film was dried at 60 °C for 15 min. The above procedure was repeated one more times to get optimized thickness of ~ 12 μm for layer-by-layer TiO2–ZrO2 films. Finally, all the prepared films of pristine TiO2, ZrO2 and TiO2–ZrO2 bilayered structure were air annealed at 450 °C for 1 h to remove the organic solvents as well as to improve the crystallinity of the film and the interfacial structures.
Preparation of natural dye
Firstly, natural chlorophyll dye was prepared from 3 g fresh leaves of fragrant screwpine (Pandanus amaryllifolius) or locally known as Pandan leaves. The leaves were cleaned with distilled water, rinsed with ethanol, and were cut into small pieces and immersed in 5 ml ethanol followed by grinding in mixer for 10 min and the same was repeated at least for 3–4 times. Again 5 ml of ethanol was then added in the above mixture and kept on a magnetic stirrer at room temperature for 1 h. Finally, the above mixture is kept for around 24 h in a closed bottle in dark at room temperature. The filtrate of the above solution was used as natural dye for sensitization of photoelectrodes towards the fabrication of DSSCs.
Sensitization of photoelectrodes
The annealed films of pristine TiO2, ZrO2 and TiO2–ZrO2 bilayered structure were then immersed in naturally prepared dye for 72 h at room temperature. Subsequently, the films were dipped in ethanol for 5–10 s to remove the excess of dye, followed by drying at room temperature.
Characterizations
The structural and optical analysis was carried out using X-ray diffractometry (XRD) (model: XRD, Rigaku ‘‘D/B max-2400’’, CuKα with λ = 1.54 Å) and UV–Vis spectrophotometer (model: JASCO V-670) was used to record optical absorption spectra of photoelectrodes using diffused reflectance (DRS) mode in wavelength range 310–800 nm at room temperature, respectively. Surface profilometer (Dektak 150) was used for the measurement of thickness of annealed photoelectrodes. The morphological properties and elemental composition was done by using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) (model: JEOL-JSM 6360-A), respectively. The BET method was used for the measurement of surface area of all samples using Quantachrome Autosorb-iQ unit. The cell performance was measured by a semiconductor characterization unit (Keithley 2420 source meter) under illumination of artificial LED light (15 mW cm−2). The electrochemical impedance spectroscopy (EIS) measurements for DSSCs were carried out using potensiostat/galvanostat (IVIUM:Vertex) in the frequency range of 1 MHz to 1 Hz under dark.
Results and discussion
Structural studies
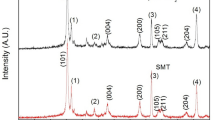
XRD patterns of the TiO2, ZrO2 and TiO2–ZrO2 bilayered films were recorded using X-ray diffractometer with 0.1° s−1 step size and scan rate of 1 s/step as shown in Fig. 1. The characteristic along (101) and (004) peaks at 25.27° and 37.8°, respectively, were clearly observed and confirm the growth of tetragonal structure (JCPDF card No. 21-1272) of TiO2 having anatase phase (a) on FTO substrate. XRD pattern of ZrO2 on FTO substrate by doctor blade method is depicted as (b). The strong diffraction peaks along (\( \bar{1}11 \)) and (\( 111 \)) were indexed to monoclinic structure (JCPDF card No. 37-1484). The XRD pattern (c) for TiO2–ZrO2 layer-by-layer film represents the combination of two sets of patterns: one from TiO2 and the other originating from the ZrO2. Thus the formation of layer-by-layer TiO2 with ZrO2 is confirmed from the XRD. Asterisk (*) shown in the pattern assigned to the FTO-coated glass substrate.
Optical absorption spectroscopy
A UV–Vis spectrophotometer (model: JASCO V-670) was used to record optical absorption spectra of photoelectrodes in diffused reflectance (DRS) mode and ethanolic solution of dye under transmission mode in the wavelength range 310–800 nm at room temperature. Figure 2 shows the absorption spectra of pristine TiO2, ZrO2, TiO2–ZrO2 films, and chlorophyll dye solution. It is observed that all the films exhibit low absorbance in the visible region of the solar spectrum. Usually, TiO2 shows the characteristic spectrum with its fundamental absorption of Ti–O bond in UV region between 320 and 400 nm [37] with characteristic peak around 350 nm (band edge) for TiO2 [38]. Whereas naturally prepared chlorophyll dye exhibits maximum absorption at 413 and 665 nm in visible region. Chlorophylls, which act as an effective photo-sensitizer in photosynthesis of green plant, has absorption maximum at 670 nm, thus, it is an attractive potential compound as a photo-sensitizer in the visible region [39]. The values for amount of dye adsorbed over different photoelectrodes used for the fabrication of DSSCs along with the thickness of photoanodes are summarized in Table 2. Chu et al. reported the enhancement of dye absorption for layer-by-layer photoelectrode and observed the improvement in performance of DSSC [40].
Surface morphology and elemental analysis
The top-view of pristine TiO2, ZrO2 and TiO2–ZrO2 bilayer films was examined using SEM to analyze the surface morphology (Fig. 3). The SEM results clearly illustrated nanoporous surface nature of TiO2, ZrO2 and TiO2–ZrO2 films which is the basic requirement of photoelectrode in DSSC. At high resolution (inset), it is observed that the samples were granular in structure and porous in nature. Figure 3c shows the TiO2–ZrO2 film has uniform surface area than the pristine ZrO2 and TiO2 which leads to better dye adsorption and results in enhancing the overall performance of the DSSC for TiO2–ZrO2 bilayered photoanode. The layer-by-layer structure of TiO2–ZrO2 bilayered photoanode exhibit moderate surface area compared with the pristine bulk materials positive sign for amount of dye adsorption and also leads to the shortened collection length for charges results into increasing efficiency [41]. The values of surface area measure by BET technique for all the samples are summarized in Table 2 and found to be in accordance with the previously reported results [42].
The energy-dispersive X-ray (EDS) spectrum of TiO2–ZrO2 bilayer film is shown in Fig. 3d. EDS confirmed that the presence of titanium, zirconium and oxygen. The atomic percentages of elements are tabulated in the inset of Fig. 3d.
Solar cell assembly
The dye-sensitized photoanodes and platinum counter electrode was separated by spacer of 10 μm thickness over photoanodes and assembled into a sandwich-type open cell using binder clip. The active cell area for DSSC was regulated to 0.25 cm2 using stainless steel mask covered from back side of photoanode over the glass side of the FTO. The mask is designed in such a way that it allowed entering the light through aperture and remains opaque for light over the remaining part of the cell. Figure 4a shows the schematic of device for chlorophyll dye-sensitized layer-by-layer TiO2–ZrO2 photoanode-based DSSC. A drop of prepared liquid electrolyte was introduced between the sensitizer and the counter electrode in such a way that no air bubble is formed. An electrolyte composed of 0.5 M potassium iodide (KI); 0.1 M iodine (I2) and 0.5 M t-butyl pyridine (C13H21N) in an acetonitrile solvent was used.
Photovoltaic study
Photovoltaic measurements of the fabricated DSSCs using natural dye as sensitizers were performed by measuring the J–V measurement as shown in Fig. 4b. In present study we have measured the J–V characteristics of each DSSCs device 3 times for 2 sets of photoanodes to check the repeatability along with the reproducibility of the device and the average values of photovoltaic performance was reported. The cell performance was recorded by a semiconductor characterization unit (Keithley 2420 source meter) under illumination of artificial light (15 mW cm−2) supplied from white LED source. The current density–voltage (J–V) characteristics under illumination conditions were measured at room temperature (27 °C). The output photovoltaic parameters of natural dye-sensitized solar cell, i.e., the short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF) and power conversion efficiency (PEC) of the devices with standard deviation are summarized in Table 2.
Electrochemical impedance spectroscopy (EIS)
Figure 4c shows the Nyquist plots and Fig. 4d represents the Bode phase plots for all three devices sensitized with natural chlorophyll dye. It is observed that the radius of distorted semicircles increases as TiO2 < ZrO2 < TiO2–ZrO2 which indicate the highest charge recombination resistance was observed for TiO2–ZrO2 photoanode-based DSSC. The electron lifetime (τe) can be calculated by the peak frequency at the minimum phase angle (fpeak) from Bode phase plots using the relationship [τe = \( \frac{1}{{2\pi f_{\text{peak}} }} \)] [43]. The shift in the phase angle maxima has been observed towards the low-frequency range as we move from the TiO2 to ZrO2 to TiO2–ZrO2 photoanode. The values of τe found to increase from bare TiO2 to TiO2–ZrO2 photoanode-based DSSC. The substantial increase in the values of electron lifetime indicates the effective suppression of back reaction between the photogenerated electrons. Superior value for τe was recorded for the TiO2–ZrO2 photoanode-based device as compare to the bare metal oxide-based devices, demonstrating the reduced charge recombination. This may be the reason towards getting higher efficiency with layer-by-layer TiO2–ZrO2 photoanode-based DSSC. The value of charge recombination resistance (Rct) was measured from Nyquist plots using second distorted semicircle in mid frequency region. Table 2 summarizes the values for τe and Rct for all the three devices. As per literature, the larger value of Rct is leads to the reduction in charge recombination rate which is favorable towards the enhancement in device efficiency [44]. The highest current density value is observed for ZrO2-based DSSCs which is may be due to the highest value of electron life time (τe) compared to other two devices.
Conclusion
Dye-sensitized solar cells (DSSCs) were assembled using extracts from fragrant screwpine (P. amaryllifolius) leaves as sensitizers with pristine TiO2, ZrO2 and TiO2–ZrO2 photoelectrodes. Photovoltaic parameters of the fabricated DSSCs were determined under illumination. It is found that the natural dye-sensitized layer-by-layer TiO2–ZrO2 photoanode exhibits maximum power conversion efficiency of 3.13% than pristine ZrO2 (3.04%) and TiO2 (0.65%) which suggest that the anchoring of chlorophyll dye goes well with ZrO2 as compared to TiO2. The improvement in efficiency is observed for TiO2–ZrO2 bilayered structure, which is due to the high surface area available for dye adsorption. From EIS studies it is observed that the electron lifetime values for layer-by-layer photoanode-based DSSC is in between pristine photoanode-based DSSC. On the other hand, the charge recombination resistance for layer-by-layer photoanode is significantly greater than pristine ZrO2 and TiO2 photoanode-based DSSCs leads to the effective suppression of photo-injected electron recombination with the excited dye and \( {\text{I}}^{ - }_{ 3} \) in the electrolyte resulted in the enhancement in efficiency for layer-by-layer TiO2–ZrO2 photoanode-based DSSC. This opens the window for use of ZrO2 as photoelectrode instead of routine metal oxides used as wide band gap materials and natural dye in spite of Ru-metal-based expensive dyes as sensitizer towards the fabrication of cost-effective and eco-friendly DSSC.
References
Mathew, S., Yella, A., Gao, P., Humphry-Baker, R., Curchod, B.F.E., Ashari-Astani, N., Tavernelli, I., Rothlisberger, U., Nazeeruddin, M.K., Gratzel, M.: Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6, 242 (2014)
Setyawati, H., Darmokoesoemo, H., Rochman, F., Permana, A.J.: Affordable dye sensitizer by waste. Mater. Renew. Sustain. Energy 6, 17 (2017)
Baviskar, P.K.: Low-cost solid-state dye-sensitized solar cell based on ZnO with CuSCN as a hole transport material using simple solution chemistry. J. Solid State Electrochem. 21, 2699–2705 (2017)
Kumari, L., Li, W.Z., Xu, J.M., Leblanc, R.M., Wang, D.Z., Li, Y., Guo, H., Zhang, J.: Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties. Cryst. Growth Des. 9, 3874–3880 (2009)
Waghmare, M.A., Naushad, M., Pathan, H.M., Ubale, A.U.: Rose bengal-sensitized ZrO2 photoanode for dye-sensitized solar cell. J. Solid State Electrochem. 21, 2719–2723 (2017)
Hu, B., Zhang, Q., Niu, L., Liu, J., Rao, J., Zhou, X.: Microsphere assembly of boron-doped Rutile TiO2 nanotubes with enhanced photoelectric performance. J. Mater. Sci. Mater. Electron. 26, 8915–8921 (2015)
Mohamed, I.M.A., Dao, V.-D., Barakat, N.A.M., Yasin, A.S., Yousef, A., Choi, H.-S.: Efficiency enhancement of dye-sensitized solar cells by use of ZrO2-doped TiO2 nanofibers photoanode. J. Colloid Interface Sci. 476, 9–19 (2016)
Sayyed, S.A.A.R., Beedri, N.I., Kadam, V.S., Pathan, H.M.: Rose Bengal sensitized bilayered photoanode of nano-crystalline TiO2–CeO2 for dye-sensitized solar cell application. Appl. Nanosci. 6, 875–881 (2016)
Lee, J.-K., Jeong, B.-H., Jang, S.-I., Yeo, Y.-S., Park, S.-H., Kim, J.-U., Kim, Y.-G., Jang, Y.-W., Kim, M.-R.: Multi-layered TiO2 nanostructured films for dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 20, S446–S450 (2009)
Jin, E.M., Park, J.-Y., Zhao, X.G., Lee, I.-H., Jeong, S.M., Gu, H.-B.: Photovoltaic properties of TiO2–ZrO2 fiber composite electrodes for dye-sensitized solar cells. Mater. Lett. 126, 281–284 (2014)
Wang, J., Jin, E.M., Park, J.-Y., Wang, W.L., Zhao, X.G., Gu, H.-B.: Increases in solar conversion efficiencies of the ZrO2 nanofiber-doped TiO2 photoelectrode for dye-sensitized solar cells. Nanoscale Res. Lett. 7, 98 (2012)
Luan, X., Wang, Y.: Ultrathin exfoliated TiO2 nanosheets modified with ZrO2 for dye-sensitized solar cells. J. Phys. Chem. C 118, 18917–18923 (2014)
Gratzel, M.: Dye-sensitized solar cells. J. Photochem. Photobiol. Photochem. Rev. 4, 145–153 (2003)
Baviskar, P., Gore, R., Ennaoui, A., Sankapal, B.: Cactus architecture of ZnO nanoparticles network through simple wet chemistry: efficient dye sensitized solar cells. Mater. Lett. 116, 91–93 (2014)
Amao, Y., Komori, T.: Bio-photovoltaic conversion device using chlorine-e6 derived from chlorophyll from Spirulina adsorbed on a nanocrystalline TiO2 film electrode. Biosensors Bioelectron. 19, 843–847 (2004)
Yazie, N., Worku, D., Reda, A.: Natural dye as light-harvesting pigments for quasi-solid-state dye-sensitized solar cells. Mater. Renew. Sustain. Energy 5, 13 (2016)
Gokilamani, N., Muthukumarasamy, N., Thambidurai, M., Ranjitha, A., Velauthapillai, D.: Basella alba rubra spinach pigment-sensitized TiO2 thin film-based solar cells. Appl. Nanosci. 5, 297–303 (2015)
Amaral, R.C., Barbosa, D.R.M., Zanoni, K.P.S., Iha, N.Y.M.: Natural sensitizers for DSCs improved with nano-TiO2 compact layer. J. Photochem. Photobiol. Chem. 346, 144–152 (2017)
Hug, H., Bader, M., Mair, P., Glatzel, T.: Biophotovoltaics: natural pigments in dye-sensitized solar cells. Appl. Energy 115, 216–225 (2014)
Isah, K.U., Jolayemi, B.J., Ahmadu, U., Kimpa, M.I., Alu, N.: Plasmonic effect of silver nanoparticles intercalated into mesoporous betalain-sensitized-TiO2 film electrodes on photovoltaic performance of dye-sensitized solar cells. Mater. Renew. Sustain. Energy 5, 10 (2016)
Win, T.T., Maung, Y.M., Soe, K.K.K.: Fabrication of TiO2–ZrO2 binary oxide electrode with natural dye (Rose) for dye sensitized solar cell application. Adv. Mater. Res. 550–553, 2036–2039 (2012)
Tomar, L.J., Bhatt, P.J., Desai, R.K., Chakrabarty, B.S., Panchal, C.J.: Improved conversion efficiency of dye sensitized solar cell using Zn doped TiO2–ZrO2 nanocomposite. AIP Conf. Proc. 1731, 050132 (2016)
Al-Alwani, M.A.M., Mohamad, A.B., Kadhum, A.A.H., Ludin, N.A., Safie, N.E., Razali, M.Z., Ismail, M., Sopian, K.: Natural dye extracted from Pandannus amaryllifolius leaves as sensitizer in fabrication of dye-sensitized solar cells. Int. J. Electrochem. Sci. 12, 747–761 (2017)
Nan, H., Shen, H.-P., Wang, G., Xie, S.-D., Yang, G.-J., Lin, H.: Studies on the optical and photoelectric properties of anthocyanin and chlorophyll as natural co-sensitizers in dye sensitized solar cell. Opt. Mater. 73, 172–178 (2017)
Siddick, S.Z., Lai, C.W., Juan, J.C.: An investigation of the dye-sensitized solar cell performance using graphene-titania (TrGO) photoanode with conventional dye and natural green chlorophyll dye. Mater. Sci. Semicond. Process. 74, 267–276 (2018)
Syafinar, R., Gomesh, N., Irwanto, M., Fareq, M., Irwan, Y.M.: Chlorophyll pigments as nature based dye for dye-sensitized solar cell (DSSC). Energy Procedia 79, 896–902 (2015)
Wongcharee, K., Meeyoo, V., Chavadej, S.: Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol. Energy Mater. Sol. Cells 91, 566–571 (2007)
Cui, Y., Zhao, W., Ogasawara, S., Wang, X.F., Tamiaki, H.: Fabrication and performance of all-solid-state dye-sensitized solar cells using synthetic carboxylated and pyridylated chlorophyll derivatives. J. Photochem. Photobiol. Chem. 353, 625–630 (2018)
Arifin, Z., Soeparman, S., Widhiyanuriyawan, D., Suyitno, S., Setyaji, A.T.: Improving stability of chlorophyll as natural dye for dye-sensitized solar cells. Jurnal Teknologi 80, 27–33 (2018)
Arifin, Z., Soeparman, S., Widhiyanuriyawan, D., Suyitno, S.: Performance enhancement of dye-sensitized solar cells using a natural sensitizer. Int. J. Photoenergy 2017, 2704864 (2017)
Pratiwi, D.D., Nurosyid, F., Kusumandari, Supriyanto, A., Suryana, R.: Performance improvement of dye-sensitized solar cells (DSSC) by using dyes mixture from chlorophyll and anthocyanin. J. Phys. Conf. 909, 012025 (2017)
Yusoff, N.H., Rosle, M.F., Buniran, S.: Fabrication of dye sensitized solar cell based on TiO2 nanoparticles and chlorophyll from Pandan leaf as active layer. Adv. Mater. Res. 545, 405–409 (2012)
Pai, A.R., Nair, B.: Synthesis and characterization of a binary oxide ZrO2–TiO2 and its application in chlorophyll dye-sensitized solar cell with reduced graphene oxide as counter electrodes. Bull. Mater. Sci. 38, 1129–1133 (2015)
Kumara, G.R.A., Kaneko, S., Okuya, M., Onwona-Agyeman, B., Konno, A., Tennakone, K.: Shiso leaf pigments for dye-sensitized solid-state solar cell. Sol. Energy Mater. Sol. Cells 90, 1220–1226 (2006)
Calogero, G., Citro, I., Crupi, C., Marco, G.D.: Absorption spectra and photovoltaic characterization of chlorophyllins as sensitizers for dye-sensitized solar cells. Spectrochim. Acta Mol. Biomol. Spectrosc. 132, 477–484 (2014)
Wang, X.F., Zhan, C.H., Maoka, T., Wada, Y., Koyama, Y.: Fabrication of dye sensitized solar cells using chlorophyll c1 and c2 from Undaria pinnatifida (Wakame). Chem. Phys. Lett. 447, 79–85 (2007)
Wu, J., Yue, G., Xiao, Y., Lin, J., Huang, M., Lan, Z., Tang, Q., Huang, Y., Fan, L., Yin, S., Sato, T.: An ultraviolet responsive hybrid solar cell based on titania/poly(3-hexylthiophene). Sci. Rep. 3, 1283 (2013)
Das, T.K., Ilaiyaraja, P., Sudakar, C.: Template assisted nanoporous TiO2 nanoparticles: the effect of oxygen vacancy defects on photovoltaic performance of DSSC and QDSSC. Sol. Energy 159, 920–929 (2018)
Zhou, H., Wu, L., Gao, Y., Ma, T.: Dye-sensitized solar cells using 20 natural dyes as sensitizers. J. Photochem. Photobiol. Chem. 219, 188–194 (2011)
Chu, L., Liu, W., Yu, A., Qin, Z., Hu, R., Shu, H., Luo, Q.P., Min, Y., Yang, J., Li, X.: Effect of TiO2 modification on urchin-like orthorhombic Nb2O5 nanospheres as photoelectrodes in dye-sensitized solar cells. Sol. Energy 15, 3584–3589 (2017)
Iqbal, K., Ikram, M., Afzal, M., Ali, S.: Efcient, low-dimensional nanocomposite bilayer CuO/ZnO solar cell at various annealing temperatures. Mater. Renew. Sustain. Energy 7, 4 (2018)
Vlazan, P., Ursu, D.H., Irina-Moisescu, C., Miron, I., Sfirloaga, P., Rusu, E.: Structural and electrical properties of TiO2/ZnO core–shell nanoparticles synthesized by hydrothermal method. Mater. Charact. 101, 153–158 (2015)
Nikam, P.R., Baviskar, P.K., Majumder, S., Sali, J.V., Sankapal, B.R.: SILAR controlled CdSe nanoparticles sensitized ZnO nanorods photoanode for solar cell application: electrolyte effect. J. Colloid Interface Sci. 524, 148–155 (2018)
Seo, K.D., You, B.S., Choi, I.T., Ju, M.J., You, M., Kang, H.S., Kim, H.K.: Dual-channel anchorable organic dyes with well-defined structures for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 1, 9947–9953 (2013)
Acknowledgements
Authors are thankful to University with Potential for Excellence (UPE)—II for partial financial support. PKB is thankful to University Grants Commission, New Delhi, India, for the award of Dr. D.S. Kothari Postdoctoral Fellowship and financial assistance (PH/16-17/0074).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pawar, K.S., Baviskar, P.K., Inamuddin et al. Layer-by-layer deposition of TiO2–ZrO2 electrode sensitized with Pandan leaves: natural dye-sensitized solar cell. Mater Renew Sustain Energy 8, 12 (2019). https://doi.org/10.1007/s40243-019-0148-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-019-0148-x