Abstract
Background
Underreporting is the major limitation of a voluntary adverse drug reaction (ADR) reporting system. Many studies have assessed the effectiveness of different interventions designed to reduce underreporting.
Objective
We aimed to conduct a critical review of papers that assessed the effectiveness of different strategies to increase ADR reporting, regardless of the health professionals or patients included.
Data Sources
Scientific papers were selected after a search of the MEDLINE-PubMed and EMBASE scientific databases up to 7 December 2010.
Study Selection
We included papers in English, French or Spanish that analysed an intervention aimed at increasing the number of reported ADRs, and quantify the results of the intervention in terms of number of reports.
Data Extraction
The abstracts retrieved in both computerized searches were reviewed independently by two of the authors. Initially selected papers were thoroughly read to evaluate if they met inclusion and exclusion criteria. Data in finally selected papers were independently extracted by both authors and set in pre-designed tables. A third author took the final decision in case of disagreement. For each study, we analysed study design, type of intervention, assessment period, and results of the intervention.
Results
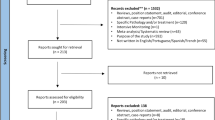
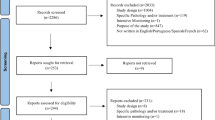
Of the 4,221 papers located that fulfilled the search criteria, 43 met the selection criteria. With the exception of one study, the interventions assessed were deemed to be effective. The vast majority of papers displayed methodological and formal limitations that lowered the grade of evidence. Multiple interventions seem to have had more impact than did single interventions. There were very few cases in which interventions were designed on the basis of inappropriate attitudes and mistaken beliefs about ADRs.
Conclusions
In general, there is a need for studies of better methodological quality in this topic, so that more evidence of the effectiveness of the respective strategies can be collected for the purpose of improving ADR reporting by health professionals. It is probable that multiple interventions cause greater increases in the ADR reporting rates than single.


Similar content being viewed by others
References
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5.
Patel KJ, Kedia MS, Bajpai D, Mehta SS, Kshirsagar NA, Gogtay NJ. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: a prospective study. BMC Clin Pharmacol. 2007;7:8.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385–96.
Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32:19–31.
Molokhia M, Tanna S, Bell D. Improving reporting of adverse drug reactions: systematic review. Clin Epidemiol. 2009;1:75–92.
Van Grootheest K, Olsson S, Couper M, de Jong-van den Berg L. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf. 2004;13:457–64.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses [online]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed 1 Jun 2012).
Committee on Safety of Medicines: annual report for 2004 [online]. http://www.mhra.gov.uk/home/groups/es-cb/documents/committeedocument/con2015695.pdf (Accessed 19 Oct 2011).
Miwa LJ, Randall RJ. Adverse drug reaction program using pharmacist and nurse monitors. Hosp Formul. 1986;21:1140–3, 1146.
Michel DJ, Knodel LC. Program coordinated by a drug information service to improve adverse drug reaction reporting in a hospital. Am J Hosp Pharm. 1986;43:2202–5.
Cantú TG, Tyler LS. Development of a hospital-based adverse drug reaction reporting program. Hosp Formul. 1988;23:658–61.
Kimelblatt BJ, Young SH, Heywood PM, Mandala AR, Gendelman S, Mehl B. Improved reporting of adverse drug reactions. Am J Hosp Pharm. 1988;45:1086–9.
Winstanley PA, Irvin LE, Smith JC, Orme LE. Breckenridge. Adverse drug reactions: a hospital pharmacy-based reporting scheme. Br J Clin Pharmacol. 1989;28:113–6.
Vorce-West TE, Barstow L, Butcher B. System for voluntary reporting of adverse drug reactions in a university hospital. Am J Hosp Pharm. 1989;46:2300–3.
Scott HD, Thacher-Renshaw A, Rosenbaum SE, Waters WJ Jr, Green M, Andrews LG, Faich GA. Physician reporting of adverse drug reactions. Results of the Rhode Island Adverse Drug Reaction Reporting Project. JAMA. 1990;263:1785–8.
Feely J, Moriarty S, O’Connor P. Stimulating reporting of adverse drug reactions by using a fee. BMJ. 1990;300(6716):22–3.
Gilroy GW, Scollins MJ, Gay CA, Harry DJ, Giannuzzi DF. Pharmacy-coordinated program that encourages physician reporting of adverse drug reactions. Am J Hosp Pharm. 1990;47:1327–33.
Chatas CA, Vinson BE. Program for improving adverse drug reaction reporting. Am J Hosp Pharm. 1990;47:155–7.
Prosser TR, Kamysz PL. Multidisciplinary adverse drug reaction surveillance program. Am J Hosp Pharm. 1990;47:1334–9.
Nazario M, Feliú JF, Rivera GC. Adverse drug reactions: the San Juan Department of Veterans Affairs Medical Center experience. Hosp Pharm. 1994;29:244–6, 249–50.
Yee WP, Norton LL, Catania HF. Developing a comprehensive medication reaction reporting system. Hosp Pharm. 1995;30:384–5, 389–91, 394–6.
Szymusiak-Mutnick BA, Ross MB. Increased adverse drug reaction reporting through wall-mounted ADR reporting cards. Formulary. 1995;30:471–3.
Saltiel E, Johnson E, Shane R. A team approach to adverse drug reaction surveillance: success at a tertiary care hospital. Hosp Formul. 1995;30:226–8, 231–2.
Orsini MJ, Orsini PA, Thorn DB, Gallina JN. An ADR surveillance program: increasing quality, number of incidence reports. Formulary. 1995;30:454–61.
Sivaram CA, Johnson S, Tirmizi SN, Robertson V, Garcia D, Sorrells E. Morning report: a forum for reporting adverse drug reactions. Jt Comm J Qual Improv. 1996;22:259–63.
Welsh CH, Pedot R, Anderson RJ. Use of morning report to enhance adverse event detection. J Gen Intern Med. 1996;11:454–60.
McGettigan P, Golden J, Conroy RM, Arthur N, Feely J. Reporting of adverse drug reactions by hospital doctors and the response to intervention. Br J Clin Pharmacol. 1997;44:98–100.
Ball D, Tisócki T. Adverse drug reaction reporting by general medical practitioners and retail pharmacists in Harare: a pilot study. Cent Afr J Med. 1998;44:190–5.
Schlienger RG, Luscher TF, Schoenenberger RA, Haefeli WE. Academic detailing improves identification and reporting of adverse drug events. Pharm World Sci. 1999;21:110–5.
Clarkson A, Ingleby E, Choonara I, Bryan P, Arlett P. A novel scheme for the reporting of adverse drug reactions. Arch Dis Child. 2001;84:337–9.
Bäckström M, Mjörndal T, Dahlqvist R. Spontaneous reporting of adverse drug reactions by nurses. Pharmacoepidemiol Drug Saf. 2002;11:647–50.
Ryan M, Gora-Harper ML, Caldwell F. Experience with an adverse drug reaction reporting program in a neurology specialty clinic. Ann Pharmacother. 2002;36:231–5.
Aspinall MB, Whittle J, Aspinall SL, Maher RL Jr, Good CB. Improving adverse-drug-reaction reporting in ambulatory care clinics at a Veterans Affairs hospital. Am J Health Syst Pharm. 2002;59:841–5.
Rosebraugh CJ, Tsong Y, Zhou F, Chen M, Mackey AC, Flowers C, et al. Improving the quality of adverse drug reaction reporting by 4th-year medical students. Pharmacoepidemiol Drug Saf. 2003;12:97–101.
Castel JM, Figueras A, Pedrós C, Laporte JR, Capellà D. Stimulating adverse drug reaction reporting: effect of a drug safety bulletin and of including yellow cards in prescription pads. Drug Saf. 2003;26:1049–55.
Perkerson KA, Quercia RA, Goldman M, Goddu G, Coleman CI. Can a multidisciplinary initiative to increase adverse drug reaction reporting work? Conn Med. 2004;68:281–4.
Bracchi RC, Houghton J, Woods FJ, Thomas S, Smail SA, Routledge PA. A distance-learning programme in pharmacovigilance linked to educational credits is associated with improved reporting of suspected adverse drug reactions via the UK yellow card scheme. Br J Clin Pharmacol. 2005;60:221–3.
Bäckström M, Mjörndal T. A small economic inducement to stimulate increased reporting of adverse drug reactions: a way of dealing with an old problem? Eur J Clin Pharmacol. 2006;62:381–5.
Granas AG, Buajordet M, Stenberg-Nilsen H, Harg P, Horn AM. Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf. 2007;16:429–34.
Figueiras A, Herdeiro MT, Polónia J, Gestal-Otero JJ. An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA. 2006;296:1086–93.
Sullivan KM, Spooner LM. Adverse-drug-reaction reporting by pharmacy students in a teaching hospital. Am J Health Syst Pharm. 2008;65:1177–9.
Ortega A, Aguinagalde A, Lacasa C, Aquerreta I, Fernández-Benítez M, Fernández LM. Efficacy of an adverse drug reaction electronic reporting system integrated into a hospital information system. Ann Pharmacother. 2008;42:1491–6.
Herdeiro MT, Polónia J, Gestal-Otero JJ, Figueiras A. Improving the reporting of adverse drug reactions: a cluster-randomized trial among pharmacists in Portugal. Drug Saf. 2008;31:335–44.
Tabali M, Jeschke E, Bockelbrink A, Witt CM, Willich SN, Ostermann T, Matthes H. Educational intervention to improve physician reporting of adverse drug reactions (ADRs) in a primary care setting in complementary and alternative medicine. BMC Public Health. 2009;9:274.
Johansson ML, Brunlöf G, Edward C, Wallerstedt SM. Effects of e-mails containing ADR information and a current case report on ADR reporting rate and quality of reports. Eur J Clin Pharmacol. 2009;65:511–4.
Pedrós C, Vallano A, Cereza G, Mendoza-Aran G, Agustí A, Aguilera C, et al. An intervention to improve spontaneous adverse drug reaction reporting by hospital physicians: a time series analysis in Spain. Drug Saf. 2009;32:77–83.
Anton C, Cox AR, Ferner RE. Improving follow-up rates in spontaneous adverse drug reaction reporting: effectiveness of a targeted letter used by a regional centre in the UK. Drug Saf. 2009;32:1135–40.
Sevene E, Mariano A, Mehta U, Machai M, Dodoo A, Vilardell D, Patel S, et al. Spontaneous adverse drug reaction reporting in rural districts of Mozambique. Drug Saf. 2008;31:867–76.
Gross R, Strom BL. Toward improved adverse event/suspected adverse drug reaction reporting. Pharmacoepidemiol Drug Saf. 2003;12:89–91.
Improving ADR reporting. Lancet. 2002;360:1435.
McCloskey BA. Improving adverse drug reaction reporting in the community setting. Med Interface. 1996;9:85–7.
Schiff GD. Using a computerized discharge summary data base check box for adverse drug reaction monitoring. QRB Qual Rev Bull. 1990;16:149–55.
Damiani DR, Swanson DP. Pharmacist-managed monitoring of adverse reactions to contrast media. Am J Hosp Pharm. 1994;51:358–63.
Schlienger RG. Management of adverse drug effects. Ther Umsch. 2000;57:584–90.
Gony M, Badie K, Sommet A, Jacquot J, Baudrin D, Gauthier P, et al. Improving adverse drug reaction reporting in hospitals. Drug Saf. 2010;33:409–16.
Valente SM. Improving professional practice through certification. J Nurses Staff Dev. 2010;26:215–9.
Kabanywanyi AM, Mulure N, Migoha C, Malila A, Lengeler C, Schlienger R, Genton B. Experience of safety monitoring in the context of a prospective observational study of artemether–lumefantrine in rural Tanzania: lessons learned for pharmacovigilance reporting. Malaria J. 2010;9:205.
Cereza G, Agustí A, Pedrós C, Vallano A, Aguilera C, Danés I, et al. Effect of an intervention on the features of adverse drug reactions spontaneously reported in a hospital. Eur J Clin Pharmacol. 2010;66:937–45.
Smith Rogers A. Strategies to promote physician reporting of adverse drug events based on the experience of an FDA-sponsored project in Maryland. J Clin Res Drug Dev. 1989;3(1):29–37.
Rosenbaum SE, Thacher-Renshaw A, Green M, Waters WJ Jr. Interventions to increase physician participation in a voluntary reporting system: the Rhode Island adverse drug reaction reporting project. Clin Res Reg Aff. 1992;9(4):261–75.
Sharkey CC, Alessandro-Battaglia R, Matuszewski J, Peker S, Lovly RM. A seven-step approach to improving ADR-reporting rates at a Department of Veterans Affairs medical center. Pharm Ther. 1999;24(7):327–31.
Colodny L, Spillane J. Toward increased reporting of adverse drug reactions. Hosp Pharm. 1999;34(10):1179–85.
Vaz I, Herdeiro MT, Figueiras A, Polonia J. Strategies for increasing spontaneous adverse drug reaction reporting rates among Portuguese health professionals. 9th ISoP Annual Meeting. From Pharmacovigilance to Risk Management™; Reims; 2009 Oct 6–9. Drug Saf. 2009;32(10):878–9.
Prakongsai N, Pongchaidecha M. Using collaborative program between pharmacists and nurses to improve spontaneous adverse drug reaction reporting system in Thailand. ISPOR; 4th Asia-Pacific Conference; Phuket. 2010 Sep 5–7. Value Health. 2010;13(7):A537.
Yen YH, Kuo LN, Hsu MH, Li YC, Cheng KJ. Evaluation of the electronic adverse drug event management system. J Exp Clin Med. 2010;2(6):287–91.
Gardner P, Watson LJ. Adverse drug reactions: a pharmacist-based monitoring system. Clin Pharmacol Ther. 1970;11:802–7.
Kilarski DJ, Xiegler B, Coarse J, Buchanan C. Adverse drug reaction reporting system: developing a well-monitored program. Hosp Formul. 1986;21:949–52.
Lee SB, Schepers GP, Goldberg KL. Electronic adverse-drug-reaction-reporting program. Am J Health Syst Pharm. 2004;61:1230, 1232–3.
Wallerstedt SM, Brunlof G, Johansson ML, Tukukino C, Ny L. Reporting of adverse drug reactions may be influenced by feedback to the reporting doctor. Eur J Clin Pharmacol. 2007;63:505–8.
Jacinto MS, Kleinmann K. Hospital pharmacy program for reporting adverse drug reactions. Am J Hosp Pharm. 1983;40:444–5.
Fincham J. A statewide program to stimulate reporting of adverse drug reactions. J Pharm Pract. 1989;2:239–44.
Griffin JP. Is better feedback a major stimulus to spontaneous adverse reaction monitoring? Lancet. 1984;2(8411):1098.
O’Connor P, Moriarty JM, Feely J. Adverse drug reactions: incentives to enhance reporting. Br J Clin Pharmacol. 1988;26:616.
Poorolajal J, Cheraghi Z, Irani AD, Rezaeian S. Quality of Cohort Studies Reporting Post the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Epidemiol Health. 2011;33:e2011005.
Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–91.
Figueiras A, Sastre I, Gestal-Otero JJ. Effectiveness of educational interventions on the improvement of drug prescription in primary care: a critical literature review. J Eval Clin Pract. 2001;7:223–41.
Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, Robertson N. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2010;(3):CD005470.
Bokhoven Van, Kok G, Van der Weijden T. Designing a quality improvement intervention: a systematic approach. Qual Saf Health Care. 2003;12:215–20.
Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, O’Brien MA, Wolf F, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009;(2):CD003030.
Boaz A, Baeza J, Fraser A, European Implementation Score Collaborative Group. Effective implementation of research into practice: an overview of systematic reviews of the health literature. BMC Res Notes. 2011;4:212.
Acknowledgments
The authors wish to express their sincere thanks to Michael Benedict for reviewing and revising the English.
Funding
This project was partly funded by Health Research Fund (Fondo de Investigación Sanitaria) grants PI081239 and PI09/90609 from the Spanish Ministry of Health, and the Mutua Madrileña insurance company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzalez-Gonzalez, C., Lopez-Gonzalez, E., Herdeiro, M.T. et al. Strategies to Improve Adverse Drug Reaction Reporting: A Critical and Systematic Review. Drug Saf 36, 317–328 (2013). https://doi.org/10.1007/s40264-013-0058-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-013-0058-2