Abstract
Objectives
Underreporting of adverse drug reactions (ADRs) limits and delays the detection of signs. The aim of this systematic review with meta-analyses was to synthesize the evidence of educational interventions (EIs) efficacy in health professionals to increase ADR reporting, attitudes, and knowledge of pharmacovigilance.
Evidence acquisition
A systematic literature review was carried out to identify randomized clinical trials evaluating the efficacy of EI in pharmacovigilance in health professionals to improve ADR reports, knowledge, and attitude toward pharmacovigilance. ADR reports were pooled by calculating Odds Ratio (OR) with a 95% confidence interval (95%CI), while pharmacovigilance knowledge and attitude were pooled by calculating a mean difference (MD) with 95%CI. In addition, the subanalysis was performed by EI type. Meta-analysis was performed with RevMan 5.4 software. PROSPERO registry CRD42021254270.
Results
Eight hundred seventy-five articles were identified as potentially relevant, and 11 were included in the systematic review. Metanalysis showed that EI increased ADR reporting in comparison with control group (OR = 4.74, [95%CI, 2.46 to 9.12], I2 = 93%, 5 studies). In subgroup analysis, the workshops (OR = 6.26, [95%CI, 4.03 to 9.73], I2 = 57%, 3 studies) increased ADR reporting more than telephone-based interventions (OR = 2.59, [95%CI, 0.77 to 8.73], I2 = 29%, 2 studies) or combined interventions (OR = 5.14, [95%CI, 0.97 to 27.26], I2 = 93%, 3 studies). No difference was observed in pharmacovigilance knowledge. However, the subanalysis revealed that workshops increase pharmacovigilance knowledge (SMD = 1.85 [95%CI, 1.44 to 2.27], 1 study). Only one study evaluated ADR reporting attitude among participants and showed a positive effect after the intervention.
Conclusion
EI improves ADR reports and increases pharmacovigilance knowledge. Workshops are the most effective EI to increase ADR reporting.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drugs are essential for the treatment of various diseases, but there are drug-related problems, such as adverse drug reactions (ADR) [1]. Post-marketing information on medicines reports a benefit-risk balance obtained from clinical studies. Nevertheless, drug surveillance is necessary to evaluate safety in real-life and long-term conditions [2]. For this reason, ADR voluntary reports are needed, thus spontaneous reporting is the pillar of pharmacovigilance. In countries with pharmacovigilance programs well-established, the report number is about 200 or more per million inhabitants [3]. However, in many countries, pharmacovigilance programs are still under development, and this fact may result in a low drug safety culture that translates into underreporting of ADR. Low notification rates make it difficult to detect signs in the general population that, limits evaluation of ADR causality and the issuance of health alerts. Underreporting can be explained by the low participation of health professionals due to a lack of knowledge and negative attitudes toward pharmacovigilance, such as ignorance (only important serious ADR reports) or lethargy (disinterest in reporting) [3,4,5].
Different strategies have been evaluated to increase ADR notification, such as the implementation of educational interventions (EI) for health professionals [6,7,8]. EI purpose is to raise awareness about drug safety issues to improve ADR reporting to obtain statistical assessments by detecting signs and issuing health alerts [9, 10]. Therefore, the aim of this systematic review with meta-analyses was to synthesize the evidence of EI efficacy in health professionals to increase ADR reporting, attitudes, and knowledge of pharmacovigilance.
Methods
A systematic review and meta-analyses were conducted according to the PRISMA statement (Suppl. 1) [11], and the protocol was prospectively registered in PROSPERO with registration number CRD42021254270.
Search strategy
A systematic literature search was carried out in the following electronic databases: PubMed, LILACS, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus and Epistemonikos. Unpublished literature was looked up in the abstracts of randomized controlled trials (RCTs) indexed in Scopus Conference Papers and ScienceDirect. Searches were conducted from inception until January 2022 and were not limited by years or language. The strategy search was constructed using the following MeSH terms and keywords: “health personnel”, “physicians”, “pharmacists”, “nurses”; “models educational”, “education medical”; “adverse drug reaction reporting systems”, “pharmacovigilance”, “adverse drug reaction reporting”. The search strategy was adapted to each database (Suppl. 2). In addition, all references identified by systematic reviews were analyzed to identify potentially relevant studies.
Study selection
Studies were included if they met the following criteria: (1) RCT, including multi-arm trials; (2) participants were health professionals (physicians, consultants, nurses, pharmacists, and dentists); (3) participants received an educational intervention in pharmacovigilance including telephone-based interventions, workshop, educational material, electronic supplementary material, letters, lectures, sessions group, email and combined intervention; in the control group, participants did not receive educational activity or received training from their pharmacovigilance unit; (4) study results were a number of ADR reports and knowledge and attitude mean scores obtained through a questionnaire, in both groups. Studies were excluded if the educational intervention was aimed at patients or if the comparison was made between health professionals and patients, as well as studies that were sponsored by the pharmaceutical industry or involved economic incentives.
Two independent reviewers (MJC and LMU) assessed all titles and abstracts to identify studies via the inclusion criteria and excluded non-relevant studies. All potentially relevant articles were retrieved and read in full text. Reviewers were blinded to each other’s decisions. Discrepancies were discussed and resolved with a third reviewer (ODC). The inter-rater reliability was evaluated using kappa coefficient.
Data extraction and risk of bias assessment
Selected studies were reviewed independently by two reviewers (MJC and LMU) to extract in an Excel database the following data: publication year, author, health professionals, EI, time of intervention, control group, the sample size of the intervention group as the control group, participants in both groups, follow-up time, the number of ADR reports, knowledge, and attitude in pharmacovigilance mean score, country, attitude, and knowledge questionnaire (validated or not), change of result over time, ADR type (severe, unexpected, high-causality and new-drugs). Discrepancies in data extraction were resolved by consensus. In case any data was not reported in the article, the authors were contacted to obtain it.
When ADR results were reported in a thousand pharmacist-months, a conversion was made to the number of ADR reports, multiplying the rate per person-month, and dividing by one thousand [12].
Potential biases related to individual RCT were assessed with the Cochrane risk-of-bias tool (RoB 2) [13]. RevMan 5.4 was used to generate the risk of bias figures. [14]. The risk of bias was assessed in duplicates by two authors independently (MJC and LMU). Any disagreement was addressed by reappraisal in conjunction with a third reviewer (ODC).
Data analysis
Statistical analyses were performed using RevMan 5.4 [14]. ADR reports were pooled using an odds’ ratio (OR) with 95% confidence intervals (95%CI). Knowledge and attitude in pharmacovigilance scores were analyzed with a standardized mean difference (SMD) with 95%CI. All analyses were performed with a random-effects approach. I² test was used to assess the heterogeneity of each evaluate results, and I² > 50% was considered with signification heterogeneity [15]. Subanalysis by type of educational intervention was performed to identify the most effective intervention, as well as to explore heterogeneity between studies. The results’ consistency was evaluated using a leave-one-out sensitivity analysis, the study with the highest bias was excluded in each comparison. Only studies that reported the ADR reports numbers (totals, serious, high probability, unexpected, and new drugs by control and intervention groups before and after the educative intervention), knowledge scores, or changes in attitude were included in the meta-analysis.
Results
Characteristics of the studies
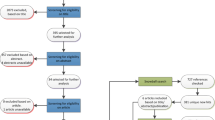
In the systematic search, a total of 875 citations were identified in databases, and the study selection process is illustrated in Fig. 1. After duplicate removal, 705 articles were screened by title and abstract for potential eligibility. In addition, 29 unpublished records were identified. No additional studies were identified in references of previously published systematic reviews. After screening, twenty-four studies were assessed for eligibility, and 13 studies were excluded [16,17,18,19,20,21,22,23,24,25,26,27,28]. Non-comparative studies were the main cause of exclusion, and all reasons are shown in the Suppl. 3. Inter-rater agreement was suitable (kappa = 0.83). Eleven studies fulfilled the inclusion criteria of the systematic review, and the characteristics of the included studies are summarized in Table 1. Two authors were contacted for data to be included in the meta-analyses [29, 30], only one responded, however the information could not be pooled. Eight studies were included in the meta-analysis [29, 31,32,33,34,35,36,37].
For country, RCTs were principally conducted in Portugal (four articles) and Sweden (two articles). Geographically, all the studies were conducted in Europe and Asia. The EI varied from one day to nine months, and follow-up ranged from 0 to 20 months. The average participation rate (a healthcare professional who agreed to participate into the study) varies in each study between 7.9 and 84.0%, and participants had more adherence to combined interventions and electronic ADR information.
Four studies involved physicians [29, 31, 33, 35], two involved nurses [29, 36], four involved pharmacists [32, 34, 37, 38], and two studies evaluated primary healthcare units that included physicians and nurses [30, 39]. The professionals mainly studied were physicians (six studies with 5097 participants and 136 primary healthcare units), followed by the pharmacist (four studies with 887 participants) (Table 1).
Workshops were the most common educative interventions used into studies [31, 33, 34, 36], followed by intervention combined (session group and educative material) [29, 32, 35], telephone-based interventions [31, 34], lecture [36], educational material (transparencies, brochures, and posters) [38], electronic information sheet of ADR [37], E-mail interventions [39] and one-page ADR information letter [30]. Three studies included continuing education by the pharmacovigilance unit as a control group [32, 35, 37], while eight studies did nothing [29,30,31,32,33,34, 36, 38, 39] (Table 1).
ADR reports
Ten studies informed the number of ADR reports [29,30,31,32,33,34,35, 37,38,39]. Five studies were excluded from the meta-analysis because these have incomplete data such as number of participants, or the total number of ADR reports [29, 30, 37,38,39]. Five studies present complete data for meta-analysis, and classified ADR as total, serious, high probability, unexpected, and new drugs by control and intervention groups [31,32,33,34,35]. Two studies presented three arms (workshop, telephone-based interventions, and control group) [31, 34], and three studies with two arms (combined intervention or workshop vs. control group) [32, 33, 35].
Educational interventions increased the reporting of all ADRs in comparison with control group (OR = 4.74, [95%CI, 2.46 to 9.12], I2 = 93%, 5 studies). In the sensitivity analysis, after removed Herdeiro et al. [31], educational interventions showed consistency in increasing ADR reporting (OR = 6.06 [95%CI, 2.50 to 14.71], I2 = 94%, 4 studies). In subgroup analysis, workshops (OR = 6.26, [95%CI, 4.03 to 9.73], I2 = 57%, 3 studies) increased ADR reporting, more than combined interventions (OR = 5.14, [95%CI, 0.97 to 27.26], I2 = 98%, 3 studies), while telephone-based interventions no showed a difference (OR = 2.59, [95%CI, 0.77 to 8.73], I2 = 29%, 2 studies) (Figs. 2).
ADR reporting change over time is shown in Table 2. In the workshop intervention, the increase in the number of reports was significant up to 16 months after IE for total and severe ADRs, but only increased over 12 months for unexpected, high-causality, and new drug ADRs. In contrast, telephone-based interventions only increased the number of total reports and serious ADRs by 4 months. Interestingly, the combined interventions increased the number of unexpected and new drug ADRs for at least 12 months, although for total, serious, and high-causality ADRs, the effect was seen from 12 months onwards.
Knowledge, and attitude in pharmacovigilance
Regarding the change in knowledge in pharmacovigilance, three studies [29, 36, 37] evaluated 4 educative interventions. The meta-analysis results showed a tendency to increase pharmacovigilance knowledge mean scores in participants who received EI in comparison with the control group (SMD = 1.12, [95%CI, -0.12 to 2.36], I2 = 98%, 4 studies). After removing the highest risk of bias study [29], participants in EI group shown an augmented their pharmacovigilance knowledge (SMD = 1.53 [95%CI, 0.58 to 2.47, I2 = 92%, 3 studies]). In subgroup analysis, the participants who received lecture (SMD = 2.23 [95%CI, 1.81 to 2.65], 1 study) and workshop (SMD = 1.85 [95%CI, 1.44 to 2.27], 1 study) increased their knowledge; this effect was not observed in those who received the combined intervention or letter with ADR information (Fig. 3).
Two studies evaluated ADR reporting attitudes among health professionals (Table 1), however, the measurement scales obtained by the questionnaire are different, so it was not possible to perform a meta-analysis. One study conducted in pharmacist showed a positive attitude toward ADR reporting after the intervention [38]. Likewise, a positive effect in behavior related to reporting was observed in physicians and nurses after educative intervention [29].
Risk of bias assessment
In risk of bias assessment (Fig. 4), 73% of studies had adequate random sequence generation [29, 32,33,34,35,36,37,38]. Only 54% describe the randomization process completely [29, 30, 35,36,37, 39], presenting low-risk allocation concealment, because the randomization was carried out by a person outside the study, or they avoided contamination between groups by randomizing health centers.
The performance bias had a high risk in at least 81% of articles, due to differences in interventions ranging from a phone call to a combined intervention [29,30,31,32,33,34,35, 38, 39]. With respect to blinding outcome assessment, in 4 studies the ADR reports evaluator was blinding [32,33,34,35]. In 63% of the studies [30,31,32,33,34, 37, 39], no missing data were seen, while reporting bias was considered a low risk in 72% of studies [30,31,32,33,34,35, 37, 39]. Additionally, in other potential sources of bias, 80% (9 of 11 studies) of the selected studies were rated with a low risk of bias [29,30,31,32,33,34, 37, 39].
Discussion
ADR report is paramount for causality analysis and drug safety assessment. Nonetheless, ADR occurrence generates distrust in health professionals due to the fear of being judged and punished [40]. To avoid this, EIs in pharmacovigilance are intended to increase knowledge about drug safety, improve attitudes towards ADRs, and consequently increase the reporting. The results of this systematic review with meta-analysis showed that EI in pharmacovigilance increases the ADR reports, and present positive changes in pharmacovigilance knowledge and attitude in health care professionals.
To synthesize the best available evidence on the role of EI in increasing ADR reporting, only RCTs were included in this systematic review. Study results show that EI increases by about four times the ADR report. Similar results were reported in a systematic review that synthesized the evidence on interventions to increase the spontaneous reporting of ADRs in healthcare professionals and patients [8]. Likewise, two previous systematic reviews, which included pre-post experimental design, quasi-experimental and RCT studies, concluded that the interventions evaluated were considered effective [6, 8]. However, no previous systematic review has evaluated efficacy by intervention type. In this study, the workshops have greater ADR reporting efficacy compared to others, that could be explained by the person-person interaction of the workshop allows a better understanding of the concept compared to reading information in a letter. In this sense, the score of knowledge observed in workshop participants is two-fold increase in comparison with participants who received a letter with an ADR information. Previous results indicated that interactive sessions enhance participant activity and provide the opportunity to practice skills can effect change in professional practice [41].
In addition, the effectiveness over time reveals that EI with interaction between people such as workshops and combined interventions maintain their effect on the ADR report for up to 16 months. This effect was not observed in telephone-based intervention, it suggests the necessity for a re-intervention.
Furthermore, educational combined intervention can reinforce and increase the understanding of pharmacovigilance issues and modify the attitude about ADR and increase the report in comparison with a simple intervention [7, 42]. Similarly, Forsetlund L., recommends using combined interventions with interactive formats that increase attention, to increase the effectiveness of the interventions [43]. It is not certain that printed educational materials, as a single intervention, can maintain the change in results over time [44]. In contrast, regular delivery of drug safety information can be an effective and inexpensive technique, but it loses its effect if delivery is stopped [45]. In this systematic review, all the studies that evaluated the combined intervention used the continuing education of the pharmacovigilance unit as a comparator. This could explain why, although there is a trend in favor of the combined intervention for the increase in the total ADR reports, this is not statistically significant.
The educative interventions dependent on complex factors such as intrapersonal, interpersonal, professional education, context, and material quality [41]. The educational intervention could work depending on the population, the objective sought, and due to the training of the participant. In RCTs included in this systematic review have no harmonization in the type of educational intervention and length. In this way, EI investigated in pharmacovigilance are different, regardless of the study design, and have durations ranging from a few minutes to six years [7]. These differences can be explained by cultural gaps, and social situations in each region that could modify the intervention type according to the context of each country, such as the geographical location and status of the pharmacovigilance system [23]. EI explored into the studies included in this systematic review were evaluated in Europe and Asia countries, appraisal of these interventions in other countries using RCTs approach may provide information on the efficacy of EI in regions whose drug safety culture may be different.
In clinical practice, the effectiveness of EI in pharmacovigilance can be increased by existence of continuous training in the study, reporting promotion by regional centers, the unit’s requirement to report cases of a new drug, an industry study, incentive programs for reporting, electronic methods of ADR report, and monetary incentives [6, 42, 46, 47]. Against, the effectiveness can be decreased due to factors such as high workload that does not allow reporting, limited time to take courses and lack of interest in pharmacovigilance [45, 48]. In this sense, the attitude to ADR underreporting can be explained by Inman and its seven deadly sins: complacency, ignorance, diffidence, financial incentives, legal aspects, lethargy, and indifference [4]. Furthermore, the fact that health professionals have a high knowledge of pharmacovigilance does not imply that they have a good attitude towards the report [49, 50]. Previous studies based on questionnaires of Knowledge, Attitude, and Practice (KAP) in pharmacovigilance support that an educational intervention could generate a change in a positive behavior on ADR report [6, 50,51,52,53]. Only one RCT in this systematic review evaluated attitude after educative intervention, with a positive effect [38].
ADR reporting in post-marketing surveillance is a cornerstone for signal detection and contribute to establish guidelines or policies for medication use. Consequently, it allows identifying serious or unexpected adverse drug reactions that represent a major problem in patient safety and increase hospital costs; thus, educative interventions sensitize health professionals about its importance [54]. In this review, the workshops and combined interventions increase the serious, unexpected, high causality, and new drug ADR reporting for at least 12 months.
Limitation of study
This systematic review has the following limitations, which should be considered when interpreting the results: (1) the educational interventions are different, such as workshops, combined interventions, telephone-based interventions, letters, or lectures; (2) the studies were evaluated with two different types of controls (continuing education and nothing); (3) No study that evaluated knowledge or attitude performed a prior validation of the questionnaire; (4) the workshop variate between brainstorming with two sessions of two hours in one day, one session of one hour, a session every month, or reminder card and report form with two sessions of 30 min.
Conclusions
The educative interventions in pharmacovigilance increased the number of ADR reports and score in the knowledge. The workshop and combined intervention are the EI with greater efficacy and duration. More RCTs are needed to assess the role of educational interventions in changing attitudes towards pharmacovigilance.
Data availability
Databases generated for this systematic review are available from the corresponding author upon reasonable request.
References
Sánchez I, Amador C, Plaza JC, Correa GAR. Assessment of an active pharmacovigilance system carried out by a pharmacist. Rev Med Chil. 2014;142:998–1005. https://doi.org/10.4067/S0034-98872014000800007.
Vlahović-Palčevski V, Mentzer D. Postmarketing surveillance. Handb Exp Pharmacol. 2011;205:339–51. https://doi.org/10.1007/978-3-642-20195-0_17.
Uppsala Monitoring Centre. Safety monitoring of medicinal products. In: Guidelines for setting up and running a Pharmacovigilance Centre. World Health Organization (WHO); 2000. https://who-umc.org/media/1703/24747.pdf. Accessed 13 Oct 2022.
Inman W. Attitudes to adverse drug-reaction reporting. Br J Clin Pharmacol. 1996;41:433–5.
Khan SA, Goyal C, Chandel N, Rafi M. Knowledge, attitudes, and practice of doctors to adverse drug reaction reporting in a teaching hospital in India: an observational study. J Nat Sci Biol Med. 2013;4:191–6. https://doi.org/10.4103/0976-9668.107289.
Li R, Zaidi STR, Chen T, Castelino R. Effectiveness of interventions to improve adverse drug reaction reporting by healthcare professionals over the last decade: a systematic review. Pharmacoepidemiol Drug Saf. 2020;29:1–8. https://doi.org/10.1002/pds.4906.
Khalili M, Mesgarpour B, Sharifi H, Daneshvar-Dehnavi S, Haghdoost AA. Interventions to improve adverse drug reaction reporting: a scoping review. Pharmacoepidemiol Drug Saf. 2020;29:965–92. https://doi.org/10.1002/pds.4966.
Paudyal V, Al-Hamid A, Bowen M, Abdul-Hadi M, Shahzad-Hasan S, Jalal Z, Stewart D. Interventions to improve spontaneous adverse drug reaction reporting by healthcare professionals and patients: systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19:1173–91. https://doi.org/10.1080/14740338.2020.1807003.
Abubakar AR, Simbak NB, Haque M. A systematic review of knowledge, attitude and practice on adverse drug reactions and pharmacovigilance among doctors. J Appl Pharm Sci. 2014;4:117–27. https://doi.org/10.7324/JAPS.2014.40121.
Faillie JL, Montastruc F, Montastruc JL, Pariente A. Pharmacoepidemiology and its input to pharmacovigilance. Therapie. 2016;71:211–6. https://doi.org/10.1016/j.therap.2016.02.016.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372. https://doi.org/10.1136/bmj.n160.
Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2008. https://www.radioterapiaitalia.it/wp-content/uploads/2017/01/cochrane-handbook-for-systematic-reviews-of-interventions.pdf. Accessed 15 Oct 2022.
Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions, version 6.3. Cochrane; 2022. Available from: https://www.training.cochrane.org/handbook. Accessed 15 Oct 2022.
The Cochrane Collaboration Review Manager (RevMan). [Computer Program]. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen, Version 5.4. Denmark: 2020. Available from: https://training.cochrane.org/online-learning/core-software/revman/revman-5-download. Accessed 15 Apr 2022.
Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions, version 6.3. Cochrane. 2022. Available from: https://www.training.cochrane.org/handbook. Accessed 15 Oct 2022.
Bäckström M, Mjörndal T. A small economic inducement to stimulate increased reporting of adverse drug reactions—a way of dealing with an old problem? Eur J Clin Pharmacol. 2006;62:381–5. https://doi.org/10.1007/s00228-005-0072-0.
Bracchi RCG, Houghton J, Woods FJ, Thomas S, Smail SA, Routledge PA. A distance-learning programme in pharmacovigilance linked to educational credits is associated with improved reporting of suspected adverse drug reactions via the UK yellow card scheme. Br J Clin Pharmacol. 2005;60:221–3. https://doi.org/10.1111/j.1365-2125.2005.02419.x.
Srikanth MS, Adeppu R. Assessment of educational intervention on knowledge, attitude and practices of rural comnunity pharmacists of Mysuru district toward adverse drug reaction reporting. Asian J Pharm Clin Res. 2018;11:242–6.
Williams GD, Muffly MK, Mendoza JM, Wixson N, Leong K, Claure RE. Reporting of perioperative adverse events by pediatric anesthesiologists at a tertiary children’s hospital: targeted interventions to increase the rate of reporting. Anesth Analg. 2017;125:1515–23.
Gumustekin M, Arici MA, Koca P, Gelal A, Tuncok Y. Impact of an educatıonal interventıon on knowledge and attıtude related to adverse drug reactıons reported by Physıcıans ın an Unıversıty Hospıtal. Clin Ther. 2017. https://doi.org/10.1016/j.clinthera.2017.05.184.
Kane-Gill SL, Hanlon JT, Fine MJ, Perera S, Culley CM, Studenski SA, Nace DA, Boyce RD, Castle NG, Handler SM. Physician perceptions of consultant pharmacist services associated with an intervention for adverse drug events in the nursing facility. Consult Pharm. 2016;31:708–20. https://doi.org/10.4140/TCP.n.2016.708.
Stoynova V, Getov IN, Naseva EK, Lebanova HV, Grigorov EE. Physicians’ knowledge and attitude towards adverse event reporting system and result to intervention randomized nested trial among Bulgarian physicians. Med Glas. 2013;10:365–72.
Gonzalez-Gonzalez C, Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Strategies to improve adverse drug reaction reporting: a critical and systematic review. Drug Saf. 2013;36:317–28. https://doi.org/10.1007/s40264-013-0058-2.
Sanghavi DR, Dhande PP, Pandit VA. Perception of pharmacovigilance among doctors in a tertiary care hospital: influence of an interventional lecture. Int J Risk Saf Med. 2013;25:197–204. https://doi.org/10.3233/JRS-130598.
Ribeiro-Vaz I, Herdeiro T, Figueiras A, Polónia J. Strategies for increasing spontaneous adverse drug reaction reporting rates among Portuguese pharmacists. Eur J Integr Med. 2009;1:250–1. https://doi.org/10.1016/j.eujim.2009.08.053.
Rosenbaum SE, Thacher-Renshaw A, Green M, Waters WJ. Interventions to increase physician participation in a voluntary reporting system: the Rhode Island adverse drug reaction reporting project. Clin Res Reg Affairs. 1992;9:261–75. https://doi.org/10.3109/10601339209005340.
Herdeiro MT, Ribeiro-Vaz I, Ferreira M, Polónia J, Figueiras A. Improving adverse drug reaction reporting through workshops and telephone education: Cluster Randomized Trial among Portuguese physicians. Basic Clin Pharmacol Toxicol. 2011;109(s1):56–164. https://doi.org/10.1111/j.1742-7843.2011.00722.x.
Jha N, Rathore DS, Shankar PR, Gyawali S, Alshakka M, Bhandary S. An educational intervention’s effect on healthcare professionals’ attitudes towards pharmacovigilance. Australas Med J. 2014;7:478–89. https://doi.org/10.4066/AMJ.2014.2235.
Potlog SM, Goldstein LH, Arcavi L, Shihmanter R, Berkovitch M, Levy A. Increasing adverse drug reaction reporting-how can we do better? PLoS ONE. 2020;15:1–15.
Johansson ML, Hägg S, Wallerstedt SM. Impact of information letters on the reporting rate of adverse drug reactions and the quality of the reports: a randomized controlled study. BMC Clin Pharmacol. 2011;11.
Herdeiro MT, Ribeiro-Vaz I, Ferreira M, Polónia J, Falcão A, Figueiras A. Workshop- and telephone-based interventions to improve adverse drug reaction reporting. Drug Saf. 2012;35:655–65.
Herdeiro MT, Polónia J, Gestal-Otero JJ, Figueiras A. Improving the reporting of adverse drug reactions: a cluster-randomized trial among pharmacists in Portugal. Drug Saf. 2008;31:335–44.
Figueiras A, Herdeiro MT, Polónia J, Gestal-Otero JJ. An educational intervention to improve physician reporting of adverse drug reactions. JAMA. 2006;296:1086–93.
Ribeiro-Vaz I, Herdeiro MT, Polónia J, Figueiras A. Strategies to increase the sensitivity of pharmacovigilance in Portugal. Rev Saude Publica. 2011;45:129–35.
Lopez-Gonzalez E, Herdeiro MT, Piñeiro-Lamas M, Figueiras A. Effect of an educational intervention to improve adverse drug reaction reporting in physicians: a cluster randomized controlled trial. Drug Saf. 2015;38:189–96.
Sarayani A, Naderi-Behdani F, Hadavand N, Javadi M, Farsad F, Hadjibabaie M, Gholami K. A 3-armed randomized controlled trial of nurses’ continuing education meetings on adverse drug reactions. J Contin Educ Health Prof. 2015;35:123–30.
Cheema E, Almualem AA, Basudan AT, Salamatullah AAK, Radhwi SO, Alsehli AS. Assessing the impact of structured education on the knowledge of hospital pharmacists about adverse drug reactions and reporting methods in Saudi Arabia: an open-label randomised controlled trial. Drugs Ther Perspect. 2019;35:296–300 Springer International Publishing.
Granas AG, Buajordet M, Stenberg-Nilsen H, Harg P, Horn AM. Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf. 2007;16:429–34.
Johansson ML, Brunlöf G, Edward C, Wallerstedt SM. Effects of e-mails containing ADR information and a current case report on ADR reporting rate and quality of reports. Eur J Clin Pharmacol. 2009;65:511–4.
Bañeres J, Cavero E, López L, Orrego C, Suñol R. Sistemas de registro y notificación de incidentes y eventos adversos. Ministerio de Sanidad y Consumo. Madrid. https://seguridaddelpaciente.es/resources/documentos/sistemasregistronotificacionincidentesea.pdf. Accessed 15 Oct 2022
Dave D, O´Brien MA, Freemantle N, Wolf FM, Mazmanian P, Taylor-Vaisey A. Impact of formal continuing medical education do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes? JAMA.1999;282:867–74.
Pagotto C, Varallo F, Mastroianni P. Impact of educational interventions on adverse drug events reporting. Int J Technol Assess Health Care. 2013;29:410–7.
Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, O’Brien MA, Wolf F, Davis D, Odgaard-Jensen J, Oxman AD. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009;2009(2):CD003030. https://doi.org/10.1002/14651858.CD003030.pub2.
Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, Makosso-Kallyth S, Wolf FM, Farmer AP, Gagnon MP. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;10:CD004398.
Biagi C, Montanaro N, Buccellato E, Roberto G, Vaccheri A, Motola D. Underreporting in pharmacovigilance: an intervention for Italian GPs (Emilia-Romagna region). Eur J Clin Pharmacol. 2013;69:237–44.
Srba J, Descikova V, Vlcek J. Adverse drug reactions: analysis of spontaneous reporting system in Europe in 2007–2009. Eur J Clin Pharmacol. 2012;68:1057–63.
Ali S, Egunsola O, Al-Dossari DS, Al-Zaagi IA. Adverse drug reaction reporting in a large tertiary hospital in Saudi Arabia: results of an incentive strategy. Ther Adv Drug Saf. 2018;9:585–90.
Salehi T, Seyedfatemi N, Mirzaee MS, Maleki M, Mardani A. Nurses’ knowledge, attitudes, and practice in relation to pharmacovigilance and adverse drug reaction reporting: a systematic review. Biomed Res Int. 2021;2021:6630404. https://doi.org/10.1155/2021/6630404.
Alshammari TM, Alamri KK, Ghawa YA, Alohali NF, Abualkol SA, Aljadhey HS. Knowledge and attitude of health-care professionals in hospitals towards pharmacovigilance in Saudi Arabia. Int J Clin Pharm. 2015;37:1104–10.
Hussain R, Hassali MA, Hashmi F, Akram T. Exploring healthcare professionals’ knowledge, attitude, and practices towards pharmacovigilance: a cross-sectional survey. J Pharm Policy Pract. 2021. https://doi.org/10.1186/s40545-020-00287-3.
Manjhi PK, Kumar M, Dikshit H, Mohan L, Mishra H. A survey on knowledge, attitude and practice of pharmacovigilance and adverse drug reaction reporting among healthcare professionals in a tertiary care hospital of Bihar, India. Int J Basic Clin Pharmacol. 2016;5:2566–71.
Herdeiro MT, Figueiras A, Polónia J, Gestal-Otero JJ. Physicians’ attitudes and adverse drug reaction reporting a case-control study in Portugal. Drug Saf. 2005;28:825–33.
Hardeep, Bajaj JK, Rakesh K. A survey on the knowledge, attitude and the practice of pharmacovigilance among the health care professionals in a teaching hospital in northern India. J Clin Diagn Res. 2013;7:97–9.
Gautier S, Bachelet H, Bordet R, Caron J. The cost of adverse drug reactions. Expert Opin Pharmacother. 2003;4:319–26.
Acknowledgements
Cervantes-Arellano Mónica Janette is a doctoral student from the Programa de Maestría y Doctorado en Ciencias Médicas, Odontológicas y de la Salud, Investigación Clínica Experimental en Salud (ICES). CMJ receive a fellowship from Consejo Nacional de Ciencia y Tecnología (CONACyT) with CVU number 777686.
Funding
No specific funding for this study was obtained.
Author information
Authors and Affiliations
Contributions
Conception of the work LMU; design of the work MJC, LMU, ODC; data acquisition MJC and LMU; data analysis MJC, LMU, ODC; data interpretation MJC, ODC, LMU, YM, JLC, OM; all authors contribute in manuscript drafting and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
This study was register in Division de Investigation at the Faculty of Medicine, UNAM FM/DI/003/21 to LMU.
Consent for participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Nothing to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cervantes-Arellano, M.J., Castelán-Martínez, O.D., Marín-Campos, Y. et al. Educational interventions in pharmacovigilance to improve the knowledge, attitude and the report of adverse drug reactions in healthcare professionals: Systematic Review and Meta-analysis. DARU J Pharm Sci 32, 421–434 (2024). https://doi.org/10.1007/s40199-024-00508-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-024-00508-z