Abstract
The influence of arc energy and filler metal composition on the microstructure of additively welded thin-walled structures of duplex stainless steels was investigated using different commercially available standard and superduplex solid wire electrodes commonly used today. As welding process, the cold metal transfer (CMT) process was used. The arc energy and cooling rate were varied by adjusting the wire feed and welding speed. Optical emission spectroscopy (OES) and carrier gas melt extraction (CGME) were used to determine the chemical composition of the specimens. The ferrite content was determined both by magnetic induction and by image analysis as a function of the wall height. In addition, the microsections were examined for intermetallic phases and precipitations. Moreover, corrosion tests were carried out according to ASTM G 48, Method A. The results indicate that an increase in arc energy leads to longer t12/8 cooling times. Depending on the filler metal composition, this leads to ferrite contents that are partially outside the values required according to ISO 17781. Furthermore, precipitates of secondary austenite are often found, which is attributed to the multiple reheating by the subsequent layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Currently, wire arc additive manufacturing (WAAM) is gaining enormous importance. Especially in the processing of high-alloyed, expensive materials, the production of near net shape objects by means of layer-by-layer deposition welding offers considerable advantages, e.g., the ability to produce complex geometric shape elements with a high degree of material utilization [1,2,3]. However, a challenge in additive arc welding is the relatively high heat input coupled with relatively low heat dissipation. This can lead to residual stresses and distortion as well as to changes in the microstructure and crack formation [4, 5]. This becomes particularly critical in the processing of materials that already require demanding handling from a welding point of view. Especially, the welding of duplex stainless steels requires an adjusted heat control to achieve material-specific properties in the weld metal. Current recommendations for welding duplex stainless steels focus on achieving a precipitation-free weld metal microstructure with balanced proportions of ferrite and austenite during joint welding and cladding [6, 7]. However, the application of these recommendations regarding arc energy and filler metals result in a predominantly austenitic microstructure in WAAM of duplex stainless steel components [8]. Reasons for the excessive precipitation of austenite, especially in thin-walled structures, are the increasing cooling time with increasing wall height as well as the repeated dwell time in the temperature range of 1200–800 °C when welding the subsequent layers [9, 10].
Since there are no reliable recommendations for the WAAM of duplex stainless steels, the objective of this study was to evaluate the influence of arc energy and filler metal composition on the microstructure of additively welded thin-walled structures of duplex stainless steels. The focus was not only on the ferrite-austenite ratio itself but also on the potential precipitation of secondary phases, such as secondary austenite.
2 Experimental
2.1 Filler metals and shielding gases
Filler metals for this study included different commercially available standard and superduplex solid wire electrodes with a diameter of 1.2 mm. The wire batch of the G Z 22 8 3 L Si was also provided with a diameter of 1.0 mm. Table 1 shows the chemical composition of the filler metals that was determined on button melts produced in an arc melter under pure argon atmosphere (99.996 %).
Based on the chemical composition of the filler metals, the expected ferrite number (FN) as a measure of the ferrite content can be estimated using the WRC-1992 diagram [11]. Table 2 shows the chromium (Eq. 1) and nickel (Eq. 2) equivalents calculated for the filler metals, which can be used to determine the FN in the WRC-1992 diagram:
Shielding gases for the additive CMT welds included an active gas mixture (Ar + 30 % He + 2 % CO2) that was used for the standard duplex wires as well as an inert gas mixture (Ar + 30 % He) that was selected for the superduplex wires, since previous investigations by Stützer et al. [12] have shown that even very low amounts of CO2 in the shielding gas can lead to pores in superduplex weld metal.
2.2 WAAM setup
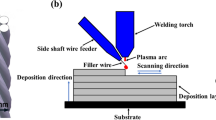
The layer-by-layer technique in combination with the GMAW-CMT process was used to produce wall structures of 20 layers with a length of 200 mm (Fig. 1). The deposition welds were performed with a neutral torch position, a contact tip to work distance of 12 mm, and a shielding gas flow rate of 18 l/min. The single layers were welded in PA (1G) position with alternating welding direction.
To evaluate the influence of the arc energy, the wire feed speed (i.e., welding current) and the travel speed were varied in the ranges given in Table 3. During the production of the wall structures, the welding parameters for the respective walls were kept constant over all layers. The welding parameters were recorded and evaluated by means of the internal documentation function of the welding power source. An external data logging system was also used for verification. The t12/8 cooling time was measured for each layer using a pyrometer. In addition, thermocouples were attached between the 3rd and 4th layer after welding the 4th layer and between the 10th and 11th layer after welding the 11th layer to record the temperature cycles for determining the dwell times in the temperature ranges that are relevant for adverse precipitations.
2.3 Characterization methods
The geometries of the welded wall structures were measured regarding their thickness and height. The ferrite content was determined in the bottom, middle, and top of the walls on cross-sections both by magnetic induction using a Fischer Feritscope® and by image analysis using micrographs. In addition, the microstructure was analyzed with regard to the occurrence of precipitations and inclusions. For the determination of the ferrite-austenite ratio by image analysis, the samples were etched using Beraha II to obtain sufficiently high contrasts. Oxalic acid and NaOH were used in addition to examine the presence of precipitations. Optical emission spectroscopy (OES) and carrier gas melt extraction (CGME) were used to determine the chemical composition of the walls and to evaluate pickup or burning loss of alloying elements. To prove the corrosion resistance of the walls, the ferric chloride pitting test was carried out according to ASTM G48, Method A [13]. Before testing, all surfaces of the specimens were milled and then polished using 120-grit abrasive paper. In accordance with ISO17781:2017 [14], the corrosion test was carried out in as-welded condition at a test temperature of 22 °C for the standard duplex specimens and 35 °C for the superduplex specimens. The acceptance criterion is a weight loss < 4 g/m2.
3 Results and discussion
3.1 Effect of welding parameters on wall geometry
For the experiments on the influence of the arc energy, both the wire feed speed (vWFS) and the travel speed (vTS) were varied in several steps. Since the control characteristic of the welding power source has sometimes led to considerable deviations (up to 0.7 m/min) between set and real wire feed speed, the measured mean values are used in this paper. As can be seen in Fig. 2a, both the travel speed and the wire feed speed directly influence the geometry of the walls. With constant travel speed and increasing wire feed speed (i.e., increasing arc energy), the wall thickness and height increase due to the increasing deposition rate. The change in travel speed also influences the wall thickness as well as the wall height at constant wire feed speed (i.e., constant deposition rate). Both wall thickness and height decrease with increasing travel speed (i.e., decreasing arc energy). This allows varying the wall thickness and height via the vWFS/vTS ratio at constant deposition rates. Thus, a specific adjustment of the wall thickness of WAAM components is possible by selecting the welding parameters. However, an increase of the seam cross-section is always coupled with an increase of the arc energy and thus with a higher heat input, which can have a negative effect on the ferrite-austenite ratio. Figure 2b compares the cross-sections of different walls. As can be seen here, not only the seam cross-section increases with increasing arc energy but also the waviness of the specimen surface increases, which can lead to major post-processing depending on the requirements of the final component.
3.2 Effect of arc energy on t 12/8 cooling time and temperature cycle
By increasing the arc energy, the seam cross-section can be increased, and thus, the number of necessary beads for the required final geometry can be reduced. However, this is coupled with an increased heat input, which results in slower cooling rates. The correlation between arc energy and t12/8 cooling time is shown in Fig. 3a as an example for different arc energies. On the one hand, the results show that an increase in arc energy leads to significantly higher t12/8 cooling times in the respective layers. On the other hand, it can be seen that the t12/8 cooling times stabilize on a certain level from about the 4th–5th layer. This indicates that above this layer height, there is only a two-dimensional heat dissipation (along and perpendicular to the welding direction), and the base material is of minor importance for heat dissipation. This leads to significantly longer cooling times compared with joint welding, as can be seen from the reference lines. These were determined at the research facility during joint welding of superduplex stainless steel using pulsed arc. The t12/8 cooling times ranged between 1.8 and 3.2 s, and the arc energy was 0.7–0.8 kJ/mm. Due to the surrounding base material and filler beads, the heat is dissipated much faster despite higher arc energies. This relationship is shown schematically in Fig. 3b.
A similar effect can be observed with the dwell times in high temperature ranges. Due to the welding of the next layers, the lower layers are repeatedly heated and pass through temperature ranges where critical phase precipitations, such as sigma phase (σ), secondary austenite (γ2), carbides, or nitrides, can form (Fig. 4). With increasing arc energy, the peak temperatures and dwell times in the reheated layers also increase. This indicates an elevated risk of adverse phase precipitations with increasing arc energy.
3.3 Effect of arc energy on microstructure
The metallographic investigations show that the ferrite-austenite ratio is partly subject to strong fluctuations. Thus, it may happen that there is much less austenite at the fusion lines between the beads than within the beads, which is due to reheating by the subsequent layers (Fig. 5). These inhomogeneities in the microstructure complicate the determination of the ferrite content using image analysis. Image analysis requires images with sufficiently high magnification and resolution to allow a proper separation of the microstructures. Because there are partly significant differences in the microstructure proportions in neighboring areas, the result strongly depends on where and how many images are taken by the operator. In the example in Fig. 5, there is a difference in the ferrite content of 20 %. In order to reduce the risk of biased results due to these influences, the images for image analysis and ferrite content determination were taken using a digital microscope. This enables panoramic images in × 500 magnification with up to 100 stitched single images. Thus, a large region of interest can be analyzed at once, and a reliable mean value for the ferrite content can be determined (Fig. 6). This was done in three different positions in the cross-section (bottom, middle, top) and at two cross-sections for each wall.
The results of the ferrite determination by Feritscope® (in FN) and image analysis (in %) as a function of arc energy and area of analysis are shown in Fig. 7 using the example of the G 22 9 3 N L and G Z 22 8 3 L Si. As can be seen, the FN and the ferrite percent show basically the same tendencies with regard to the influence of the arc energy. An increase in the arc energy obviously leads to lower ferrite contents. This is due to the cooling times and temperature cycles described above, which are significantly influenced by the arc energy. When looking at the results with regard to the area of analysis, it is obvious that the FN and the ferrite percent also show mostly the same tendencies, but in some cases, there are contrary tendencies. However, the majority of the results indicate that the ferrite content is higher in the upper layers than in the middle and lower layers. One possible reason for this is that the 18th–20th layers were included in the analysis of the upper layers. The 19th layer experienced only one and the 20th layer no reheating by subsequent layers into temperature ranges that are relevant for austenite precipitation, so that the ferrite content here is higher than in the layers below. Furthermore, it is evident that ferrite percent contents may be obtained which do not meet the requirements of ISO17781 [14]. For example, in the case of the G 22 9 3 N L, it is to be expected that unacceptably high austenite contents will occur at arc energies above 0.3 kJ/mm. In contrast to that, the G Z 22 8 3 L Si seems to provide an acceptable ferrite-austenite ratio over a wide range of different arc energies.
3.4 Effect of filler metal composition on microstructure
To determine the influence of the filler metal composition on the ferrite-austenite ratio, walls were produced with different filler metals (cf. Table 1) but same welding parameters. Due to the previously described control characteristic of the welding power source, the actual values of the different walls differ slightly, but the arc energies are at a comparable level. Figure 8 shows the influence of the filler metal composition on the ferrite content (in FN and %). As can be seen, the FN and the ferrite percent basically reflect the same trends with regard to the influence of the filler metal composition. For example, it is apparent that the highest ferrite contents in the wall structures can be achieved with the nickel-reduced G Z 22 5 3 L and the higher chromium-alloyed G Z 29 8 2 L. In the case of the G Z 22 5 3 L, the ferrite content may even become unacceptably high. In contrast, significantly lower ferrite contents are achieved with the standard duplex filler metal G 22 9 3 N L.
In principle, the influence of the filler metal composition can also be estimated using the WRC-1992 diagram. However, this requires knowledge of the exact chemical composition of the weld metal. Otherwise, relatively strong deviations may occur, as illustrated in Fig. 9. Here, the measured FN is compared with the pre-calculated FN (based on the chemical composition of the filler metals) and the re-calculated FN (based on the chemical composition of the weld metal). A major reason for the deviations between pre-calculated and measured FN is the pickup and burning loss of alloying elements during welding. For example, a significant pickup of nitrogen (+ 516 ppm) was detected in the wall of G Z 22 5 3 L (Table 4). Furthermore, a slight to moderate burning loss of chromium was determined for all filler metals. Since these elements are included in the calculation of the chromium and nickel equivalents (cf. Eqs. 1 and 2), the prediction is subject to inaccuracies. However, if the chemical compositions of the walls are then used to predict the FN values (re-calculated FN), taking into account the pickups and burning losses, it can be seen that the predicted and measured FN values are very close to each other.
The metallographic examination with regard to the presence of unwanted phase precipitations shows that secondary austenite (γ2) is often formed. A specific quantification of the γ2 contents is not possible on the basis of the micrographs. However, from a qualitative point of view, the quantity of γ2 depends mainly on the filler metal composition and to a minor extent on the arc energy. γ2 was found in all specimens, with the exception of the walls made of G Z 25 10 4 L and G Z 29 8 2 L, in which at most, a beginning γ2 formation at very few spots can be suspected. Sporadic indications of γ2 were identified in the specimens of G Z 22 5 3 L, while larger amounts of γ2 were detected in the specimens of G 25 9 4 N L and G 22 9 3 N L (Fig. 10). A particularly strong γ2 formation was found in the WAAM walls of G Z 22 8 3 L Si (Fig. 10). A possible reason for the strong γ2 formation in the G Z 22 8 3 L Si is the increased silicon content compared with the other filler metals (cf. Table 1). When comparing the specimens of G Z 25 10 4 L and G 25 9 4 N L, there is a significant difference in the tungsten content. While the G Z 25 10 4 L does not contain any quantifiable tungsten, the more γ2 susceptible G 25 9 4 N L contains significantly more tungsten with 0.53 wt.%. Both silicon and tungsten shift the precipitation of γ2 to shorter times [16]. In the last welded layer (20th layer), no γ2 was found in any of the WAAM walls, which suggests that γ2 is in fact only caused by the reheating from the subsequent layers.
In the wall structures of G Z 22 5 3 L, fine nitride precipitations were also found in addition to γ2. This is because of the low solubility of nitrogen in the ferrite in combination with the high ferrite content in the wall of the G Z 22 5 3 L. Thus, the excess nitrogen cannot diffuse into the austenite [17]. Evidence of intermetallic phases, such as σ phase, could not be found in any of the walls, which is in good agreement with simulations made by Hosseini et al. [15].
3.5 Corrosion behavior
In the literature, both secondary austenite and nitrides are often described as detrimental with regard to corrosion resistance [18,19,20]. However, in the present investigations, no significant corrosion phenomena can be detected under the required test conditions with the ferric chloride pitting test according to ASTM G48, Method A. As the results in Fig. 11 show, there is no significant weight loss in any of the specimens. There is also no visual evidence of pitting.
4 Conclusions
The influence of arc energy and filler metal composition on the microstructure of additively welded thin-walled structures of duplex stainless steels was investigated. The arc energy was varied between 0.09 and 0.66 kJ/mm using the CMT process. Three different standard duplex and three different superduplex filler metals were used to evaluate the influence of the chemical composition. The following conclusions can be drawn:
-
1
The wall thickness of WAAM components can be varied via the vWFS/vTS ratio when using GMAW processes. Thus, an increase in the seam cross-section leads to an increase in arc energy.
-
2
The t12/8 cooling times and dwell times in high temperature ranges increase significantly with an increase in arc energy. Furthermore, the cooling time extends with increasing wall height (number of layers) due to the changed heat dissipation. After about the 5th layer, the t12/8 time stabilizes at a certain level that depends on the arc energy.
-
3
The ferrite content tends to decrease with increasing arc energy. At high arc energies, the filler metals optimized for joint welding can result in unacceptably high austenite contents. However, an approximate prediction of the FN can be made on the basis of the WRC-1992 diagram if the final chemical composition of the WAAM walls is known with sufficient accuracy, considering pickups and burning losses of alloying elements.
-
4
In addition to unacceptable ferrite-austenite ratios, undesirable phase precipitations can also occur. Among the tested filler metal compositions, secondary austenite and partly also nitrides are to be expected in particular. Other detrimental phase precipitations were not found.
-
5
The corrosion tests carried out in accordance with the requirements of ISO 17781 and ASTMG48, Method A did not show any degradation of the corrosion resistance under the required test conditions.
-
6
The results of the superduplex wires of the type G Z 25 10 4 L and G Z 2 9 8 2 L indicate a potential suitability for WAAM due to the achieved ferrite-austenite ratio and the extensive absence of secondary austenite. In the case of standard duplex wires, an adjustment of the alloy composition seems to be necessary to reduce the risk of γ2 formation on the one hand and to enable a balanced ferrite-austenite ratio over a wide range of arc energies on the other hand. The authors recommend the testing of an adapted alloying concept based on the G 22 9 3 N L with the nominal composition: Cr ≈ 23.0 %, Ni ≈ 7.5 %, Mo ≈ 3.1 %, Mn ≈ 1.6 %, Si ≤ 0.4 %, Cu ≤ 0.1 %, W ≤ 0.01 %, N ≈ 0.16 % (values in wt.%).
References
Allen J (2006) An investigation into the comparative costs of additive manufacture vs. machine from solid for aero engine parts. Cost Effective Manufacture via Net-Shape Processing: 17-1–17-10.
Gebhardt A (2013) Generative Fertigungsverfahren [Generative manufacturing processes]. Carl Hanser Verlag, München
Lachmayer R, Lippert RB, Fahlbusch T (2016) 3D-Druck beleuchtet [3D printing illuminated]. Springer Vieweg, Berlin Heidelberg. https://doi.org/10.1007/978-3-662-49056-3
Ding J, Colegrove P, Mehnen J, Ganguly S, Sequeira Almeida PM, Wang F, Williams S (2011) Thermo-mechanical analysis of wire and arc additive layer manufacturing process on large multi-layer parts. Comput Mater Sci 50:3315–3322. https://doi.org/10.1016/j.commatsci.2011.06.023
Colegrove PA, Coules HE, Fairman J, Martina F, Kashoob T, Mamash H, Cozzolino LD (2013) Microstructure and residual stress improvement in wire and arc additively manufactured parts through high-pressure rolling. J Mater Process Technol 213:1782–1791. https://doi.org/10.1016/j.jmatprotec.2013.04.012
Messer B, Oprea V, Wright A (2007) Duplex stainless steel welding: best practices. Stainless Steel World. Fluor Canada Ltd., Canada, pp 53–63
Karlsson L (2012) Welding duplex stainless steels — a review of current recommendations. Weld World 56:65–76. https://doi.org/10.1007/BF03321351
Posch G, Chladil K, Chladil H (2017) Material properties of CMT — metal additive manufactured duplex stainless steel blade-like geometries. Weld World 61:873–882. https://doi.org/10.1007/s40194-017-0474-5
Hoefer K, Haelsig A, Mayr P (2018) Arc-based additive manufacturing of steel components—comparison of wire- and powder-based variants. Weld World 62:243–247. https://doi.org/10.1007/s40194-017-0527-9
Stützer J, Zinke M, Jüttner S, Findeklee P (2018) Additive Fertigung von Bauteilen aus Duplexstahl durch Schutzgasschweißen [Additive manufacturing of components made of duplex stainless steel by gas shielded arc welding]. Proceedings of the DGM-Conference: Materials and Additive Manufacturing: 103–108.
Kotecki DJ, Siewert TA (1992) WRC-1992 constitution diagram for stainless steel weld metals: a modification of the WRC-1988 diagram. Weld J 71:171–178
Stützer J, Zinke M, Jüttner S (2017) Studies on the pore formation in super duplex stainless steel welds. Weld World 61:351–359. https://doi.org/10.1007/s40194-016-0409-6
ASTM G48-11 (2015) Standard test methods for pitting and crevice corrosion resistance of stainless steels and related alloys by use of ferric chloride solution. ASTM International, West Conshohocken
ISO 17781 (2017) Petroleum, petrochemical and natural gas industries – test methods for quality control of microstructure of ferritic/austenitic (duplex) stainless steels. Beuth, Berlin
Hosseini VA, Karlsson L, Engelberg D, Wessman S (2018) Time-temperature-precipitation and property diagrams for super duplex stainless steel weld metals. Weld World 62:517–533. https://doi.org/10.1007/s40194-018-0548-z
Lippold JC, Kotecki DJ (2005) Welding metallurgy and weldability of stainless steels. John Wiley & Sons, Inc., Hoboken
Knyazeva M, Pohl M (2013) Duplex steels. Part II: carbides and nitrides. Metallogr Microstruct Anal 2:343–351. https://doi.org/10.1007/s13632-013-0088-2
Nilsson J-O, Karlsson L, Andersson J-O (1995) Secondary austenite formation and its relation to pitting corrosion in duplex stainless steel weld metal. Mater Sci Technol 11:276–283. https://doi.org/10.1179/mst.1995.11.3.276
Ramirez AJ, Brandi SD, Lippold JC (2004) Secondary austenite and chromium nitride precipitation in simulated heat affected zones of duplex stainless steels. Sci Technol Weld Join 9:301–313. https://doi.org/10.1179/136217104225021715
Nilsson JO, Wilson A (1993) Influence of isothermal phase transformations on toughness and pitting corrosion of super duplex stainless steel SAF 2507. Mater Sci Technol 9:545–554. https://doi.org/10.1179/mst.1993.9.7.545
Acknowledgments
Open Access funding enabled and organized by Projekt DEAL. The present contribution is a part of the AiF project IGF-No. 20.361B/DVS-No. 01.3060 of the German Research Association on Welding and Allied Processes (DVS). It was kindly supported by the AiF (German Federation of Industrial Research Associations) within the program for promoting the Industrial Collective Research (IGF) of the Federal Ministry for Economic Affairs and Energy (BMWi), based on a decision by the German Bundestag. Sincere thanks are given for this support and to the representing companies actively involved in the project committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Recommended for publication by Commission II - Arc Welding and Filler Metals
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wittig, B., Zinke, M. & Jüttner, S. Influence of arc energy and filler metal composition on the microstructure in wire arc additive manufacturing of duplex stainless steels. Weld World 65, 47–56 (2021). https://doi.org/10.1007/s40194-020-00995-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-020-00995-z