Abstract
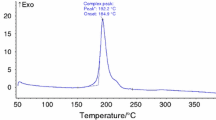
The thermal decomposition behaviors of 1,2,3-triazole nitrate were studied using a Calvet Microcalorimeter at four different heating rates. Its apparent activation energy and pre-exponential factor of exothermic decomposition reaction are 133.77 kJ mol−1 and 1014.58 s−1, respectively. The critical temperature of thermal explosion is 374.97 K. The entropy of activation (ΔS ≠), the enthalpy of activation (ΔH ≠), and the free energy of activation (ΔG ≠) of the decomposition reaction are 23.88 J mol−1 K−1, 130.62 kJ mol−1, and 121.55 kJ mol−1, respectively. The self-accelerating decomposition temperature (T SADT) is 368.65 K. The specific heat capacity was determined by a Micro-DSC method and a theoretical calculation method. Specific heat capacity equation is \( C_{\text{p}} \left( {{\text{J mol}}^{ - 1} {\text{ K}}^{ - 1} } \right) = - 42.6218 + 0.6807T \) (283.1 K < T < 353.2 K). The adiabatic time-to-explosion is calculated to be a certain value between 98.82 and 100.00 s. The critical temperature of hot-spot initiation is 637.14 K, and the characteristic drop height of impact sensitivity (H 50) is 9.16 cm.



Similar content being viewed by others
References
Gao HX, Ye CF, Piekarski CM. Computational characterization of energetic salts. J Phys Chem C. 2007;111:10718–26.
Agrawal JP. Recent trends in high-energy materials. Prog Energ Combust Sci. 1998;24:1–13.
Huang HF, Meng ZH, Zhou ZM, Gao HX, Zhang J, Wu YK. Energetic salts and energetic ionic liquids. Prog Chem. 2009;21:152–61 (in Chinese).
Drake G, Kaplan G, Hall L, Hawkins T, Larue J. A new family of energetic ionic liquids 1-amino-3-alkyl-1,2,3-triazolium nitrates. J Chem Crys. 2007;37:15–22.
Ye CF, Shreeve JM. Rapid and accurate estimation of densities of room-temperature ionic liquids and salts. J Phys Chem A. 2007;111:1456–61.
Tong B, Liu QS, Tan ZC, Urs WB. Thermochemistry of alkyl pyridinium bromide ionic liquids: calorimetric measurements and calculation. J Phys Chem A. 2010;114:3782–7.
Krossing I, Slattery JM, Daguenet C, Dyson PJ, Oleinikova A, Weingärtner H. Why are ionic liquids liquid? A simple explanation based on lattice and salvation energies. J Am Chem Soc. 2006;128:13427–34.
Kolaski M, Lee HM, Pak C, Kim KS. Charge-transfer-to-solvent-driven dissolution dynamics of 1-(H2O)2-5 upon excitation: excited-state ab initio molecular dynamics simulations. J Am Chem Soc. 2008;130:103–12.
Mel’yanenko EVN, Verevkin SP, Heintz A. Imidazolium-based ionic liquids. 1-Methyl imidazolium nitrate: thermochemical measurements and Ab initio calculations. J Phys Chem B. 2009;113:9871–81.
Urszula D, Andrzej M. Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-ethyl-3-methylimidazolium trifluoroacetate. J Phys Chem B. 2007;111:11984–8.
Fischer G, Holl G, Klapötke TM, Weigand JJ. A study on thermal decomposition behavior of derivatives of 1,5-diamino-1H-tetrazole (DAT): a new family of energetic heterocyclic-based salts. Thermochim Acta. 2005;437:168–75.
Chowdhury A, Thynell ST. Confined rapid thermolysis/FTIR/ToF studies of triazolium-based energetic ionic liquids. Thermochim Acta. 2007;466:1–11.
Chowdhury A, Thynell ST, Lin P. Confined rapid thermolysis/FTIR/ToF studies of triazolium-based energetic ionic liquids. Thermochim Acta. 2009;485:1–12.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JG. Thermal analysis kinetics. 2nd ed. Beijing: Science Press; 2008. (in Chinese).
Xing XL, Xue L, Zhao FQ, Gao HX, Hu RZ. Thermochemical properties of 1,1-diamino-2,2-dinitroethylene (FOX-7) in dimethyl sulfoxide (DMSO). Thermochim Acta. 2009;35:491–7.
Gao HX, Zhao FQ, Hu RZ, Zhao HA, Zhang H. Estimation of the critical temperature of thermal explosion for azido-acetic-acid-2-(2-azido-acetoxy)-ethylester using non-isothermal DSC. J Therm Anal Calorim. 2009;95:477–82.
Xu KZ, Song JR, Zhao FQ, Ma HX, Gao HX, Chang CR, Ren YH, Hu RZ. Thermal behavior, specific heat capacity and adiabatic time-to explosion of G(FOX-7). J Hazard Mater. 2008;158:333–9.
Xue L, Zhao FQ, Xing XL, Gao HX, Xu SY, Hu RZ. Dissolution properties of 1,3,3-trinitroazetidine (TNAZ) in ethyl acetate and N,N-dimethylformamide. Acta Phy Chim Sin. 2009;25:2413–21.
Xue L, Zhao FQ, Hu RZ, Gao HX. A simple method to estimate the critical temperature of thermal explosion for energetic materials using nonisothermal DSC. J Energ Mater. 2010;28:17–31.
Li JZ, Fan XZ, Hu RZ, Zhao FQ, Gao HX. Thermal behavior of copper(II) 4-nitroimidazolate. J Therm Anal Calorim. 2009;96:01–195.
Xu SY, Zhao FQ, Yi JH, Hu RZ, Gao HX, Li SW, Hao HX, Pei Q. Thermal behavior and non-isothermal decomposition reaction kinetics of composite modified double base propellant containing CL-20. Acta Phy Chim Sin. 2008;24:1371–9.
Arkady MK, Liubov PS. Molar heat capacities of the (water + acetonitrile) mixtures at T = (283.15, 298.15, 313.15, and 328.15) K. J Chem Thermodyn. 2010;42:1209–12.
Dong HS, Zhao FF. Performances of high explosive and its related materials. Beijing: Science Press; 1989.
Dong HS, Hu RZ, Yao P, Zhang XX. Thermograms of energetic materials. Beijing: National Defence Industry Press; 2001.
Acknowledgements
The financial supports received from the National Natural Science Foundation of China (Grant No. 20573098), and the Science and Technology Foundation of the National Key Lab of Science and Technology on Propellant and Explosive Combustion in China (Grant No. 9140C3501020901) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, L., Zhao, FQ., Xing, XL. et al. Thermal behavior of 1,2,3-triazole nitrate. J Therm Anal Calorim 104, 999–1004 (2011). https://doi.org/10.1007/s10973-010-1231-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1231-9