Abstract
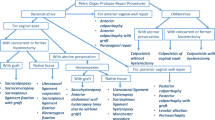
Pelvic floor disorders (PFDs) affect a significant number of women during their lifetime with approximately 11 % of all women seeking surgical intervention for PFDs. Women seeking surgical intervention for PFDs have a variety of procedures, many of which can be performed using a minimally invasive approach. Among the array of options, utilization of the robotic platform continues to garner more interest from both patients and surgeons. Initial studies have shown the safety and feasibility of procedures adapted for the robotic platform. Newer studies have begun to investigate the long-term efficacy and functional outcomes of robotic-assisted surgery. Other investigations demonstrate varying cost differences between robotic pelvic floor procedures compared to laparotomy and conventional laparoscopy procedures. An attractive aspect of the robotic platform is a significantly shorter learning curve for surgeons compared with the prolonged learning curve for conventional laparoscopy. As surgeons decide whether they will ultimately adopt this surgical modality, it is imperative that level 1 evidence and other well designed studies be available for surgeons to assess the robotic platform objectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 11 % of women in the United States will require surgical intervention for pelvic floor disorders (PFDs) [1]. As the population ages, it is estimated that by 2050, over 50 million women will have at least one pelvic floor disorder [2]. Traditional vaginal and abdominal procedures to correct pelvic floor disorders are increasingly being replaced by alternative minimally invasive approaches in order to decrease morbidity associated with open surgery.
The conventional laparoscopic approach to correcting pelvic floor disorders provides many clinical advantages compared with open abdominal surgery. However, a lengthy learning curve associated with the advanced skill set required to perform reconstructive procedures has limited the adoption of this technique. Recently, the development of robotic platforms in assisting with complex minimally invasive procedures has become widely adopted as an alternative minimally invasive approach to conventional laparoscopy.
The daVinci surgical system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was approved by the Food and Drug Administration in 2005 and its use in many surgical fields has grown considerably over the past several years. A recent retrospective study suggests that the introduction of robotics has shifted management of pelvic organ prolapse towards this surgical modality [3]. While early adoption of this platform has allowed many surgeons to provide minimally invasive approaches to reconstructive pelvic surgery, various factors must be considered before choosing this surgical approach to pelvic floor disorders. The objective of this article is to discuss the various procedures currently performed using the robotic approach when treating pelvic floor disorders and to review the current evidence available in the literature.
Advantages of the Robotic Approach
The robotic approach is a modification of the conventional laparoscopic approach to reconstructive pelvic surgery that affords technical advantages to the surgeon. Among these advantages are enhanced three-dimensional visualization, increased freedom of motion with wristed instruments, and tremor-filtrated movement. Compared to the conventional laparoscopic approach, which is associated with a longer learning curve due to rigid non-articulating instruments, the wristed instruments allow for an easier way to introduce the advanced skill of intracorporeal knot-tying and assistance with difficult dissection into deep or difficult to approach spaces into a surgeon’s practice.
The design of the robotic console provides an additional ergonomic advantage to the surgeon that may decrease strain compared to conventional laparoscopy. Studies in multiple surgical specialties have shown a shorter learning curve for robotic procedures when compared with conventional laparoscopic procedures. This provides another attractive feature for surgeons looking to learn minimally invasive approaches to treat pelvic floor disorders and introduce these procedures into their armamentarium.
Disadvantages of the Robotic Approach
While there are many advantages to the robotic platform for correction of pelvic floor disorders, there remain unique limitations. One such limitation is the lack of tactile haptics in robotic surgery. The surgeon must be adept at interpreting visual cues regarding the amount of force being applied to the tissue. Additionally, as with the adoption of any new medical technology, the surgeon must obtain proper training, which requires both time and cost. In addition to cost for training, there are significant costs to the platform, required instrumentations, robotic platform upkeep, and support personnel in the hospital.
Successful and efficient docking requires familiarity with the robotic platform by the entire surgical team. Set up of the robot requires precision and proper planning to allow the surgeon to take advantage of the wristed instruments. Instrument ports and robotic arms must be placed a specified distance apart in order to prevent intraoperative collisions that will hinder the surgeon from using the full motion of the instruments. Furthermore, the docking procedure places the patient in a fixed trendelenburg position rather than allowing for intraoperative position changes. If the patient’s position needs to be altered intraoperatively, the robot must be undocked in order to perform this safely.
Robotic-Assisted Laparoscopic Sacrocolpopexy
The abdominal sacrocolpopexy (ASC) is most often cited as the gold standard for reconstructive procedures to correct postoperative vaginal prolapse. In a recent Cochrane review, ASC was shown to provide a more durable repair compared with vaginal procedures, but at the cost of increased recovery time [4]. Development of laparoscopic sacrocolpopexy (LSC) allowed surgeons to perform a durable prolapse repair that previously required an open procedure. Despite the advantages of the laparoscopic approach, the learning curve remained prolonged, which deterred some surgeons from adopting this approach. Robotic-assisted laparoscopic sacrocolpopexy (RALSC) was introduced with the hopes of providing the same benefits of conventional laparoscopy while allowing more surgeons to obtain the necessary skills to perform the procedure.
Multiple single-institution studies have shown the feasibility and safety of robotic-assisted laparoscopic sacrocolpopexy [5–7]. While there are no randomized studies comparing the robotic approach with the open approach to sacrocolpopexy, several prospective and retrospective studies have shown equivalent anatomic outcomes between the abdominal and robotic approaches [8, 9]. Furthermore, the robotic approach has been shown to have less blood loss and shorter postoperative hospital stays than the open approach.
When the robotic approach is compared with the conventional laparoscopic approach, anatomic outcomes appear to be similar. Paraiso et al., have published the first randomized control study of 78 women for RALSC or LSC and showed similar anatomic and functional improvements from baseline, with the robotic approach resulting in significantly longer operating times, pain, and cost. While there appear to be advantages to the conventional laparoscopic approach, it is important to understand that the authors were advanced conventional laparoscopic surgeons and had performed a required 10 RALSC procedures prior to enrolling in the trial [10••].
One concern regarding RALSC is the operative time required for performing the procedure. Additional time is needed for the setup, docking, and undocking of the robot. Mean operative times for RALSC have been reported to be between 150 and 227 min [11–15]. Complications that can arise from RALSC are similar to those experienced with the conventional laparoscopic approach.
While abdominal placement of mesh for prolapse is not included in the recent FDA report on mesh erosion, patients have become increasingly worried about the possibility of mesh erosion. Mesh erosion is a known complication for sacrocolpopexy with a rate of 3.4 % being reported in the literature [16]. However, several studies have suggested that concomitant hysterectomy places patients at an increased risk. Several studies have shown up to a fivefold increase (8–10 %) in risk for mesh erosion when total hysterectomy is performed with sacrocolpopexy, regardless of the surgical route used [17–19]. A recent retrospective study of 102 women undergoing concomitant total hysterectomy and RALSC showed a mesh erosion rate of 14 % for the total hysterectomy group versus 0 % for the supracervical hysterectomy group [20]. While data are sparse, we advocate for supracervical hysterectomy, if concomitant hysterectomy is performed with RALSC, surgeons should ensure patients have had proper screening for cervical disease. Preoperative counseling about the risks of mesh erosion should be performed, especially if total hysterectomy is indicated. Recently, an alternative method of mesh fixation using vaginal approach with subsequent sacral fixation by laparoscopy was investigated. The preliminary data showed similar short-term anatomic outcomes and complications to traditional laparoscopic approach, including a mesh erosion rate of 2.3 % [21].
Few studies have addressed the long-term functional outcomes of RALSC, but studies have shown that the short-term functional outcomes are significantly improved [8, 10••, 22]. Most recently, long-term follow-up from a prospective cohort study comparing ASC and RALSC showed similar improvements in pelvic floor symptoms and sexual function. With a mean follow-up time of 44.2 months, functional outcomes, including Pelvic Floor Distress Inventory (PFDI-20), Pelvic Floor Impact Questionnaire (PFIQ-7), and Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ-12), showed considerable improvement [23••].
Robotic-Assisted Laparoscopic Sacrohysteropexy
Uterine preservation at the time of prolapse repair remains a controversial topic. While many different procedures have been described, they remain poorly studied. There is currently no outcome data regarding robotic-assisted laparoscopic sacrohysteropexy (RALSHx). With respect to conventional laparoscopic hysteropexy, a few studies have reported an objective cure rate of 94.7–100 % [24, 25]. In one recent study of 51 consecutive laparoscopic hysteropexies using single bifurcated anterior mesh, 98 % of patients had objective success (absence of uterine prolapse of >grade 1 on the Baden–Walker classification) and showed improvement on their International Consultation on Incontinence Modular Questionnaire—Vaginal Symptoms (ICIQ-VS) scores [26].
Leaving the uterus in situ presents some unique considerations. Patient selection and preoperative counseling regarding uterine sparing procedures must include the possibility of future pregnancies and its effect on prolapse repair and the continued need for screening regarding cervical and uterine pathology. Patients should also be counseled that future hysterectomy would required advanced surgical skills and may be associated with increased complications due to scarring and mesh implantation.
For pre-menopausal women, correction of pelvic organ prolapse is often recommended to be performed after they have completed childbearing. While successful pregnancy has been reported after hysteropexy [27], there are very little data to allow for evidence-based recommendations regarding pregnancy after a prolapse procedure. A multidisciplinary approach involving the patient, pelvic surgeon, and obstetrician should include obstetrical considerations, the possibility of complications, and the lack of data available regarding the effect pregnancy would have on any prior prolapse repair.
While the presence of prolapse or subsequent repair of prolapse is not known to be a risk factor for cervical or uterine pathology, surgeons should continue to utilize current screening guidelines. Patients with a current or prior history of cervical or uterine pathology should be counseled appropriately if they are considering RALSHx.
There is no recommendation regarding workup in patients who are asymptomatic. In one study, the risk of unanticipated uterine pathology for women undergoing hysterectomy at the time of prolapse repair was 2.6 % [28].
Robotic Colposuspension and Paravaginal Defect Repair
While primary surgical treatment of stress urinary incontinence (SUI) has been largely been replaced by midurethral slings, open Burch colposuspension was largely regarded as the gold standard for correction of SUI. Despite this, familiarity with procedures that require access to the retropubic space may be necessary for special situations (failure of prior slings, recurrent mesh erosion, patient preference), especially in light of the recent FDA warning issued on vaginal mesh. A Cochrane review of open colposuspension showed at 1 year, overall continence rate is approximately 85 to 90 % and approximately 70 % at 5 years [29]. When laparoscopic colposuspension was compared with the open approach, similar subjective cure rates were seen but objective cure rates favored the open approach. Laparoscopic colposuspension was associated with less perioperative complications, hospital stay and return to activity but required additional operating time and cost [30]. While results seem promising for laparoscopic colposuspension, the difficulty of the procedure likely deterred wider adoption. Both the retropubic dissection and difficult angle for suturing prohibit many surgeons from performing the procedure laparoscopically. Robotic-assisted colposuspension have potential technical difficulties associated with the conventional laparoscopic approach to the retropubic space. Despite this potential advantage, only case reports have been published regarding robotic-assisted colposuspension [31].
Like laparoscopic colposuspension, paravaginal vaginal defect has not been widely adopted. Few studies address isolated laparoscopic paravaginal repair for the correction of the anterior vaginal wall. One study of 212 patients who underwent laparoscopic bilateral paravaginal repair for anterior prolapse, with 45 undergoing concomitant uterosacral ligament vaginal vault suspension, reported an objective cure rate of 76 % at a mean follow-up of 14.2 months [32]. No current studies have been published evaluating robotic-assisted paravaginal defect repair.
Robotic-assisted Laparoscopic Rectopexy
Treatment for rectal prolapse has followed a similar pattern of evolution to surgical treatments for pelvic organ prolapse. Transabdominal procedures have generally been considered to be more effective than their transperineal counterparts. Subsequently, adaptation to conventional laparoscopy has been shown to have similar effectiveness to open technique with earlier recovery and less postoperative pain [33–35]. Given the favorable outcomes for conventional laparoscopic rectopexy, adaptation to the robotic platform was the next evolutionary step for the procedure.
Several cohort studies have compared the robotic approach with the conventional laparoscopic approach and have found similar anatomic outcomes and complication rates, but at the expense of longer operating times and higher costs [36]. A recent prospective study of open, laparoscopic, and robotic-assisted laparoscopic rectopexy (RALR) showed similar improvements in validated scales for constipation and impact on daily living, but at the expense of a higher recurrence rate in the laparoscopic and robotic groups [37]. While the safety and feasibility of RALR have been previously assessed, level 1 evidence is still lacking.
The RALR has recently been expanded to treat posterior vaginal compartment defects. One study has shown RALR to provide satisfactory anatomic results and resolution of symptoms for complex rectoceles [38•, 39]. A follow-up study by the same authors showed significant relief from symptoms such as vaginal bulge and dyspareunia. Furthermore, there was no development of de novo dyspareunia [38•]. Despite these results, there remain very little data on quality of life outcomes with regards to RALR.
Costs
The cost of robotic surgery is an important factor to consider when choosing a surgical approach. Several costs analysis studies have shown mixed results regarding RALSC. Some studies suggest that there may be cost savings when compared with the open approach. Elliott et al., were able to show a 10 % cost savings for RALSC compared with ASC. According to the study, the cost savings depended on shorter postoperative length of stays and whether there was sufficient institutional volume [11]. Furthermore, a recent study comparing ASC and RALSC showed median costs to be significantly less when using the robotic versus the open approach [40•]. Yet when compared with LSC, several studies have shown RALSC to be significantly more expensive than LSC [41, 42•, 43] with the increased costs mainly being attributed to increased duration of surgery and robotic instrumentation. Paraiso et al., showed that the RALSC cost was $1946 greater than LSC, an analysis that did not include the cost of the robotic platform or upkeep (approximately $100,000 annually). As surgical times decrease with increased robotic experience, the cost gap may become smaller. There are no data comparing robotic-assisted retropubic procedures with open or conventional laparoscopy.
Data regarding cost comparisons for ventral rectopexy is limited. However, one recent study has shown significantly greater costs for the robotic approach when compared to the conventional laparoscopic approach. This increased cost was mainly attributed to increased time consumption and increased material costs [36]. Additional studies need to be performed regarding the cost of robotic rectopexy in order to identify areas in which the cost differential can be decreased.
Conclusions
Utilization of the robotic approach for the treatment of pelvic floor disorders is growing as it is becoming more accessible to surgeons. As patients increasingly desire minimally invasive procedures, more surgeons will adopt the robotic approach. Short-term data regarding anatomic outcomes, functional outcomes, and complications of the robotic approach appear to be similar to those from the laparoscopic approach. While previous studies have indicated a significant cost burden when utilizing robotic surgery, newer studies suggest that the cost gap is closing. Due to decreased work hours and surgical experience, robotic-assisted surgery is likely to become more widely adopted due to lack of conventional laparoscopy training despite increased cost. Recent studies are beginning to show promising long-term data with respect to surgical outcomes. Due to the natural history of pelvic floor disorders, additional level 1 evidence with long-term outcomes will be helpful in assessing the recurrence and satisfaction rates for procedures utilizing the robotic approach.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–6. doi:10.1016/S0029-7844(97)00058-6.
Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US women: 2010 to 2050. Obstet Gynecol. 2009;114:1278–83. doi:10.1097/AOG.0b013e3181c2ce96.
Carroll AW, Lamb E, Hill AJ, Gill EJ, Matthews CA. Surgical management of apical pelvic support defects: the impact of robotic technology. Int Urogynecol J. 2012;. doi:10.1007/s00192-012-1749-4.
Maher CM, Feiner B, Baessler K, Glazener CM. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445–57. doi:10.1007/s00192-011-1542-9.
Di Marco DS, Chow GK, Gettman MT, Elliott DS. Robotic-assisted laparoscopic sacrocolpopexy for treatment of vaginal vault prolapse. Urology. 2004;63:373–6. doi:10.1016/j.urology.2003.09.033.
Elliott DS, Frank I, Dimarco DS, Chow GK. Gynecologic use of robotically assisted laparoscopy: sacrocolpopexy for the treatment of high-grade vaginal vault prolapse. Am J Surg. 2004;188:52S–6S. doi:10.1016/j.amjsurg.2004.08.022.
Daneshgari F, Kefer JC, Moore C, Kaouk J. Robotic abdominal sacrocolpopexy/sacrouteropexy repair of advanced female pelvic organ prolaspe (POP): utilizing POP-quantification-based staging and outcomes. BJU Int. 2007;100:875–9. doi:10.1111/j.1464-410X.2007.07109.x.
Geller EJ, Parnell BA, Dunivan GC. Pelvic floor function before and after robotic sacrocolpopexy: one-year outcomes. J Minim Invasive Gynecol. 2011;18:322–7. doi:10.1016/j.jmig.2011.01.008.
Siddiqui NY, Geller EJ, Visco AG. Symptomatic and anatomic 1-year outcomes after robotic and abdominal sacrocolpopexy. Am J Obstet Gynecol. 2012;206:435.e1–5. doi:10.1016/j.ajog.2012.01.035.
•• Paraiso MF, Jelovsek JE, Frick A, Chen CC, Barber MD. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol. 2011;118:1005-1013. doi: 10.1097/AOG.0b013e318231537c. This is the only randomized controlled trial comparing laparoscopic and robotic sacrocolpopexy.
Elliott CS, Hsieh MH, Sokol ER, Comiter CV, Payne CK, Chen B. Robot-assisted versus open sacrocolpopexy: a cost-minimization analysis. J Urol. 2012;187:638–43. doi:10.1016/j.juro.2011.09.160.
Gocmen A, Sanlikan F, Ucar MG. Robotic-assisted sacrocolpopexy/sacrocervicopexy repair of pelvic organ prolapse: initial experience. Arch Gynecol Obstet. 2012;285:683–8. doi:10.1007/s00404-011-2032-5.
Moreno Sierra J, Ortiz Oshiro E, Fernandez Perez C, Galante Romo I, Corral Rosillo J, Prieto Nogal S, Castillon Vela IT, Silmi Moyano A, Alvarez Fernandez-Represa J. Long-term outcomes after robotic sacrocolpopexy in pelvic organ prolapse: prospective analysis. Urol Int. 2011;86:414–8. doi:10.1159/000323862.
Benson AD, Kramer BA, Wayment RO, Schwartz BF. Supracervical robotic-assisted laparoscopic sacrocolpopexy for pelvic organ prolapse. JSLS. 2010;14:525–30. doi:10.4293/108680810X1292446600806.
Matthews CA, Carroll A, Hill A, Ramakrishnan V, Gill EJ. Prospective evaluation of surgical outcomes of robot-assisted sacrocolpopexy and sacrocervicopexy for the management of apical pelvic support defects. South Med J. 2012;105:274–8. doi:10.1097/SMJ.0b013e318254d0c6.
Nygaard IE, McCreery R, Brubaker L, Connolly A, Cundiff G, Weber AM, Zyczynski H, Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805–23. doi:10.1097/01.AOG.0000139514.90897.07.
Cundiff GW, Varner E, Visco AG, Zyczynski HM, Nager CW, Norton PA, Schaffer J, Brown MB, Brubaker L, Pelvic Floor Disorders Network. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008;199:688.e1–5. doi:10.1016/j.ajog.2008.07.029.
Bensinger G, Lind L, Lesser M, Guess M, Winkler HA. Abdominal sacral suspensions: analysis of complications using permanent mesh. Am J Obstet Gynecol. 2005;193:2094–8. doi:10.1016/j.ajog.2005.07.066.
Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011;22:205–12. doi:10.1007/s00192-010-1265-3.
Osmundsen BC, Clark A, Goldsmith C, Adams K, Denman MA, Edwards R, Gregory WT. Mesh erosion in robotic sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2012;18:86–8. doi:10.1097/SPV.0b013e318246806d.
von Pechmann WS, Aungst MJ, Gruber DD, Ghodsi PM, Cruess DF, Griffis KR. A pilot study on vaginally assisted laparoscopic sacrocolpopexy for patients with uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2011;17:115–9. doi:10.1097/SPV.0b013e318216379d.
Seror J, Yates DR, Seringe E, Vaessen C, Bitker MO, Chartier-Kastler E, Roupret M. Prospective comparison of short-term functional outcomes obtained after pure laparoscopic and robot-assisted laparoscopic sacrocolpopexy. World J Urol. 2012;30:393–8. doi:10.1007/s00345-011-0748-2.
•• Geller EJ, Parnell BA, Dunivan GC. Robotic vs abdominal sacrocolpopexy: 44-month pelvic floor outcomes. Urology. 2012;79:532–536; doi: 10.1016/j.urology.2011.11.025. This prospective cohort study provides long-term pelvic floor outcomes.
Krause HG, Goh JT, Sloane K, Higgs P, Carey MP. Laparoscopic sacral suture hysteropexy for uterine prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:378–81. doi:10.1007/s00192-005-0019-0.
Rosenblatt PL, Chelmow D, Ferzandi TR. Laparoscopic sacrocervicopexy for the treatment of uterine prolapse: a retrospective case series report. J Minim Invasive Gynecol. 2008;15:268–72. doi:10.1016/j.jmig.2008.01.001.
Price N, Slack A, Jackson SR. Laparoscopic hysteropexy: the initial results of a uterine suspension procedure for uterovaginal prolapse. BJOG. 2010;117:62–8. doi:10.1111/j.1471-0528.2009.02396.x.
Lewis CM, Culligan P. Sacrohysteropexy followed by successful pregnancy and eventual reoperation for prolapse. Int Urogynecol J. 2012;23:957–9. doi:10.1007/s00192-011-1631-9.
Frick AC, Walters MD, Larkin KS, Barber MD. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507.e1–4. doi:10.1016/j.ajog.2010.01.077.
Lapitan MC, Cody JD. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev. 2012;6:CD002912. doi:10.1002/14651858.CD002912.pub5.
Dean NM, Ellis G, Wilson PD, Herbison GP. Laparoscopic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev. 2006;(3):CD002239; doi: 10.1002/14651858.CD002239.pub2.
Khan MS, Challacombe B, Rose K, Dasgupta P. Robotic colposuspension: two case reports. J Endourol. 2007;21:1077–9. doi:10.1089/end.2005.0025.
Behnia-Willison F, Seman EI, Cook JR, O’Shea RT, Keirse MJ. Laparoscopic paravaginal repair of anterior compartment prolapse. J Minim Invasive Gynecol. 2007;14:475–80. doi:10.1016/j.jmig.2006.12.002.
Byrne CM, Smith SR, Solomon MJ, Young JM, Eyers AA, Young CJ. Long-term functional outcomes after laparoscopic and open rectopexy for the treatment of rectal prolapse. Dis Colon Rectum. 2008;51:1597–604. doi:10.1007/s10350-008-9365-6.
D’Hoore A, Cadoni R, Penninckx F. Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg. 2004;91:1500–5. doi:10.1002/bjs.4779.
Collinson R, Wijffels N, Cunningham C, Lindsey I. Laparoscopic ventral rectopexy for internal rectal prolapse: short-term functional results. Colorectal Dis. 2010;12:97–104. doi:10.1111/j.1463-1318.2009.02049.x.
Heemskerk J, de Hoog DE, van Gemert WG, Baeten CG, Greve JW, Bouvy ND. Robot-assisted vs. conventional laparoscopic rectopexy for rectal prolapse: a comparative study on costs and time. Dis Colon Rectum. 2007;50:1825–30. doi:10.1007/s10350-007-9017-2.
de Hoog DE, Heemskerk J, Nieman FH, van Gemert WG, Baeten CG, Bouvy ND. Recurrence and functional results after open versus conventional laparoscopic versus robot-assisted laparoscopic rectopexy for rectal prolapse: a case-control study. Int J Colorectal Dis. 2009;24:1201–6. doi:10.1007/s00384-009-0766-3.
• Wong MT, Abet E, Rigaud J, Frampas E, Lehur PA, Meurette G. Minimally invasive ventral mesh rectopexy for complex rectocoele: impact on anorectal and sexual function. Colorectal Dis. 2011;13:e320–6. doi: 10.1111/j.1463-1318.2011.02688.x. This prospective cohort is one of only a few studies that address sexual function after ventral rectopexy.
Wong MT, Meurette G, Rigaud J, Regenet N, Lehur PA. Robotic versus laparoscopic rectopexy for complex rectocele: a prospective comparison of short-term outcomes. Dis Colon Rectum. 2011;54:342–6. doi:10.1007/DCR.0b013e3181f4737e.
• Hoyte L, Rabbanifard R, Mezzich J, Bassaly R, Downes K. Cost analysis of open versus robotic-assisted sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2012;18:335–339. doi: 10.1097/SPV.0b013e318270ade3. Cost analysis study showing significant costs savings utilizing the robotic approach compared with open sacrocolpopexy.
Patel M, O’Sullivan D, Tulikangas PK. A comparison of costs for abdominal, laparoscopic, and robot-assisted sacral colpopexy. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:223–8. doi:10.1007/s00192-008-0744-2.
• Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010;17:493–499. doi: 10.1016/j.jmig.2010.03.011. This cost analysis study evaluates sacrocolpopexy performed in all three modalities using a decision model allowing for incorporation of outcomes and costs.
Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Robotic-assisted and laparoscopic sacrocolpopexy: comparing operative times, costs and outcomes. Female Pelvic Med Reconstr Surg. 2011;17:44–9. doi:10.1097/SPV.0b013e3181fa44cf.
Disclosure
Nathan Kow and Marie Fidela R. Paraiso declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kow, N., Paraiso, M.F.R. Robotic Approach to Pelvic Floor Disorders. Curr Surg Rep 1, 197–202 (2013). https://doi.org/10.1007/s40137-013-0011-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40137-013-0011-4