Abstract
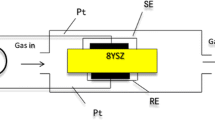
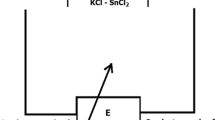
To quantify the oxygen content in molten salts, we examined the performance of an yttria-stabilized zirconia solid electrolyte oxygen sensor with a Bi/Bi2O3 reference electrode, focusing on its output accuracy. When the above sensor was tested in a flow of gas with known oxygen partial pressure, \(p_{O_2}\), a linear relationship between \(lgp_{O_2}\) and the electromotive force (EMF) was observed, and the correlation slope exhibited a positive deviation from Nernstian behavior. EMF measurements performed in molten NaCl–KCl indicated that the oxygen content of this salt mixture increased with increasing oxygen partial pressure in the covering gas, in agreement with Henry’s law. Moreover, the EMF exhibited a linear decrease with increasing melt temperature of molten NaCl–KCl, in agreement with the theoretical model. Finally, a relationship between the structure of molten NaCl–KCl and its oxygen diffusion behavior was established. As a result, the developed sensor was demonstrated to be well suited for determining the oxygen content of molten salts.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Serrano-López R, Fradera J, Cuesta-López S. Molten salts database for energy applications. Chem Eng Process 2013, 73: 87–102.
Liu Q, Bai Z, Sun J, et al. Thermodynamics investigation of a solar power system integrated oil and molten salt as heat transfer fluids. Appl Therm Eng 2016, 93: 967–977.
Wang W, Li H, Guo Y, et al. Mullite whiskers prepared by molten salt method using Si powders. J Adv Ceram 2012, 1: 283–289.
Herrmann U, Kelly B, Price H. Two-tank molten salt storage for parabolic trough solar power plants. Energy 2004, 29: 883–893.
García López J, Respaldiza MA, Siejka J, et al. Use of nuclear microanalysis to study the oxygenation mechanism of Y1Ba2Cu3O7-x thin films: Estimation of the oxygen diffusion coefficients. Nucl Instrum Meth B 2002, 190: 661–666.
Cho Y-Z, Yang H-C, Park G-H, et al. Treatment of a waste salt delivered from an electrorefining process by an oxidative precipitation of the rare earth elements. J Nucl Mater 2009, 384: 256–261.
Nunes VMB, Lourenco MJV, Santos FJV, et al. Importance of the accurate data on viscosity and thermal conductivity in molten salts applications. J Chem Eng Data 2003, 48: 446–450.
Frangini S, Scaccia S. The role of foreign cations in enhancing the oxygen solubility properties of alkali molten carbonate systems: Brief survey of existing data and new research results. Int J Hydrogen Energ 2014, 39: 12266–12272.
Imanaka N, Masui T, Jyoko K. Selective liquid phase oxidation of cyclohexane over Pt/CeO2–ZrO2–SnO2/SiO2 catalysts with molecular oxygen. J Adv Ceram 2015, 4: 111–117.
Gao X, Liu T, Yu J, et al. Limiting current oxygen sensor based on La0.8Sr0.2Ga0.8Mg0.2O3−δ as both dense diffusion barrier and solid electrolyte. Ceram Int 2017, 43: 6329–6332.
Lovering DG. Molten Salt Technology. NY: Plenum Press, 1982.
Papatheodorou GN. Molten salts for two centuries. Molten Salts Bull 2005, 83: 1–6.
Gaune-Escard M. Molten Salts: From Fundamentals to Applications. Dordrecht: Kluwer Acad. Publ., 2002.
Volkovich VA, Griffiths TR, Fray DJ, et al. Oxidation of ceramic uranium dioxide in alkali metal carbonate-based melts: A study using various oxidants and comparison with UO2 powder. J Nucl Mater 1998, 256: 131–138.
Kearney D, Kelly B, Herrmann U, et al. Engineering aspects of a molten salt heat transfer fluid in a trough solar field. Energy 2004, 29: 861–870.
Menéndez RP, Martínez JÁ, Prieto MJ, et al. A novel modeling of molten-salt heat storage systems in thermal solar power plants. Energies 2014, 7: 6721–6740.
Volkovic VA, Griffiths TR, Fray DJ, et al. A new method for determining oxygen solubility in molten carbonates and carbonate–chloride mixtures using the oxidation of UO2 to uranate reaction. J Nucl Mater 2000, 282: 152–158.
Phongikaroon S, Bezzant RW, Simpson MF. Measurements and analysis of oxygen bubble distributions in LiCl–KCl molten salt. Chem Eng Res Des 2013, 91: 418–425.
Acknowledgements
The author thanks the Shanghai Institute of Ceramics and Chinese Academy of Sciences for support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access The articles published in this journal are distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zheng, H., Nian, H., Xia, J. et al. Bi/Bi2O3 sensor for quantitation of dissolved oxygen in molten salts. J Adv Ceram 7, 1–4 (2018). https://doi.org/10.1007/s40145-017-0250-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-017-0250-4