Abstract
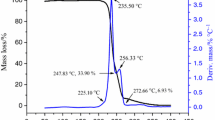
Ammonium 3,3′-dinitrimino-5,5′-bis(1H-1,2,4-triazole) (ADNABT) was synthesized and characterized by IR spectroscopy, 1H/13C NMR and single-crystal X-ray diffraction. The thermal decomposition of ADNABT was investigated by thermogravimetry–differential thermal analysis (TG–DTA) and accelerating rate calorimeter (ARC). The kinetic parameters (activation energy, pre-exponential factor, mechanism functions) by DTA and ARC tests were simulated by Thermal Safety Software (TSS). The simulated results revealed that the exothermic decomposition of ADNABT under non-isothermal and adiabatic conditions all followed a full autocatalysis model. In order to ensure the safety of production, transportation and storage, several thermal hazard indicators such as time to maximum rate (TMR), reaction temperature at which TMR is 24 h (TD24), time to conversion limit and self-accelerating decomposition temperature (SADT) were also simulated by TSS on the kinetic model. The TD24 and SADT50 kg were calculated as 183.37 and 167.00 °C, respectively.









Similar content being viewed by others
References
Wang RH, Xu HY, Guo Y, Sa RJ, Shreeve JM. Bis[3-(5-nitroimino-1,2,4-triazolate)]-based energetic salts: synthesis and promising properties of a new family of high-density insensitive materials. J Am Chem Soc. 2010;132:11904–5.
Fischer N, Izsák D, Klapötke TM, Rappenglück S, Stierstorfer J. Nitrogen-rich 5,5′-bistetrazolates and their potential use in propellant systems: a comprehensive study. Chem Eur J. 2012;18:4051–62.
Dippold AA, Klapötke TM, Winter N. Insensitive nitrogen-rich energetic compounds based on the 5,5′-dinitro-3,3′-bi-1,2,4-triazol-2-ide anion. Eur J Inorg Chem. 2012;21:3474–84.
Dippold AA, Klapötke TM. A study of dinitro-bis-1,2,4-triazole-1,1′-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N-oxides. J Am Chem Soc. 2013;135:9931–8.
Dippold AA, Klapötke TM, Oswald M. Asymmetrically substituted 5,5′-bistriazoles-nitrogen-rich materials with various energetic functionalities. Dalton Trans. 2013;42:11136–45.
Rao GN, Feng W, Zhang J, Wang SY, Chen LP, Guo ZC, Chen WH. Simulation approach to decomposition kinetics and thermal hazards of hexamethylenetetramine. J Therm Anal Calorim. 2019;135:2447–56.
Mi WZ, Wei RC, Zhou TN, He JJ, Wang J. Experimental study on the thermal decomposition of two nitrocellulose mixtures in different forms. Mater Sci Medzg. 2019;25:60–5.
Wei RC, Huang SS, Wang Z, He Y, Yuen R, Wang J. Estimation on the safe storage temperature of nitrocellulose with different humectants. Propellants, Explos, Pyrotech. 2018;43:1122–8.
Gan XY, Yang S, Wang SY, Guo XY, Chen LP, Chen WH. Thermal behavior of benzoyl peroxide mixed with NaOH solution. Thermochim Acta. 2018;670:13–7.
Shen SJ, Wu SH, Chi JH, Lin CC, Horng JJ, Shu CM. Simulation of solid thermal explosion and liquid thermal explosion of dicumyl peroxide using calorimetric technique. Simul Model Pract Theory. 2011;19:1251–7.
Shen SJ, Wu SH, Chi JH, Wang YW, Shu CM. Thermal explosion simulation and incompatible reaction of dicumyl peroxide by calorimetric technique. J Therm Anal Calorim. 2010;102:569–77.
You ML, Liu MY, Wu SH, Chi JH, Shu CM. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J Therm Anal Calorim. 2009;96:777–82.
Dippold AA, Klapötke TM. Nitrogen-rich bis-1,2,4-triazoles-a comparative study of structural and energetic properties. Chem Eur J. 2012;18:16742–53.
Zhang CY, Jin SH, Chen SS, Li LJ, Zhou C, Zhang Y, Shu QH. Thermal behavior and thermo-kinetic studies of 5,5′-bistetrazole-1,1′-diolate (1,1-BTO). J Therm Anal Calorim. 2017;129:1265–70.
Bao F, Zhang GZ, Jin SH. Thermal decomposition behavior and thermal stability of DABT·2DMSO. J Therm Anal Calorim. 2018;131:3185–91.
Bao F, Zhang GZ, Jin SH, Zhang CY, Niu H. Thermal decomposition and safety assessment of 3,3′-dinitrimino-5,5′-bis(1H-1,2,4-triazole) by DTA and ARC. J Therm Anal Calorim. 2018;132:805–11.
Vyazovkin S, Burnham AK, Criado JM, Pérez MLA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Lin HY, Tsai SY, Tseng YL, Lin CP. Gamma irradiation for improving functional ingredients and determining the heat treatment conditions of Cordyceps militaris mycelia. J Therm Anal Calorim. 2015;120:439–48.
Tseng JM, Lin CP. Prediction of incompatible reaction of dibenzoyl peroxide by isothermal calorimetry analysis and green thermal analysis technology. J Therm Anal Calorim. 2012;107:927–33.
Kossoy AA, Belokhvostov VM, Koludarova EY. Thermal decomposition of AIBN: part D: verification of simulation method for SADT determination based on AIBN benchmark. Thermochim Acta. 2015;621:36–43.
Cao CR, Liu SH, Das M, Shu CM. Evaluation for the thermokinetics of the autocatalytic reaction of cumene hydroperoxide mixed with phenol through isothermal approaches and simulations. Process Saf Environ. 2018;117:426–38.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Kossoy A, Sheinman I. Effect of temperature gradient in sample cells of adiabatic calorimeters on data interpretation. Thermochim Acta. 2010;500:93–9.
Kossoy AA, Singh J, Koludarova EY. Mathematical methods for application of experimental adiabatic data-an update and extension. J Loss Prevent Proc. 2015;33:88–100.
Hsueh KH, Chen WT, Chu YC, Tsai LC, Shu CM. Thermal reactive hazards of 1,1-bis(tert-butylperoxy) cyclohexane with nitric acid contaminants by DSC. J Therm Anal Calorim. 2012;109:1253–60.
Tsai SY, Lin HY, Hong WP, Lin CP. Evaluation of preliminary causes for vitamin D series degradation via DSC and HPLC analyses. J Therm Anal Calorim. 2017;130:1357–69.
Cheng YF, Liu SH, Shu CM, Zhang B, Li YF. Energy estimation and modeling solid thermal explosion containment on reactor for three organic peroxides by calorimetric technique. J Therm Anal Calorim. 2017;130:1201–11.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bao, F., Jin, S., Li, Y. et al. Thermal decomposition and thermal kinetic simulation of ammonium 3,3′-dinitrimino-5,5′-bis(1H-1,2,4-triazole). J Therm Anal Calorim 146, 911–917 (2021). https://doi.org/10.1007/s10973-020-10038-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10038-w