Abstract
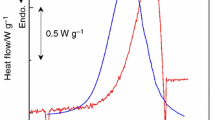
The thermal decomposition kinetics of 1-amino-1,2,3-triazolium nitrate(ATZ-NO3) was investigated by non-isothermal TG-DTG at various heating rates(2, 5, 10, 15 and 20 °C/min). The results show that the thermal decomposition of ATZ-NO3 consist of two stages corresponding to the losing of nitrate anion, substituent group and the splitting of triazole ring respectively. The kinetic triplets of the two stages were described by a three-step method. First, the differential Kissinger and intergral Ozawa methods were used to calculate the apparent activation energies(E) and pre-exponential factors(A) of the two decomposition stages. Second, two calculation methods(intergral Šatava-Šesták and differential Achar methods) were used to obtain several probable decomposition mechanism functions. Third, three judgment methods(average, double-extrapolation and Popescu methods) were used to confirm the most probable decomposition mechanism functions. Both reaction models of the two stages were random-into-nucleation and random-growth mechanisms with n=3/2 for the first stage and n=1/3, m=3 for the second stage. The kinetic equations for the two decomposition stages of ATZ-NO3 may be expressed as \(\frac{{da}} {{dt}} = 10^{13.60} \times e^{ - \frac{{128970}} {{RT}}} (1 - a)\left[ { - \ln (1 - a)} \right]^{ - \frac{1} {2}}\) and \(\frac{{da}} {{dt}} = 10^{11.41} \times e^{ - \frac{{117370}} {{RT}}} (1 - a)\left[ { - \ln (1 - a)} \right]^{\frac{2} {3}}\). The thermodynamic parameters including Gibbs free energy of activation(ΔG ≠), entropy of activation(ΔS ≠) and enthalpy of activation(ΔH ≠), for the thermal decomposition reaction were also derived.
Similar content being viewed by others
References
Sikder A. K., Sikder N., J. Hazard. Mater., 2004, 112(1/2), 1
Singh R. P., Gao H., Meshri D. T., Shreeve J. M., Structure and Bonding, 2007, 125, 35
Singh R. P., Verma R. D., Meshri D. T., Shreeve J. M., Angew. Chem. Inter. Ed., 2006, 45(22), 3584
Steinhauser G., Klapötke T. M., Angew. Chem., 2008, 120(18), 3376
Qi S. Y., Zhang J. G., Zhang T. L., Cui Y., Yang L., Yu K. B., Shu Y. J., Chem. J. Chinese Universities, 2009, 30(10), 1935
Zhao F. Q., Chen S. P., Fan G., Xie G., Jiao B. J., Gao S. L., Chem. J. Chinese Universities, 2008, 29(8), 1519
Garg S., Gao H., Joo Y. H., Parrish D. A., Huang Y., Shreeve J. M., J. Am. Chem. Soc., 2010, 132(26), 8888
Joo Y. H., Twamley B., Shreeve J. M., Chemistry-A European Journal, 2009, 15(36), 9097
Klapöetke T. M., Stierstorfer J., J. Am. Chem. Soc., 2009, 131(3), 1122
Klapötke T. M., Mayer P., Schulz A., Weigand J. J., J. Am. Chem. Soc., 2005, 127(7), 2032
Xue H., Gao Y., Twamley B., Shreeve J. M., Chem. Mater., 2005, 17(1), 191
Zeng Z., Gao H., Twamley B., Shreeve J. M., J. Mater. Chem., 2007, 17(36), 3819
Zhang Y., Gao H., Guo Y., Joo Y. H., Shreeve J. M., Chemistry-A European Journal, 2010, 16(10), 3114
Lin Q. H., Li Y. C., Li Y. Y., Wang Z., Liu W., Qi C., Pang S. P., J. Mater. Chem., 2012, 22(2), 666
Pagoria P. F., Lee G. S., Mitchell A. R., Schmidt R. D., Thermochimica Acta, 2002, 384(1), 187
Xue L., Zhao F. Q., Xing X. L., Zhou Z. M., Wang K., Gao H. X., Yi J. H., Xu S. Y., Hu R. Z., J. Therm. Anal. Calor., 2011, 104(3), 999
Hu R. Z., Gao S. L., Zhao F. Q., Shi Q. Z., Zhang T. L., Zhang J. J., Thermal Analysis Kinetics, Science Press, Beijing, 2008, 151
Kissinger H. E., Anal. Chem., 1957, 29(11), 1702
Jiménez A., Berenguer V., Lépez J., Sánchez A., J. Appl. Polym. Sci., 1993, 50(9), 1565
Škvára F., Šesták J., J. Therm. Anal. Calor., 1975, 8(3), 477
Achar B. N., Sharp J. H., Proceedings of the International Clay Conference, Jerusalem, 1966, (1), 67
Sharp J. H., Wentworth S. A., Anal. Chem., 1969, 41(14), 2060
Pan Y. X., Guan X. Y., Feng Z. Y., Li X. Y., Yan Z., Chinese J. Chem. Phys., 1998, 14(12), 1088
Pan Y. X., Guan X. Y., Feng Z. Y., Li X. Y., Wu Y. S., Chinese J. Inorg. Chem., 1999, 15(2), 111
Popescu C., Thermochimica Acta, 1996, 285(2), 309
Zhang J. J., Ren N., Bai J. H., Chinese Journal of Chemistry, 2006, 24(3), 360
Garcìa-Pèrez M., Chaala A., Yang J., Roy C., Fuel, 2001, 80(9), 1245
Starink M. J., Thermochimica Acta, 1996, 288(1/2), 97
Zhao F. Q., Xue L., Xing X. L., Hu R. Z., Zhou Z. M., Gao H. X., Yi J. H., Xu S. Y., Pei Q., Scientia Sinica Chimica, 2010, 40(9), 1430
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Defense Pre-Research Foundation of China(Nos.40406050101, 62201070103).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Du, X., Li, X., Yang, R. et al. Thermal kinetics and decomposition mechanism of 1-amino-1,2,3-triazolium nitrate. Chem. Res. Chin. Univ. 30, 130–136 (2014). https://doi.org/10.1007/s40242-014-3299-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-014-3299-4