Abstract
Purpose of Review
Soft tissue sarcomas (STS) are relatively rare cancers, commonly occurring in the extremities. Over the last decades a shift in the treatment of extremity STS has occurred, from limb amputations toward so-called limb salvage surgery (LSS). This review provides an overview of the current surgical treatment options for lower extremity STS and how the role of reconstructive surgery may evolve in the coming years toward optimizing functional outcomes and improving the quality of life in these patients.
Recent Findings
Recent research has shown that having a low threshold for advanced reconstructive techniques, with the goal of bringing well-vascularized tissue to the defect, may lower the wound complication rates, especially in preoperatively irradiated tumors. Careful preoperative planning should not only include an optimal balance between complete surgical resection and preservation of vital structures, but also the possibilities of restoring anticipated loss of function. Such functional reconstructions may include the use of free functional muscle transfers but also tendon transfers and any type of nerve reconstruction. A more recent development is lymphatic surgery for STS patients to prevent or treat lymphorrhea and lymphedema. Future prospective studies should further study the indications, the timing of the surgery, and measure the outcomes of the lymphatic surgical techniques to further gain insights into the efficacy.
Summary
Novel techniques such as functional reconstructions, nerve transfers, and lymphatic surgery are within the realms of reconstructive options and therefore, it is important that patients with a STS are taken care of in centers that have a multidisciplinary team with a reconstructive surgeon as a member.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas (STS) are relatively rare cancers, commonly occurring in the extremities [1]. They often present as aggressive tumors and may harbor dismal prognosis. Over the last decades a shift was made in the treatment of extremity STS, from limb amputations toward so-called limb salvage surgery (LSS). LSS is currently the standard of care whenever possible, often combined with the use of radiotherapy to decrease local recurrence rates [2]. The use of preoperative irradiation in treating STS is on the rise, despite showing equivalent oncological outcomes, due to fact that the radiation field can be smaller and the total radiation dose lower [3•]. Although preoperative radiotherapy is associated with a higher incidence of wound complications, the lower total radiation dose does lead to a lower incidence of physical impairment and secondary fibrosis [3•]. Recent research has shown that having a low threshold for advanced reconstructive techniques, with the goal of bringing well-vascularized tissue to the defect, may lower the wound complication rates, especially in preoperatively irradiated tumors [4•].
Most surgical oncologists advocate for microscopically complete tumor resections (R0) in any STS. In both upper- and lower extremity tumor sites this may pose difficulties, as tumors are often near critical structures, such as neurovascular bundles, bone, or muscles/tendons. The preservation of these structures may call for close margin resections, including planned microscopically positive margins (R1), and is therefore often combined with the use of radiotherapy. In some cases, however, the resection of functional structures is inevitable due to tumor size, complete encasement of a vital structure, or simply because the tumor originates from it like in malignant peripheral nerve sheath tumors (MPNST). Reconstruction of these structures are called functional reconstructions and is a more common practice in traumatic cases yielding good functional outcomes [5•].
Besides the aim of reconstructive surgery for (preservation of) a functional lower limb, other reconstructive procedures may aid the functional outcome of the lower extremity in terms of health-related quality of life [6]. Such procedures include lymphaticovenous anastomosis (LVA) for lower limb lymphedema and free vascularized lymph node flap transfer (VLNT).
As STS patients are generally young at time of diagnosis (mean age < 50), and treatment options are slowly improving (even for high-grade tumors), the number of long-term survivors with serious disabilities will increase. This review will give an overview of the current surgical treatment options for lower extremity STS and how the role of reconstructive surgery may evolve in the coming years toward optimizing functional outcomes and improving the quality of life in this patient group.
Evolution of Treatment in Soft Tissue Sarcoma
Data (1998–2012) from a nationwide cancer registry (US) showed that of all STS, the lower extremity is most frequently affected (74.7%). Although limb salvage is achieved in 80–90% of cases, amputations are still a frequent necessity (5–7%, historically 40%) [7, 8]. Amputations are more likely to occur in adolescents/young adults (compared to children of adults), in advanced disease (grade III or grade IV), or in high-volume centers (compared to low volume), and in academic centers (compared to community hospitals) [9]. Interestingly, patients with the highest income and treated at community centers were significantly less likely to receive an amputation [9]. Since surgical multidisciplinary teams nowadays aspire to LSS treatments and divert from amputations and disarticulations, reconstructive techniques are becoming more important [10]. Moreover, (neo)adjuvant therapies such as chemotherapy and radiotherapy inadvertently come with downsides affecting the surgical site, thereby making the involvement of reconstructive surgeons early in the process even more important and necessary as part of multidisciplinary care.
Radiotherapy
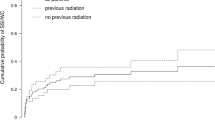
The rise of neoadjuvant radiotherapy is associated with a higher incidence of major wound complications in STS surgery compared to adjuvant therapy (24–44% vs. 5–17%), although the definition of wound complications differed between studies [11•, 12, 13]. The timing of surgery after completion of neoadjuvant radiotherapy does not seem to influence the risk of developing wound complications [14••]. As preoperative radiotherapy is becoming standard of care, we as surgeons need to be aware of the impact that neoadjuvant radiotherapy has on developing wound complications and adjust our reconstructive plan to minimize the risk for developing complications.
Reconstructive Ladder vs. Elevator
Limb salvage in patients with a STS is a multidisciplinary team effort between the surgical oncologists, medical oncologists, radiation oncologists, and plastic and reconstructive surgeons. A tension-free closure of the surgical defect with well-vascularized tissue and management to limit the amount of “dead space” are all well-known basic principles in reducing postoperative wound complications. In STS patients, a simple reconstruction frequently violates one or more of these basic principles and therefore advanced reconstructions are often indicated. The concept of having a lower threshold for ascending the reconstructive ladder to more advanced reconstructions in STS patients is not a new concept [3•, 12, 13, 15,16,•, 16•, 17]. Abouarab et al. published an overview of therapeutic options and postoperative wound complications after extremity STS resection and postoperative external beam radiotherapy in 2017 [15•]. The authors state in their conclusion that advanced reconstruction with flaps helps to decrease wound complications by introducing vascularized tissue.
Of note is that most of the articles that are included in the overview article of Abourab et al. administered the radiation therapy in a postoperative setting to their patients instead of preoperative. Adding the fact that neoadjuvant radiation therapy leads to an increase in wound complications makes the argument of a lower threshold for an advanced reconstruction in STS defects even stronger. Hesitation to perform free-flap surgery in an irradiated limb is abandoned, since free tissue transfer is regarded safe in the radiated limb regarding quality of recipient vessels, recipient bed, and flap survival [18]. The timing of the soft tissue reconstruction, however, is important, as evidence suggests that microvascular reconstruction is time sensitive to radiotherapy treatment, with a preference for early reconstruction to forestall associated vessel changes and tissue fibrosis [18]. In STS, the timing of the reconstruction is depicted by the timing of the definitive resection. After radiotherapy, an interval of 3–6 weeks is required to decrease the risk of wound complications and to cool down the acute reactions [19]. Nevertheless, it is not recommended either to create an interval that is too long because this can lead to the development of late fibrosis which can hamper surgery [20]. If the created defect calls for a free tissue transfer, it has been shown that this can be safely performed after 5.9 ± 1.9 weeks, after completing the preoperative radiation therapy, using irradiated vessels as the recipient vessels [21•]
Functional Reconstructions
Even though LSS is often achieved in lower extremity STS, many patients will experience significant functional impairment postoperatively. Such impairments may include loss of certain motor function, leading in the worst cases to the inability to walk, but loss of sensation and strength can also contribute to serious impairment. Careful preoperative planning should therefore not only include an optimal balance between complete surgical resection and preservation of vital structures, but in some cases also the possibilities of reconstructing anticipated loss of function. Such functional reconstructions may include the use of free functional muscle transfers but also tendon transfers and any type of nerve reconstruction.
Current Use of Functional Reconstructions
Functional reconstructions form the next step in the era of LSS. While the loss of complete motor groups in the legs does not always lead to the inability to walk, orthoses and walking aids do impair patients’ functioning. Which is why, whenever possible and reasonable, loss of function should be reconstructed. The use of functional reconstruction is nonetheless not widespread. This can be attributed to several factors, including the main goal of oncological safety, prolonging survival, and minimizing risk of local recurrence; however, the lack of involvement of a reconstructive surgeon in multidisciplinary teams in some centers may also contribute to this.
Conversely, some reconstructive surgeons are reluctant to perform functional reconstructions because of multimodal treatment or the lack of knowledge on oncological outcome compared to postoperative rehabilitation [22]. In one survey regarding the use of functional reconstruction in MPNST, different surgical specialties, including surgical oncologists and reconstructive surgeons agreed upon a life expectancy of three years before considering these reconstructions [22]. This is well below the average expectancy for any STS treated with curative intent [2].
Functional reconstructions are vital to restore lost motor and sensory function after resection of lower extremity STS. Nevertheless, the resection of a single muscle belly may in some cases not necessitate reconstruction. Resection of a single quadriceps muscle or a single muscle from the hamstrings for instance do not result in significant motor deficits that require reconstruction [23, 24]. When (near)-complete muscle compartments of the upper leg are resected, however, replacement of muscle function can be done depending on required strength and size of additional skin paddle. For STS specifically, the innervated latissimus dorsi flap has been described numerous times with good clinical outcomes for both hamstring and quadriceps replacement [25•, 26,27,28,29]. Some studies also showed reasonable outcomes with a contralateral free composite anterolateral thigh (ALT), including vastus lateralis muscle [28, 29]. Other options described in the literature for STS, include using a rectus abdominis muscle or transferring the biceps femoris muscle anteriorly as a replacement for the quadriceps muscle [23, 25•, 26], but theoretically any functional reconstruction performed in upper leg reconstruction could be considered in sarcoma surgery.
Lower leg functional reconstructions are rarely described, but are certainly possible [5]. Reconstruction of the anterior compartment, responsible for dorsiflexion of the foot and hallux, can often be achieved with an innervated gracilis flap [25•, 26]. A composite ALT has also been described and an innervated (segmental) latissimus dorsi could also be a good option. Tendon transfers are possible in selected cases in which soft tissue and skin reconstruction are not necessary or in distal tumor resections [30]. Reconstruction of posterior compartments may be necessary in case both the gastrocnemius muscles and soleus muscle are resected. Their replacement is commonly achieved with a free latissimus dorsi [26].
Nerve Reconstructions
Nerve reconstructions are even less frequently performed and studied after STS resection [31•]. They do however pose the only option to restore sensory function whenever this is lost due to the resection of, for instance, branches of the sciatic, tibial, or peroneal nerve. Not only does the reconstruction of damaged nerves pose the possibility of restoring sensory and motor function, but it may also prevent the formation of a symptomatic neuroma, in turn diminishing residual limb pain [31•]. While losing sensation of cutaneous branches such as the saphenous nerve, femoral cutaneous nerve, or sural nerve in general do not impair physical functioning, resulting neuromas may certainly do so. Because our knowledge on nerve reconstructions has made great leaps over the last few decades and techniques are still evolving, not all reconstructive surgeons are aware of the wide array of possibilities that lay within this field. Prevention of symptomatic neuroma will become increasingly more important in the coming years, especially in cases of necessary amputation, but techniques to do so go beyond the scope of this review. Reconstructing nerves for recovering motor and sensory function after resection of STS in lower extremities have classically involved nerve grafting. Newer techniques learned from brachial plexus surgery include nerve transfers, which grant shorter recovery times and in STS surgery specifically, the possibility to perform these transfers outside of irradiated fields or at likely locations for local recurrence. One study reported on an obturator to femoral nerve transfer after oncological resection [32]. Both the patients’ knee extension and stabilization of gait were restored.
Reconstruction of a full sciatic nerve defect may be difficult and is also dependent on the level of transection. Nerve grafting for the sciatic nerve has been a subject of debate, because of its long distance to the motor end plates. In the past, loss of sciatic nerve function was seen as an indication for amputation, but studies have shown that the functionality can be acceptable and patients prefer salvage over amputation [33]. Loss of foot sensation in STS patients is associated with an increased risk of a secondary amputation, similar to that seen in patients with diabetes [34]. Reports on sciatic nerve reconstruction after STS resection have shown that regaining lower leg motor function is possible yet uncommon, but protective sensation of the foot is more likely and should thus be the major goal [35,36,37].
Some surgeons may be hesitant to perform nerve reconstructions when radiotherapy and/or chemotherapy are administered [22]. Several preclinical studies, however, have shown that neither radiotherapy nor chemotherapy seem to affect the success rate of nerve regeneration in rat models [38,39,40,41]. Although large comparative studies have not been performed, reviews investigating outcomes of nerve reconstruction in STS have not shown lower functional outcomes or higher failure rates in patients receiving multimodal therapy [5•, 31•]. Basic nerve reconstruction principles do obviously apply as nerves are more likely to regenerate properly in well-vascularized wound beds, which may need additional resection in some cases of fibrous tissue in patients that received preoperative radiotherapy [41]. Altogether, in resected nerves, nerve stumps should be adequately treated to prevent painful neuromas, using targeted muscle reinnervation (TMR) and regenerative peripheral nerve interface (RPNI), like techniques, especially when myoelectric prosthesis in amputee cases are a possibility. But whenever possible and needed, nerve gaps should be bridged with grafts. Grafts can be either autografts, like sural nerves or allografts, depending on patient and defect characteristics. In some motor reconstructions, nerve transfers can be performed to restore nerve function loss. Nerve transfers have a shorter time to reinnervation, as opposed to nerve grafts (depending on the location and the length of the defect) and can often be performed outside the irradiated or oncological field.
Lymphatic Reconstructions
Reconstructive lymphatic surgery, also known as lymphatic microsurgery or LVA, is a surgical technique used in the context of lymphedema management after treatments for cancer (e.g., breast, melanoma), including STS [42, 43••]. Lymphedema severely diminishes health-related quality of life and has, for instance, shown to be the greatest hazard for morbidity in breast cancer patients in a large Swedish cancer registry [44]. Lymphedema can arise after sentinel node biopsy, lymph node dissection, or radiation therapy. In STS treatment, a sentinel node biopsy or a lymph node dissection are less frequently indicated as opposed to in other types of malignancies; however, irradiation in combination with large resections (in especially the medial thigh) can transect the main lymphatic channels of a lower extremity resulting in lymphedema.
The primary goal of lymphatic surgery is to restore lymphatic drainage, thereby improving associated symptoms. This is done either by bypassing blocked or damaged lymph channels by performing a LVA or a VLNT to the affected area. Both procedures require precise (super-)microsurgical techniques and are not yet widely available in many institutions. Surgical intervention is typically considered for patients with lymphedema for whom conservative measures, such as compression therapy, physical therapy, or lymphatic massage, bring insufficient relieve of symptoms. Lymphatic reconstructions can either be performed immediately during the resection or secondarily as a therapeutic intervention for lymphedema developing due to the initial resection.
Immediate Lymphatic Reconstructions
Immediate lymphatic reconstructions (ILR) are globally on the rise, after smaller studies claimed high success rates, and multiple larger studies are currently being performed [45,46,47,, 46, 47••, 48••]. Most studies have been performed in patients undergoing axillary lymph node dissections (ALND) in whom an immediate LVA (ILR) was performed. A recent meta-analysis by Fraser Hill et al. reported that patients who received an ILR concurrently with their ALND had a 6.7% risk of developing lymphedema vs. a 34% risk of developing lymphedema in cases where an ILR had not been performed [47••]. In STS surgery lymphedema and large seromas (due to lymphorrhea) are major causes of wound complications, especially after resections in the adductor compartment of the medial thigh [49•, 50].
Prevention of these complications might be achieved when incorporating simultaneous immediate LVA or VLNT surgery during the oncological resection. Small studies reporting good outcomes about ILR or lymphatic flow-through flaps in STS resection have been published [50,51,52,53••]. The long-term advantage and benefits of performing an ILR, concurrent with the resection, is not yet clear [53••].
Secondary Lymphatic Reconstructions
As opposed to ILR, a lot more research has been done in surgically treating already developed lymphedema. Besides the more known conservative treatment modalities for lymphedema, the surgical options entail LVA, VLNT, liposuction, or even the Charles procedure [54]. Lymph node flaps described for VLNT include jejunal, thoracic, omental/gastroepiploic, supraclavicular, submental, and groin donor sites. There is still a debate about which patients may benefit most from which surgical intervention and combining two types of interventions, for instance, LVA and VLNT, is not uncommon [55]. Most studies included in a recent review on these flaps showed a reduction in excess limb volume, a lower risk of cellulitis, and a lower demand of garments [43••].
Future prospective studies should further study the indications, the timing of the surgery, and measure the outcomes of the various surgical techniques, aimed at reducing lymphedema, to further gain insights in the efficacy of each treatment option.
Conclusion
Soft tissue sarcoma in the lower extremity often result in large complex defects requiring reconstructive techniques to limit functional loss and to optimize the postoperative quality of life. Preoperative radiotherapy poses additional challenges and therefore we argue that reconstructive surgeons should have a low threshold for advanced reconstructive techniques in order to add well-vascularized tissue to the defect and subsequently limit major wound complications. Novel techniques such as functional reconstructions, nerve transfers, and lymphatic surgery are within the realms of reconstructive options and therefore, it is important that patients with a STS are taken care of in centers that have a multidisciplinary team with a reconstructive surgeon as a member.
Data availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zagars GK, Ballo MT, Pisters PWT, et al. Prognostic factors for patients with localized soft- tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97(10):2530–43. https://doi.org/10.1002/cncr.11365.
Gronchi A, Miah AB, Dei Tos AP, et al. Soft tissue and visceral sarcomas: ESMO- EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(11):1348–65. https://doi.org/10.1016/j.annonc.2021.07.006.
•O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet (London, England) 2002;359(9325):2235–41. https://doi.org/10.1016/S0140-6736(02)09292-9. This RCT is significant in that it compared outcomes between sarcoma patients who received radiotherapy preoperative vs. postoperative.
•Slump J, Bastiaannet E, Halka A, et al. Risk factors for postoperative wound complications after extremity soft tissue sarcoma resection: a systematic review and meta-analyses. J Plast Reconstr Aesthet Surg. 2019;72(9):1449–64. https://doi.org/10.1016/j.bjps.2019.05.041. This article is a rather recent meta-analysis about the risk factors for wound complications after a sarcoma resection.
•Martin E, Dullaart MJ, van de Sande MAJ, van Houdt WJ, Schellekens PPA, Coert JH. Resuscitating extremities after soft tissue sarcoma resections: Are functional reconstructions an overlooked option in limb salvage? A systematic review. Eur J Surg Oncol. 2019;45(10):1762–9. https://doi.org/10.1016/j.ejso.2019.05.024. This article emphasizes that functional reconstructions are often not consider, however these types of reconstructions are quite often an option.
Kask G, Repo JP, Tukiainen EJ, Blomqvist C, Barner-Rasmussen I. Soft tissue sarcoma of lower extremity: functional outcome and quality of life. Ann Surg Oncol. 2021;28(11):6892–905. https://doi.org/10.1245/s10434-021-09774-6.
Ghert MA, Abudu A, Driver N, et al. The indications for and the prognostic significance of amputation as the primary surgical procedure for localized soft tissue sarcoma of the extremity. Ann Surg Oncol. 2005;12(1):10–7. https://doi.org/10.1007/s10434-004-1171-3.
Mann GN. Less is (usually) more: when is amputation appropriate for treatment of extremity soft tissue sarcoma? Ann Surg Oncol. 2005;12(1):1–2. https://doi.org/10.1245/ASO.2005.10.907.
Dilday JC, Nelson DW, Fischer TD, Goldfarb M. Disparities in amputation rates for non- metastatic extremity soft tissue sarcomas and the impact on survival. Ann Surg Oncol. 2021;28(1):576–84. https://doi.org/10.1245/s10434-020-08586-4.
Shiu MH, Castro EB, Hajdu SI, Fortner JG. Surgical treatment of 297 soft tissue sarcomas of the lower extremity. Ann Surg. 1975;182(5):597–602. https://doi.org/10.1097/00000658-197511000-00011.
•Cannon CP, Ballo MT, Zagar GK, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer. 2006;107(10):2455–61. https://doi.org/10.1002/cncr.22298. This article is significant because it looked at the short term and the long- term outcomes after preoperative or postoperative radiation for sarcomas.
Miller ED, Mo X, Andonian NT, et al. Patterns of major wound complications following multidisciplinary therapy for lower extremity soft tissue sarcoma. J Surg Oncol. 2016;114(3):385–91. https://doi.org/10.1002/jso.24313.
Griffin AM, Dickie CI, Catton CN, et al. The influence of time interval between preoperative radiation and surgical resection on the development of wound healing complications in extremity soft tissue sarcoma. Ann Surg Oncol. 2015;22(9):2824–30. https://doi.org/10.1245/s10434-015-4631-z.
••Krijgh DD, Smith JM, Tilney G, et al. Identifying risk factors and analyzing reconstructive outcomes in patients with lower extremity soft tissue sarcoma. J Plast Reconstr Aesthet Surg. 2023. https://doi.org/10.1016/j.bjps.2023.12.015. This recent article gives a good overview of the risk factors for developing surgical complications in patients who undergo sarcoma resections.
•Abouarab MH, Salem IL, Degheidy MM, et al. Therapeutic options and postoperative wound complications after extremity soft tissue sarcoma resection and postoperative external beam radiotherapy. Int Wound J. 2018;15(1):148–58. https://doi.org/10.1111/iwj.12851. This is a systematic review of the literature that concludes that it is important to add well vascularized tissue to the area where the sarcoma has been resection to optimize wound healing.
•Agrawal N, Wan D, Bryan Z, Boehmler J, Miller M, Tiwari P. Outcomes analysis of the role of plastic surgery in extremity sarcoma treatment. J Reconstr Microsurg. 2013;29(2):107–11. https://doi.org/10.1055/s-0032-1329920. This article shows that including a plastic surgeon to a multidisciplinary approach in sarcoma care may result in lower wound complications.
Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evanse GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer. 2002;94(8):2256–64. https://doi.org/10.1002/cncr.10419.
Townley WA, Mah E, O’Neill AC, et al. Reconstruction of sarcoma defects following pre- operative radiation: free tissue transfer is safe and reliable. J Plast Reconstr Aesthet Surg. 2013;66(11):1575–9. https://doi.org/10.1016/j.bjps.2013.06.029.
Hoefkens F, Dehandschutter C, Somville J, Meijnders P, van Gestel D. Soft tissue sarcoma of the extremeties: pending questions on surgery and radiotherapy. Radiat Oncol. 2016;11:136. https://doi.org/10.1186/s13014-016-0668-9.
Von Mehren M, Lor Randall R, DeLaney T, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) soft tissue sarcoma. 2014;2.2014:126.
•Chao AH, Chang DW, Shuaib SW, Hanasono MM. The effect of neoadjuvant versus adjuvant irradiation on microvascular free flap reconstruction in sarcoma patients. Plast Reconstr Surg. 2012:129(3):675–82. https://doi.org/10.10197/PRS.0b013e3182412a39. This is important study showing that tissue transfers can safely be performed in patients who underwent a lower extremity sarcoma resection with preoperative radiation therapy.
Martin E, Slooff WBM, van Houdt WJ, van Dalen T, Verhoef C, Coert JH. Surgical strategies and the use of functional reconstructions after resection of MPNST: an international survey on surgeons’ perspective. Orthoplastic Surg. 2021;4:12–9. https://doi.org/10.1016/j.orthop.2021.03.001.
Fischer S, Soimaru S, Hirsch T, et al. Local tendon transfer for knee extensor mechanism reconstruction after soft tissue sarcoma resection. J Plast Reconstr Aesthet Surg. 2015;68(5):729–35. https://doi.org/10.1016/j.bjps.2015.01.002.
Gu X, Ricketts S, Mah E. Functional outcomes after single quadriceps muscle resection in patients with soft tissue sarcoma of the anterior compartment of the thigh. ANZ J Surg. 2023;93(1–2):288–93. https://doi.org/10.1111/ans.18205.
•Doi K, Kuwata N, Kawakami F, Hattori Y, Otsuka K, Ihara K. Limb-sparing surgery with reinnervated free-muscle transfer following radical excision of soft-tissue sarcoma in the extremity. Plast Reconstr Surg. 1999;104(6):1679–87. https://doi.org/10.1097/00006534-199911000-00011. This is case report showing that functional muscle reconstructions may be considered after a lower extremity sarcoma resection.
Grinsell D, Di Bella C, Choong PFM. Functional reconstruction of sarcoma defects utilising innervated free flaps. Sarcoma. 2012;2012:315190. https://doi.org/10.1155/2012/315190.
Innocenti M, Abed YY, Beltrami G, Delcroix L, Balatri A, Capanna R. Quadriceps muscle reconstruction with free functioning latissimus dorsi muscle flap after oncological resection. Microsurgery. 2009;29(3):189–98. https://doi.org/10.1002/micr.20607.
Stranix JT, Lee Z-H, Lam G, Mirrer J, Rapp T, Saadeh PB. Limb-sparing sarcoma reconstruction with functional composite thigh flaps. Microsurgery. 2018;38(5):466–72. https://doi.org/10.1002/micr.30254.
Walley KC, Taylor EM, Anderson M, Lozano-Calderon S, Iorio ML. Reconstruction of quadriceps function with composite free tissue transfers following sarcoma resection. J Surg Oncol. 2017;115(7):878–82. https://doi.org/10.1002/jso.24594.
Gunterberg B, Markhede G, Stener B. Function after anterolateral resection of the lower leg for extirpation of tumors. Extension and pronation of the foot restored by transfer of the tibialis posterior muscle. Acta Orthop Scand. 1981;52(1):95–8. https://doi.org/10.3109/17453678108991766.
•Martin E, Dullaart MJ, Verhoef C, Coert JH. A systematic review of functional outcomes after nerve reconstruction in extremity soft tissue sarcomas: a need for general implementation in the armamentarium. J Plast Reconstr Aesthetic Surg. 2020;73(4):621–32. https://doi.org/10.1016/j.bjps.2019.12.010. This article stresses the importance of nerve reconstruction in lower extremity sarcoma resection in case functional nerves need to be resected as well.
O’Brien AL, West JM, Zewdu A, Grignol VP, Scharschmidt TJ, Moore AM. Nerve transfers to restore femoral nerve function following oncologic nerve resection. J Surg Oncol. 2021;124(1):33–40. https://doi.org/10.1002/jso.26487.
Nambisan RN, Rao U, Moore R, Karakousis CP. Malignant soft tissue tumors of nerve sheath origin. J Surg Oncol. 1984;25(4):268–72. https://doi.org/10.1002/jso.2930250410.
Brooks AD, Gold JS, Graham D, et al. Resection of the sciatic, peroneal, or tibial nerves: assessment of functional status. Ann Surg Oncol. 2002;9(1):41–7. https://doi.org/10.1245/aso.2002.9.1.41.
Lee GW, Mackinnon SE, Brandt K, Bell RS. A technique for nerve reconstruction following resection of soft-tissue sarcoma. J Reconstr Microsurg. 1993;9(2):139–44. https://doi.org/10.1055/s-2007-1006662.
Melendez M, Brandt K, Evans GR. Sciatic nerve reconstruction: limb preservation after sarcoma resection. Ann Plast Surg. 2001;46(4):375–81. https://doi.org/10.1097/00000637-200104000-00004.
Tokumoto H, Akita S, Kubota Y, Kuriyama M, Mitsukawa N. Use of vascularized sural nerve grafts for sciatic nerve reconstruction after malignant bone and soft tissue tumor resection in the lower legs. Ann Plast Surg. 2018;80(4):379–83. https://doi.org/10.1097/SAP.0000000000001315.
Evans GR, Brandt K, Ang KK, et al. Peripheral nerve regeneration: the effects of postoperative irradiation. Plast Reconstr Surg. 1997;100(2):375–80. https://doi.org/10.1097/00006534-199708000-00015.
Brandt K, Evans GR, Johnson M, et al. The effects of cisplatinum and vincristine on peripheral nerve regeneration. Plast Reconstr Surg. 1999;104(2):464–9. https://doi.org/10.1097/00006534-199908000-00019.
Brandt K, Evans GR, Gurlek A, et al. The effects of preoperative irradiation on peripheral nerve regeneration. Ann Plast Surg. 1998;40(3):277–82. https://doi.org/10.1097/00000637-199803000-00014.
Evans GRD, Brandt K. Peripheral nerve regeneration: the effects of postoperative irradiation. Plast Reconstr Surg. 2003;111(6):2023–4. https://doi.org/10.1097/01.PRS.0000056837.37545.58.
Patel KM, Lin C-Y, Cheng M-H. A prospective evaluation of lymphedema-specific quality- of-life outcomes following vascularized lymph node transfer. Ann Surg Oncol. 2015;22(7):2424–30. https://doi.org/10.1245/s10434-014-4276-3.
••Gasteratos K, Morsi-Yeroyannis A, Vlachopoulos NC, Spyropoulou G-A, Del Corral G, Chaiyasate K. Microsurgical techniques in the treatment of breast cancer-related lymphedema: a systematic review of efficacy and patient outcomes. Breast Cancer. 2021;28(5):1002–15. https://doi.org/10.1007/s12282-021-01274-5. This recent systematic review bundles all the known literature about lymphatic reconstructions and shows that choosing the right lymphatic reconstruction therapy can be beneficial to the patient.
Yang H, Pawitan Y, He W, et al. Disease trajectories and mortality among women diagnosed with breast cancer. Breast Cancer Res. 2019;21(1):95. https://doi.org/10.1186/s13058-019-1181-5.
Xia TY, Cakmakoglu C, Kwiecien G, Gastman BR. Prohylactic lymphaticovenous anastomosis performed with lymphadenectomy is oncologically safe for melanoma. Ann Surg Oncol. 2023;30(3):1823–39. https://doi.org/10.1245/s10434-022-12791-8.
Chung JH, Kwon SH, Jung SP, Park SH, Yoon ES. Assessing the preventive effect of immediate lymphatic reconstruction on the upper extremity lymphedema. Gland Surg. 2023;12(3):334–43. https://doi.org/10.21037/gs-22-554.
••Fraser Hill WK, Deban M, Platt A, et al. Immediate lymphatic reconstruction during axillary node dissection for breast cancer: a systematic review and meta-analysis. Plast Reconstr Surg Glob Open. 2022;10(5);e4291. https://doi.org/10.1097/GOX.0000000000004291. This recent meta-analysis discusses immediate lymphatic reconstructions after axillary lymph node dissections and concludes that there is a clear signal indicating the benefit of these immediate reconstructions.
••Chun MJ, Saeg F, Meade A, et al. Immediate lymphatic reconstruction for prevention of secondary lymphedema: a meta-analysis. J Plast Reconstr Aesthet Surg. 2022;75(3):1130–41. https://doi.org/10.1016/j.bjps.2021.11.094. This is another recent meta-analysis that discusses immediate lymphatic reconstructions after axillary lymph node dissections and concludes that it is an effective treatment.
•Wu P, Elswick SM, Arkhaven A. Risk factors for Lymphedema after thigh Sarcoma Resection and Reconstruction. Plast Reconstr Surg Glob Open. 2020;23(8):e2912. https://doi.org/10.1097/GOX.0000000000002912. This recent article shows that there is also a risk of lymphedema after a sarcoma resection.
Kobayashi H, Iida T, Yamamoto T, et al. Lymphaticovenous anastomoses for lymphedema complicated by severe lymphorrhea following resection of soft-tissue sarcomas of the adductor compartment: a report of two cases. JBJS Case Connect. 2017;7(4):e80. https://doi.org/10.2106/JBJS.CC.17.00078.
di Summa PG, Guillier D. The Lymphatic Flow-Through (LyFT) flap: proof of concept of an original approach. J Plast Reconstr Aesthet Surg. 2020;73(5):983–1007. https://doi.org/10.1016/j.bjps.2020.01.014.
Scaglioni MF, Meroni M, Fritsche E, Fuchs B. Combined pedicled superficial circumflex iliac artery perforator (SCIP) flap with lymphatic tissue preservation and lymphovenous anastomosis (LVA) for defect reconstruction and lymphedema-lymphocele prevention in thigh sarcoma surgery: preliminary results. J Surg Oncol. 2021;123(1):96–103. https://doi.org/10.1002/jso.26228.
••Wan R, Hussain A, Kuruoglu D, Houdek MT, Moran SL. Prophylactic lymphaticovenous anastomosis (LVA) for preventing lymphedema after sarcoma resection in the lower limb. A report of three cases and literature review. Microsurgery. 2023;43(3):273–80. https://doi.org/10.1002/micr.30975. This recent article shows that there is also a risk of lymphedema after a sarcoma resection and that immediate lymphatic reconstructions may be beneficial.
Hassan K, Chang DW. The Charles procedure as part of the modern armamentarium against lymphedema. Ann Plast Surg. 2020;85(6):e37–43. https://doi.org/10.1097/SAP.0000000000002263.
Granzow JW, Soderberg JM, Kaji AH, Dauphine C. Review of current surgical treatments for lymphedema. Ann Surg Oncol. 2014;21(4):1195–201. https://doi.org/10.1245/s10434-014-3518-8.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
WDR and EM contributed equally to this review and share a first authorship. They helped DDK in background research and writing this review. DDK was involved in all aspects of creating the paper.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Human and Animal Rights
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rinkel, W.D., Martin, E. & Krijgh, D.D. The Role of Reconstructive Surgery in Lower Extremity Soft Tissue Sarcoma. Curr Surg Rep 12, 76–82 (2024). https://doi.org/10.1007/s40137-024-00391-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40137-024-00391-2