Abstract
Introduction
This study aims to evaluate the long-term clinical outcomes of excisional goniotomy with the Kahook Dual Blade (KDB) in the management of various types of glaucoma.
Methods
This was a retrospective, noncomparative chart review of 90 eyes of 53 patients with glaucoma that underwent standalone KDB goniotomy (KDB-alone group) or KDB goniotomy with concomitant phacoemulsification (KDB-phaco group) between October 2015 and October 2017. Surgical success was defined as an intraocular pressure (IOP) reduction by ≥ 20% at the last follow-up with no surgical reinterventions required and a final IOP ≥ 4 mmHg and ≤ 21 mmHg. We also report on changes from baseline in IOP, number of glaucoma medications, best-corrected visual acuity (BCVA), and visual field parameters, for up to 72 months.
Results
At 72 months, mean (standard deviation [SD]) IOP was reduced from 17.5 (5.7) to 13.6 (3.0) mmHg (P < 0.0001) in the KDB-phaco group and from 23.3 (5.9) to 15.1 (6.2) mmHg (P = 0.0593) in the KDB-alone group. The mean (SD) number of glaucoma medications was reduced from 1.3 (1.0) to 0.8 (0.9) (P < 0.0001) in the KDB-phaco group and from 1.2 (1.0) to 0.7 (0.8) (P = 0.3409) in the KDB-alone group. During the 72-month follow-up, surgical success was achieved in 24 of the 52 available eyes (46.2%). Four eyes underwent a glaucoma surgical reintervention by 72 months.
Conclusions
Excisional goniotomy with the KDB effectively lowered the IOP (by an average of 28.0% from baseline) and maintained or further reduced glaucoma medication burdens (by an average of 30.8% from baseline) under an excellent safety profile, independent of phacoemulsification status. The procedure exhibited favorable success for up to 6 years, providing valuable insights into its long-term efficacy as a glaucoma treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Within the literature, there is an abundance of reports documenting short-term outcomes of Kahook Dual Blade (KDB) excisional goniotomy; however, the longest study (5-year follow-up) within the current literature is limited to the inclusion of successful outcomes. |
We aimed to provide a study with the longest follow-up duration currently documented with real-world data. |
This study aims to assess the therapeutic capability of the KDB for the long-term management of various glaucoma etiologies, as measured by a mean 28.0% reduction from baseline in intraocular pressure (IOP) and a mean 30.8% reduction from baseline in glaucoma medication burden. |
The outcomes of this study demonstrate an exceptional safety profile and a 46% success rate over 6 years of follow-up. |
This work underscores the favorable selection of KDB among a plethora of minimally invasive glaucoma surgeries (MIGSs) and confirms its utility as a safe and effective therapy for glaucoma. This research may help direct further investigation on the safety and efficacy of KDB excisional goniotomy as a standalone procedure, as opposed to with concomitant phacoemulsification. |
Introduction
Within the past two decades, the glaucoma surgical armamentarium has seen a significant expansion with the introduction of minimally invasive glaucoma surgeries (MIGSs). MIGSs are advantageous in that they can be performed in earlier stages of the glaucoma disease course and delay the need for more invasive incisional procedures, with up to 10 years of stable intraocular pressure (IOP) maintenance currently reported within the literature [1, 2]. Additionally, these procedures possess lower procedural risks, shorter operation times, and faster patient recovery times when compared to traditional surgical interventions [3]. Performing MIGS can reduce the vision-threatening complications associated with traditional incisional procedures, such as endophthalmitis, hypotony, and choroidal effusion [4]. Typically, MIGS procedures fall into one of three categories: (1) MIGSs that increase uveoscleral outflow; (2) MIGSs that reroute the flow of aqueous humor to the subconjunctival space; and (3) MIGSs that divert the flow of aqueous humor to the Schlemm’s canal [5].
The Kahook Dual Blade (KDB; New World Medical Inc. Rancho Cucamonga, CA, USA) is an “angle-based” MIGS device that aims to increase conventional outflow by diverting aqueous egress to the Schlemm’s canal. Similar to gonioscopy-assisted transluminal trabeculotomy, this procedure is bleb-independent and minimally invasive [6]. The KDB is intended to perform excisional goniotomy and possesses two parallel blades that allow for a complete and uniform excision of a strip of trabecular meshwork, limiting the inflammation and scleral damage seen in traditional goniotomy procedures [7]. The current literature reports primarily on the outcomes of KDB goniotomy—with and without phacoemulsification—in open-angle glaucoma (OAG) [8,9,10,11,12,13,14], but the procedure has also demonstrated success in angle-closure glaucoma (ACG) [15,16,17,18]. Studies within the literature support a favorable safety and efficacy profile for KDB goniotomy, reporting notable reductions in IOP by 20–30% from baseline and a decrease in glaucoma medication burden by 1–3 medications [8,9,10,11,12,13,14].
Currently, long-term outcomes of KDB goniotomy require further investigation. Within the PubMed-indexed literature, there is one study evaluating 5-year outcomes with the device [14], but its findings are limited by the inclusion of successful outcomes only. This study aims to document clinical safety and efficacy outcomes of KDB goniotomy with or without concomitant phacoemulsification over a 6-year period in a real-world setting.
Methods
A retrospective, single surgeon (S.D.), noncomparative study was conducted at the Mayo Clinic. The study protocol was reviewed and approved by the Mayo Clinic Institutional Review Board (IRB) on February 28, 2018 (IRB Number: 18-000006). This study was conducted in accordance with the tenets of the Declaration of Helsinki of 1964 and its later amendments. All patient data were deidentified, and informed consent was waived by the Mayo Clinic IRB. Patients aged ≥ 18 years diagnosed with glaucoma that received excisional goniotomy with the KDB between October 1, 2015 and October 31, 2017 were included within this study. All glaucoma subtypes were eligible for inclusion.
Excisional goniotomy with the KDB was carried out in accordance with the manufacturer’s guidelines. The procedure began with the sharp tip of the device being introduced through a clear corneal incision, targeting the nasal angle. The tip was used to puncture the trabecular meshwork while the heel of the device was positioned against Schlemm’s canal. The device was then moved for four clock hours in a clockwise or counterclockwise direction, creating two parallel incisions to remove a strip of trabecular meshwork, ensuring no damage to surrounding structures. After the excision, the KDB blade was extracted from the eye. In cases involving combined phacoemulsification, KDB goniotomy was executed after the successful implantation of the intraocular lens.
Participants were evaluated preoperatively, intraoperatively, and postoperatively on day 1, week 1, and months 1, 3, 6, 12, 24, 36, 48, 60, and 72. Pre- and postoperative data included IOP, number of glaucoma medications required, best-corrected visual acuity (BCVA), and visual field parameters—pattern standard deviation (PSD) and mean deviation (MD). Medications utilized for glaucoma management included alpha-agonists, beta-blockers, carbonic anhydrase inhibitors, prostaglandin analogs, pilocarpine, and various combination eyedrops of the aforementioned medications. An assessment of IOP, cup–disc ratio, and visual fields were utilized to determine the number of medications necessary. IOP was measured using Goldmann applanation tonometry, and BCVA was assessed using a Snellen eye chart at 20 feet. Baseline measurements were assessed one day prior to surgery for all patients. Slit-lamp and fundus examinations were used to visualize and evaluate the anterior and posterior segments of each eye. Standard automatic perimetry was performed using a Humphrey field analyzer (Carl Zeiss Meditec Inc., Dublin, CA, USA). Glaucoma severity was classified based upon the International Classification of Diseases, Tenth Revision [19].
Standard descriptive statistics were used to report demographics and baseline characteristics, with means and standard deviations (SD) used for continuous variables, and percentages used for categorical variables. Normality of continuous variables was confirmed, and subsequent paired two-tailed t tests were used wherein a p value of less than 0.05 was considered statistically significant. Kaplan–Meier survival curves were utilized to analyze the surgical success probability in the cumulative cohort. Surgical success was defined as an IOP reduction of ≥ 20% with no surgical reintervention required and a final IOP ≥ 4 mmHg and ≤ 21 mmHg. All statistical analyses were performed with GraphPad Prism (GraphPad Version 10.2.3; GraphPad Software, Boston, MA, USA).
Results
Demographic and Preoperative Glaucoma Status
Ninety eyes of 53 patients that underwent KDB excisional goniotomy with or without concomitant phacoemulsification with ≥ 12 months of follow-up were eligible for inclusion. Data from 52 eyes (58%) were available at 72 months.
Demographic information of patients and baseline characteristics are detailed in Table 1. The mean (SD) age of all patients was 71.2 (7.4) years and the majority of patients were White (72%) and female (66%). There were 72 eyes (80%) that underwent combined KDB and phacoemulsification (KDB-phaco group), while 18 eyes (20%) underwent KDB as a standalone procedure (KDB-alone group). All eyes that underwent standalone KDB were pseudophakic. Various glaucoma etiologies were represented, including POAG (63%), pseudoexfoliative glaucoma (4%), steroid-induced glaucoma (7%), and PACG (26%). Of the total sample, ten eyes (11%) had mild glaucoma, 55 eyes (61%) had moderate glaucoma, and 25 eyes (28%) had severe glaucoma. Thirty-six eyes (40%) were surgically naïve at baseline (Table 1).
Mean IOP, Glaucoma Medication Use, and BCVA at Baseline and Each Postoperative Visit in All Eyes
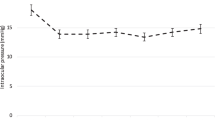
Table 2 outlines the mean IOP, glaucoma medication burden, and BCVA in all eyes from baseline to 72 months. IOP progression of the total sample can be found in Fig. 1. IOP ranged from 13.3–15.7 mmHg (Fig. 1) across months 6–72, representing mean percent reductions ranging from 12.4–31.1% (P < 0.05 at every time point). The mean (SD) IOP decreased from 18.6 (6.2) mmHg at baseline to 13.9 (3.9) mmHg at 72 months (− 28.0%). Medication dependency progression of the total sample can be found in Fig. 2. Mean medication dependency ranged from 0.8 to 1.2 medications (Fig. 2) between months 6–72. The mean (SD) number of glaucoma medications required at baseline was 1.3 (1.0) and was reduced to 0.8 (0.9) at 72 months (− 30.8%). LogMAR BCVA was recorded at each follow-up visit; however, significant changes from baseline in BCVA were not observed at 72 months (Table 2).
Mean IOP, Glaucoma Medication Use, and BCVA at Baseline and Each Postoperative Visit in KDB-Phaco Group
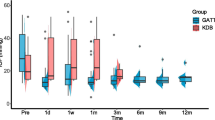
IOP, glaucoma medication, and BCVA outcomes for the KDB-phaco group are outlined in Table 3. Reductions in IOP ranged from 2.9–5.6 mmHg between months 6–72, representing mean percent reductions ranging from 16.6–32.0% (P < 0.05 at every follow-up). The mean (SD) baseline IOP of 17.5 (5.7) mmHg was reduced to 13.6 (3.0) mmHg at 72 months (− 27.4%). Across all time points, the largest reduction in medication from baseline use occurred at 12 months (− 53.8%; P < 0.0001) and the smallest reduction occurred at 60 months (− 15.4%; P = 0.2290) (Table 3). The mean (SD) number of glaucoma medications was significantly reduced from 1.3 (1.0) at baseline to 0.8 (0.9) at 72 months (P < 0.0001). Baseline logMAR BCVA for KDB-phaco group was 0.283,and subsequent visits yielded a BCVA of 0.107, 0.121, 0.115, 0.142, 0.167, 0.227, and 0.247 logMAR at months 6, 12, 24, 36, 48, 60, and 72, respectively (P < 0.05 at months 6–48; P = 0.0696 at month 60; and P = 0.1734 at month 72).
Mean IOP, Glaucoma Medication Use, and BCVA at Baseline and Each Postoperative Visit in KDB-alone Group
IOP, glaucoma medication, and BCVA outcomes for the KDB-alone group are reported in Table 4. The KDB-alone cohort included 18 eyes at baseline, with 11 remaining after 6 years. Across all time points, the average reduction in IOP was 24.4%. Statistical significance was achieved at 6, 12, and 60 months (P < 0.05). The mean (SD) IOP of 23.3 (5.9) mmHg was reduced to 15.1 (6.2) mmHg (− 28.8%) at 72 months. A scatterplot of IOP progression across all visits can be found in the Supplementary Material. The baseline mean (SD) number of glaucoma medications was reduced from 1.2 (1.0) to 0.7 (0.8) at 72 months (− 41.7%). Statistical significance for medication burden reduction was not achieved at any follow-up visit. Changes in BCVA for eyes within this group were not reported.
Visual Field Outcomes
Changes in visual field mean deviation (VF-MD) and pattern standard deviation (VF-PSD) can be found in Table 5, Figs. 3, and 4. At baseline, VF-MD was − 3.56 dB, which over 72 months dropped to − 5.32 dB (P = 0.0222) (Fig. 3). VF-PSD did not yield significant differences from 3.83 dB at baseline to 4.93 at 72 months (P = 0.1464) (Fig. 4).
Surgical Success
At 72 months, 24 of the 52 remaining eyes (46.2%) met surgical success, as defined by the success criteria of > 20% IOP reduction from baseline with no surgical reintervention and an IOP between 4 and 21 mmHg. The success rate for the total sample from the baseline 90 patients using the last follow-up visit of the remaining 52 patients was 26.7%. Four eyes underwent a surgical reintervention and were deemed to have failed. Median duration of surgical success was 48 months. A Kaplan–Meier survival curve of surgical success probability can be found in Fig. 5.
Complications
A summary of postoperative complications can be found in Table 6. All complications were minor and self-limited, with no severe or sight-threatening complications observed. IOP spike (defined as an increase in IOP > 10 mmHg or a transient measurement of 30 mmHg within 1-month postoperatively) was observed in three eyes (3.3%) and resolved spontaneously. Four eyes (4.4%) required surgical re-intervention with canaloplasty (Table 6).
Discussion
In this retrospective study, we report clinical and safety efficacy data from a cohort of patients who were followed longitudinally over 6 years after undergoing KDB excisional goniotomy, either as a standalone procedure or combined with cataract surgery. All procedures were performed at the same center by a single surgeon, ensuring consistency and validity of the surgical technique and results. To the best of our knowledge, this analysis represents the longest published follow-up of KDB goniotomy outcomes to date.
Compared to the existing literature on outcomes of KDB with and without phacoemulsification [8,9,10,11,12,13,14,15,16,17,18, 20,21,22,23,24,25,26], similar outcomes in terms of average IOP (− 28.0%) and medication reductions (− 30.8%) were observed. The current literature indicates that an average IOP reduction of 20–37% is expected and commonly seen with follow-up periods ranging from 12 months until 36 months [8,9,10,11,12,13,14,15,16,17]. Moreover, the preoperative IOP (18.6 mmHg) and postoperative IOP (13.9 mmHg) of the total sample was generally similar to the range reported in the existing publications on trabecular MIGSs [3, 8,9,10, 12,13,14,15,16,17,18, 22, 24, 27]. Statistically and clinically significant reductions in IOP and medication burden were observed in the cumulative cohort after 6 years, demonstrating the long-term efficacy of the KDB procedure. Additionally, minimal early post-operative and intraoperative complications were observed, highlighting the excellent safety profile of the KDB. Notably, there was a complete absence of post-operative endophthalmitis, hypotony, Descemet membrane detachment, and posterior capsule opacification, as would commonly be expected with other MIGSs [28].
The KDB-phaco group exhibited statistically significant reductions in IOP at all follow-up visits. Apart from the 4- and 5-year visits, this cohort also showed statistically significant reductions in medication burden. The variability in medication dependence is noteworthy and can likely be attributed to poor medication compliance and reduced communication with physicians, compounded by the COVID-19 pandemic. Patients underwent KDB excisional goniotomy between 2015 and 2017, causing several 4- and 5-year follow-up visits to occur between 2020 and 2021. Poor compliance with medication during this period may have resulted in unexpected disease progression as indicated by visual field changes, despite stable IOPs, necessitating an increase in prescribed medications.
The KDB-alone group yielded promising reductions from baseline in IOP, with percent decreases ranging from 11.2–39.0% across all follow-up visits. However, these reductions did not reach statistical significance at 2, 3, 4, or 6 years. Notably, at 12 and 24 months, a transient increase in IOP was observed, illustrated by the scatter plot shown in the Supplementary Material. This occurred primarily due to a single patient who experienced surgical failure requiring reintervention, with IOP measurements of 36 and 38 mmHg at 12 and 24 months, respectively. This cohort experienced a greater variability in IOP, likely due to its comparatively smaller sample size. As seen in the KDB-phaco group, several patients in the standalone group had follow-up visits scheduled during the COVID-19 pandemic, leading to inconsistencies in medication compliance, communication, and timely pressure monitoring. Additionally, it has been established that medication compliance is reduced in multi-drug regimens for glaucoma, exacerbating disease progression [29].
BCVA remained stable in the KDB-alone group and statistically significant improvements were noted in the KDB-phaco group, for up to 4 years of follow-up. Between 5 and 6 years, visual acuity appeared to approach the baseline value (0.283 logMAR) in KDB-phaco eyes. This trend can be attributed to the normal progression of ocular aging, with BCVA changes likely resulting from concurrent pathologies such as age-related macular degeneration (AMD) and diabetic retinopathy (DR) [30,31,32].
Visual fields were assessed using VF-MD and VF-PSD. At 6 years, VF-MD (SD) significantly decreased to − 5.32 (7.70) (P < 0.05) dB while PSD (SD) increased to 4.93 (4.11) dB (P = 0.1464), albeit not significantly. Significant changes in VF-MD and VF-PSD were observed at the 2-, 3-, and 4-year follow-up visits, but reintervention was avoided due to stable IOPs and cup-to-disc ratios in the affected eyes. It is worth noting that visual field measurements can be highly variable due to changes in testing settings and circumstances such as eye fatigue, movement within the testing room, and glaucoma severity [33, 34]. Furthermore, current literature states that the normal glaucomatous process and aging can produce significant changes is VF-MD up to − 0.80 dB per year in treated patients [35,36,37,38,39,40]. The results within this study fall well below this threshold throughout the follow-up period.
A study by Bravetti et al. discusses surgical success of KDB excisional goniotomy over a 12-month period with a similar success criteria of a ≥ 20% reduction in IOP [27]. Within this study, 37.5% of eyes exhibited surgical success over a substantially shorter follow-up duration [27], whereas our study exhibited a more favorable success rate of 46.2% at 6 years in the remaining eyes. With a median success of 4 years, KDB goniotomy provides promising long-term reliability for the management of glaucoma. This current study underscores the durability of treatment with KDB with exceptional 6-year success and can be interpreted as a safe and reliable option for management of various glaucoma subtypes.
This study offers valuable insights into the long-term safety and efficacy outcome of KDB excisional goniotomy. Advantages of this study include the inclusion of real-world outcomes encompassing both failures and successes, as well as the consistency and validity ensured by having all procedures performed by a single surgeon at the same center.
Limitations
Several limitations are present in this study. Firstly, the retrospective nature of this study did not allow for randomization. Secondly, several patients were lost to follow-up, affecting overall consistency of the results. Additionally, medication regimens were not standardized, potentially influencing the number of medications used. The various forms of glaucoma represented in this study may also serve as a potential limitation as it can cause variation in clinical outcomes. Provided the seniority of the patient population and convenience of concomitant cataract surgery with goniotomy, the KDB-alone group was relatively small. Finally, baseline measurements of IOP were not at a standardized time of day, resulting in potential for inconsistencies as the literature has established that IOP tends to peak in the early hours of the day—shortly after awakening from sleep [41]. Future studies are needed to evaluate the long-term efficacy and safety of standalone excisional goniotomy with the KDB.
Conclusions
Combined KDB excisional goniotomy and phacoemulsification demonstrated clinically significant reductions in IOP and clinically reduced medication burdens in patients with glaucoma. The procedure showed favorable success, even at 6 years post-operatively. KDB goniotomy appears to be a promising long-term therapy for correcting angle-closure and various forms of open-angle glaucoma.
References
Ahmed IIK. MIGS and the FDA: what’s in a name? Ophthalmology. 2015;122:1737–9.
Neuhann TH, Neuhann RT, Hornbeak DM. Ten-year effectiveness and safety of trabecular micro-bypass stent implantation with cataract surgery in patients with glaucoma or ocular hypertension. Ophthalmol Ther. 2024;13:2243–54.
Balas M, Mathew DJ. Minimally invasive glaucoma surgery: a review of the literature. Vision. 2023;7:54.
Zaifar A, Pratomo TG, Suryono AN. Comparison between MIGS with trabeculectomy in the management of open-angle glaucoma with cataract: a systematic review. Indian J Ophthalmol. 2024;72:S345–53.
Wagner IV, Stewart MW, Dorairaj SK. Updates on the diagnosis and management of glaucoma. Mayo Clin Proc Innov Qual Outcomes. 2022;6:618–35.
Tekcan H, İmamoğlu S, Mangan MS. Anterior segment changes and refractive outcomes after cataract surgery combined with gonioscopy-assisted transluminal trabeculotomy in open-angle glaucoma. Turk J Ophthalmol. 2023;53:369–76.
Dorairaj S, Radcliffe NM, Grover DS, Brubaker JW, Williamson BK. A review of excisional goniotomy performed with the Kahook Dual Blade for glaucoma management. J Curr Glaucoma Pract. 2022;16:59–64.
Laroche D, Nkrumah G, Ugoh P, Ng C. Real-world outcomes of Kahook Dual Blade goniotomy in Black and Afro-Latinx adult patients with glaucoma: a 6-month retrospective study. J Natl Med Assoc. 2021;113:230–6.
Dorairaj SK, et al. 12-Month outcomes of goniotomy performed using the Kahook Dual Blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther. 2018;35:1460–9.
Barkander A, Economou MA, Jóhannesson G. Kahook Dual-Blade goniotomy with and without phacoemulsification in medically uncontrolled glaucoma. Clin Ophthalmol Auckl NZ. 2023;17:1385–94.
Miller VJ, et al. Outcomes of Kahook Dual Blade goniotomy for uveitis associated open-angle glaucoma or ocular hypertension. J Glaucoma. 2022;31:903–8.
Iwasaki K, et al. Long-term outcomes of a Kahook Dual Blade procedure combined with phacoemulsification in Japanese patients with open-angle glaucoma. J Clin Med. 2022;11:1354.
Albuainain A, Al Habash A. Three-year clinical outcomes of phacoemulsification combined with excisional goniotomy using the Kahook Dual Blade for cataract and open-angle glaucoma in Saudi Arabia. Saudi J Ophthalmol. 2022;36:213–7.
Wagner IV, et al. Long-term efficacy of successful excisional goniotomy with the Kahook Dual Blade. Clin Ophthalmol Auckl NZ. 2024;18:713–21.
Dorairaj S, Tam MD. Kahook Dual Blade excisional goniotomy and goniosynechialysis combined with phacoemulsification for angle-closure glaucoma: 6-month results. J Glaucoma. 2019;28:643–6.
Dorairaj S, Tam MD, Balasubramani GK. Twelve-month outcomes of excisional goniotomy using the Kahook Dual Blade® in eyes with angle-closure glaucoma. Clin Ophthalmol Auckl NZ. 2019;13:1779–85.
Dorairaj S, Tam MD, Balasubramani GK. Two-year clinical outcomes of combined phacoemulsification, goniosynechialysis, and excisional goniotomy for angle-closure glaucoma. Asia-Pac J Ophthalmol Phila Pa. 2020;10:183–7.
Al Habash A, Albuainain A. Long term outcome of combined phacoemulsification and excisional goniotomy with the Kahook Dual Blade in different subtypes of glaucoma. Sci Rep. 2021;11:10660.
American Academy of Ophthalmology. ICD-10-CM Quick Reference Guide for Glaucoma. San Francisco: American Academy of Ophthalmology; 2015.
Radwan L, et al. Outcomes of phacoemulsification with or without Kahook Dual Blade goniotomy for glaucoma patients with cataract. J Glaucoma. 2024. https://doi.org/10.1097/IJG.0000000000002429.
Irie A, et al. Trabeculotomy using the Kahook Dual Blade for exfoliation glaucoma and primary open-angle glaucoma: comparison of outcomes according to incision range. J Glaucoma. 2024;33:270–6.
Espinoza G, et al. Twelve-month outcomes of Kahook Dual Blade goniotomy combined with cataract surgery in Latino patients. Int Ophthalmol. 2024;44:44.
Birnbaum F, et al. Postoperative management of Kahook Dual Blade goniotomy with phacoemulsification cataract extraction. J Curr Glaucoma Pract. 2023;17:169–74.
Mochizuki T, et al. Surgical outcomes of ab interno trabeculotomy without phacoemulsification. Clin Ophthalmol Auckl NZ. 2024;18:9–16.
Richter GM, et al. Trabecular procedures combined with cataract surgery for open-angle glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2024;131:370–82.
Salimi A, et al. Outcomes and risk factors for Kahook Dual Blade excisional goniotomy with concomitant phacoemulsification: a multicentre Canadian study. Can J Ophthalmol. 2023;S0008–4182(23):00249–51. https://doi.org/10.1016/j.jcjo.2023.08.004.
Bravetti GE, et al. Surgical outcomes of excisional goniotomy using the Kahook Dual Blade in severe and refractory glaucoma: 12-month results. Eye. 2023;37:1608–13.
Harvey BJ, Khaimi MA. A review of canaloplasty. Saudi J Ophthalmol. 2011;25:329–36.
Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144:533-540.e2.
Rodica L. Peculiarities of ocular and systemic pathology in the elderly. J Ophthalmol Adv Res. 2022. https://doi.org/10.46889/JOAR.2022.3206.
Carter TL. Age-related vision changes: a primary care guide. Geriatrics. 1994;49:37–42, 45 (quiz 46–47)
Ehrlich R, et al. Age-related ocular vascular changes. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2009;247:583–91.
Wyatt HJ, Dul MW, Swanson WH. Variability of visual field measurements is correlated with the gradient of visual sensitivity. Vis Res. 2007;47:925–36.
Rabiolo A, et al. Quantification of visual field variability in glaucoma: implications for visual field prediction and modeling. Transl Vis Sci Technol. 2019;8:25.
Giammaria S, et al. Rates of visual field change in patients with glaucoma and healthy individuals. JAMA Ophthalmol. 2022;140:504–11.
Garway-Heath DF, et al. Evaluation of visual field and imaging outcomes for glaucoma clinical trials (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2017;115:T4.
Smith SD, Katz J, Quigley HA. Analysis of progressive change in automated visual fields in glaucoma. Investig Ophthalmol Vis Sci. 1996;37:1419–28.
Chauhan BC, Nicolela MT, Artes PH. Incidence and rates of visual field progression after longitudinally measured optic disc change in glaucoma. Ophthalmology. 2009;116:2110–8.
Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol (Copenh). 2013;91:406–12.
Aptel F, et al. Progression of visual field in patients with primary open-angle glaucoma—ProgF study 1. Acta Ophthalmol (Copenh). 2015;93:e615-620.
Machiele R, Motlagh M, Zeppieri M, Patel BC. Intraocular pressure. In: Pi J (ed.) StatPearls. Treasure Island: StatPearls Publishing; 2024.
Acknowledgements
We thank the participants of the study. The authors acknowledge Ms. Joyce Baker for her generous contributions to the Department of Ophthalmology, Mayo Clinic, Jacksonville, FL, USA.
Author Contribution
Pranav Vasu—statistical analysis, drafting manuscript, data curation. Yazan Abubaker—data curation, drafting manuscript. Nithya Boopathiraj—concept and design, data curation. Isabella V. Wagner—concept and design, data curation, drafting manuscript. P. Connor Lentz—concept and design, data curation. Emily Dorairaj—data curation, drafting manuscript. Aya Shokair—drafting manuscript. Ibrahim Qozat—supervision, manuscript review. Darby D Miller—supervision, manuscript review. Syril Dorairaj—supervision, manuscript review.
Funding
No funding or sponsorship was received for the study or publication of this article. The Rapid Service Fee was funded by the authors.
Data Availability
The datasets generated during and/or analyzed during the current study may be available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Pranav Vasu, Yazan Abubaker, Nithya Boopathiraj, Isabella V. Wagner, P. Connor Lentz, Emily Dorairaj, Aya Shokair, Ibrahim Qozat, Darby D Miller, and Syril Dorairaj all have nothing to disclose.
Ethical Approval
The study protocol was reviewed and approved by the Mayo Clinic Institutional Review Board (IRB) on 28 February 2018 (IRB 18-000006). This study was conducted in accordance with the tenets of the Declaration of Helsinki of 1964 and its later amendments. Informed consent was waived by the Mayo Clinic IRB. All patient data has been deidentified, and no identifying information is present within this report.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Vasu, P., Abubaker, Y., Boopathiraj, N. et al. Clinical Outcomes of Excisional Goniotomy with the Kahook Dual Blade: 6-Year Results. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-01016-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-01016-8