Abstract
Objectives
To describe the efficacy and safety of goniotomy with trabecular meshwork excision using the Kahook Dual Blade (KDB, New World Medical Inc., Rancho Cucamonga, CA) in patients with severe or refractory glaucoma.
Methods
This retrospective multicentre case series reports on 40 eyes with severe or refractory open-angle glaucoma that underwent standalone or combined KDB goniotomy and were followed for 12 months post-operatively in the United-States, Mexico and Switzerland. Surgical success was defined as an intraocular pressure (IOP) reduction ≥20% from baseline at 12 months, with fewer medications than preoperatively. Mean IOP and antiglaucoma medication reduction, probabilities of achieving an IOP ≤16 or 18 mmHg, and adverse events were also analysed.
Results
Mean IOP decreased from 18.1 ± 5.0 mmHg at baseline to 14.8 ± 3.7 mmHg at 12 months (18.2% reduction, P < 0.001). Concomitantly, the mean number of glaucoma medications decreased from 2.5 ± 1.4 to 1.7 ± 1.2 (32% reduction, P = 0.002). The proportion of eyes achieving an IOP reduction of more than 20% from baseline was 37.5% (n = 15) at 12 months. At 12 months, 67.5% and 82.5% achieved a medicated IOP ≤ 16 and ≤18 mmHg, respectively. No severe complications were reported.
Conclusion
Excisional goniotomy with KDB achieves a statistically significant IOP and antiglaucoma medication reduction in severe or refractory glaucoma over a period of 12 months. While its efficacy decreases with time, its favourable safety profile makes it a potentially useful primary or adjunctive procedure in high-risk eyes.
Similar content being viewed by others
Introduction
Elevated intraocular pressure (IOP) is a major risk factor for glaucoma, and its reduction is the cornerstone of glaucoma management [1]. Filtering surgery, glaucoma drainage devices (GDDs) and cyclodestructive procedures are traditionally performed for the treatment of severe and refractory glaucoma when maximal medical therapy or laser interventions fail [2]. Despite relatively high rates of complications, those techniques provide greater IOP reduction compared to less invasive alternatives. Most adverse events are related to the formation of a subconjunctival or retro-orbital bleb, and include fibrosis, uncontrolled IOP, wound leak, hypotony and infection [3]. Due to these post-operative risks, bleb-creating procedures have generally been reserved for patients with moderate-to-advanced disease [4].
In recent years, several minimally invasive glaucoma surgery (MIGS) techniques have emerged in order to provide clinicians with a safe, effective, and less invasive surgical alternative, encouraging an early transition to surgical treatment, while delaying, or avoiding, more invasive surgeries [5]. Some MIGS target the sub-conjunctival filtration pathway with the creation of a filtering bleb, while others avoid bleb formation by shunting aqueous humour into Schlemm’s canal via stents or routing aqueous into the suprachoroidal space [6]. While the literature abounds with evidence of efficacy and safety for MIGS techniques in mild-to-moderate glaucoma [7,8,9,10,11,12,13,14,15,16], data on MIGS procedures for the treatment of severe and refractory glaucoma are scarce.
The juxta-canalicular trabecular meshwork (TM), most adjacent to Schlemm’s canal, is considered to be the main site of aqueous outflow resistance, especially in glaucomatous eyes [17, 18]. Based on this assumption, incising or removing TM should lower IOP. In recent years, multiple studies have explored the notion of reducing TM resistance using various procedures and devices. Some approaches achieve this by incising sections of TM with little or no actual tissue removal (trabectome and gonioscopy-assisted transluminal trabeculotomy), while others merely penetrate the TM to dilate Schlemm’s canal (suture canaloplasty or viscocanalostomy).
Goniotomy with the Kahook Dual Blade (KDB; New World Medical, Inc, Rancho Cucamonga, CA) is a relatively recent surgical method that differs from other TM procedures in that it involves the ab interno removal of a strip of TM tissue providing a wide opening between the anterior chamber and Schlemm’s canal, with no permanent device implanted in the eye. It is designed with a taper at the tip of the blade to allow for smooth entry into Schlemm’s canal. The device is then advanced along Schlemm’s canal, as the ramp at the distal end of the instrument elevates the TM tissue and guides it toward the blades on either side of the device, which incises trabecular tissues to allow for removal [19]. The aim of KDB is to perform safe and effective goniotomy with TM excision (GTE), while minimising collateral damage [20]. Goniotomy with the KDB is intended to be used both as a standalone procedure or combined with cataract surgery.
The aim of this study was to assess the 12-month safety and efficacy of GTE using KDB in patients with severe or refractory glaucoma.
Materials and methods
Study design
This was a retrospective, multicentre study. Data were collected from 11 surgeons in 11 centres in the United States, Mexico and Switzerland using standardised de-identified data forms. The study complies with the tenets of the Declaration of Helsinki and written informed consent was obtained from all included patients.
Study population
Every patient who had undergone standalone or combined KDB goniotomy for severe or refractory glaucoma at one of the investigation centres between January and September 2017 were retrospectively included if 12-month post-operative data were available. Every effort was made to include every suitable patient as per the inclusion and exclusion criteria. Severe glaucoma was defined as per the ICD-10 definition as optic nerve abnormalities consistent with glaucoma, and glaucomatous visual field abnormalities in both hemifields and/or loss within 5 degrees of fixation in at least one hemifield [21]. Refractory glaucoma was defined as eyes with glaucoma that were uncontrolled by maximal medical therapy and failed one or more incisional intraocular glaucoma procedure or cycloablative procedure. Inclusion criteria were a diagnosis of primary or secondary glaucoma classified as severe or refractory, and uncontrolled, which was defined as either progressive or not achieving individual IOP targets. Exclusion criteria were pre-operative IOP < 6 mmHg or > 40 mmHg, and age under 18 years. In bilateral cases, both eyes of a given patient were included if both met these eligibility criteria.
Baseline measurements
For each included patient, the following demographics and clinical characteristics were collected: age, gender, ethnicity, glaucoma diagnosis and surgical history, best corrected visual acuity (BCVA) in logMAR, number of antiglaucoma medications, central corneal thickness (CCT), IOP, mean RNFL thickness, visual field mean deviation (MD) and root square of loss variance (sLV). Baseline IOP was defined as the mean of the last two pre-operative measurements by Goldmann applanation tonometry, without medication washout.
Single-use dual blade
The KDB has a microengineered profile which allows for insertion into the eye through a clear cornea microincision, to reach the TM across the anterior chamber. The device has a sharp distal tip designed for smooth entry of the blade through the TM and into Schlemm’s canal. Its ramp rises from the distal tip, elevates and stretches the TM tissue, guiding it toward two parallel blades. The dual blades excise a strip of TM and the foot plate prevents damage to anterior wall of the canal and facilitates smooth motion. According to the manufacturer, elevating the TM above its natural position and stretching it before cutting it removes the tissue more cleanly and minimises damage to adjacent structures. The device was described in more detail elsewhere [22].
Surgical technique
Surgical procedures were performed under topical anaesthesia in the operating room, and all participating surgeons reported using the same surgical protocol. A clear corneal incision was made temporally with a 15-degree knife. Viscoelastic was injected in the anterior chamber (AC). The patient’s head was rotated 30 to 45 degrees away from the surgeon and the microscope was tilted 30 to 45 degrees in the opposite direction. Under gonioscopy, the KDB was inserted through the corneal incision and introduced nasally into the TM. The device was advanced along Schlemm’s canal, excising TM. After excising sufficient TM, the dual blade was rotated 180 degrees and positioned a few clock hours away from the first treatment area. The reverse procedure was performed to join the initial excision. The total treatment area was approximately 90-to-110 degrees. A floating strip of TM was created and visible in the angle, as well as the posterior wall of Schlemm’s canal. The KDB was removed and the free-floating TM strip was either removed with forceps or aspirated by irrigation-aspiration during removal of the viscoelastic. When phacoemulsification was indicated, a principal corneal incision was made at its steepest axis, and 2 paracentesis incisions were performed 1 mm from the limbus, in nasal and infero-temporal positions. Phacoemulsification was performed in a standard manner until the end of the emulsification stage when AC viscoelastic was left in place, and the KDB procedure was performed through the temporal paracentesis as previously described.
Postoperative management
All antiglaucoma medications were withdrawn after the procedure. Postoperative management included a combination of topical antibiotic with a steroid and/or nonsteroidal anti-inflammatory drug for 1 to 2 months at the discretion of each surgeon. Postoperative follow-up visits were scheduled weekly for the first month, then at 3-month, 6-month, 9-month, and 12-month. If necessary, antiglaucoma topical medications were reintroduced at the surgeons’ discretion.
The following data were collected from medical notes for each follow-up visit: IOP, adverse events (AEs), BCVA and antiglaucoma medications. IOP was assessed using Goldmann applanation tonometry. Fixed combination medications were documented according to the number of active ingredients.
Outcome measures
Several definitions of surgical success were considered. Complete success was defined as either unmedicated IOP at last follow-up visit ≤12 mmHg, ≤16 mmHg, or ≤18 mmHg and at least a 20% reduction from baseline; or as qualified success if medicated IOP met the same thresholds. Additional success rates were reported for the combinations of IOP reduction ≥ 20% and fewer medications than baseline. Loss of light perception, serious irreversible complications, IOP > 18 mmHg or any subsequent glaucoma surgical intervention were considered surgical failure. Secondary efficacy and safety outcome measures included the mean reduction in IOP and topical hypotensive medications, and the rate of surgical failure. Safety endpoints included the rate of intraoperative complications and post-operative AEs during the entire follow-up.
Statistical analysis
Descriptive statistics included mean and standard deviation (SD) for normally distributed variables, and median and interquartile range (IQR) for non-normally distributed variables. Kaplan–Meier survival curves were used to assess the cumulative probability of success. Associations between failure and demographic or clinical variables such as age, gender, ethnicity, diagnosis, number of preoperative treatments or surgeries were assessed using multivariate Cox proportional hazard regression model. All tests were two-tailed and a P-value less than 0.05 was considered statistically significant. Statistical analyses were performed with commercially available software (Stata version 13.1; StataCorp, College Station, TX).
Results
Baseline characteristics of study population
A total of 83 eyes of 74 patients were included. Data from 40 eyes (48.2%) of 34 patients were available at 12 months. Out of them, 32 (80%) had severe glaucoma and 8 (20%) refractory glaucoma. The mean age at baseline was 75.4 ± 8.3 years, 55.9% (n = 19) were female, and the majority of patients (50%, n = 17) were Caucasian. In all, 67.5% of eyes had a diagnosis of POAG, followed by angle-closure glaucoma (15%); 19 eyes (47.5%) were pseudophakic. Selective laser trabeculoplasty was previously performed on 20% of the eyes (n = 8). Eight eyes (20%) experienced previous glaucoma surgery, including 2 combined phacoemulsification-filtering surgery, 3 deep sclerectomies and 1 trabeculectomy. Out of 40 eyes, 52.5% underwent standalone KDB surgery, and 47.5% underwent KDB combined with phacoemulsification. Demographics and baseline characteristics of the study patients are summarised in Table 1.
Safety
Only 1 case (2.5%) of serious intraoperative complications in the form of iridodialysis was observed in the cohort. Thirteen eyes (32.5%) presented perioperative hyphaema. None of the combined procedures were associated with posterior capsule rupture or required anterior vitrectomy.
Intraocular pressure and medication use
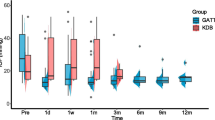
Mean medicated IOP at baseline and at 12 months were 18.1 ± 5.0 mmHg and 14.8 ± 3.7 mmHg, respectively (−18.2%; P < 0.001). Postoperative IOP reduction was statistically significant at all postoperative timepoints through 12 months (P < 0.05). The number of anti-glaucoma medications concomitantly dropped from 2.5 ± 1.4 at baseline to 1.7 ± 1.2, representing a reduction of 32.0% (P = 0.003). The proportion of patients whose regimen decreased by more than 1 medication at 12 months was 57.5% (n = 23). At the last follow-up visit, 85% of eyes (n = 34) required antiglaucoma medications to achieve target IOP. Antiglaucoma medications and IOP progression throughout the follow-up period are presented in Figs. 1, 2.
Primary outcome: surgical success
At 12 months, 15 eyes (37.5%) achieved an IOP reduction of 20% or more with fewer medications than preoperatively. Complete success at last follow-up visit was achieved in 7.5% of eyes using the strictest absolute threshold of 12 mmHg or less, whereas 15% of eyes achieved an unmedicated IOP of 18 mmHg or less. Qualified success was achieved in 15% of eyes using the 12 mmHg or less definition, while 67.5% and 82.5% of eyes achieved a medicated IOP ≤ 16 mmHg and ≤ 18 mmHg, respectively. The Kaplan–Meier survival curves are presented in Fig. 3. In total, 7 eyes (17.5%) were classified as complete failure due to uncontrolled IOP above 18 mmHg despite medical treatment. The average time of complete failure was 9.0 ± 3.6 months after surgery. Table 2 (A) presents the surgical success and failure rates against all definitions. Association analysis showed no statistically significant association between surgical outcomes and any of the patients’ demographics or recorded clinical data.
Postoperative complications
Amongst the 13 eyes in which perioperative blood reflux was observed, spontaneous resorption was observed in 76.9% after 1 week, and in 100% after 1 month. One day after surgery, corneal oedema was observed in 4 eyes (10.0%). Four cases (10.0%) presented with IOP spike above 21 mmHg at week 1, which persisted through 1 month in 2 cases (5.0%) and required reinstatement of antiglaucoma therapy. One case of intravitreal haemorrhage was reported at day 1 with spontaneous resolution. No severe or sight threatening complications were reported. Seven eyes (17.5%) experienced refractory intraocular hypertension during the follow-up, and amongst those, one eye required secondary glaucoma filtering surgery at 9 months. Table 2 (B) shows the ocular adverse events observed in the study.
Discussion
The present study constitutes the first study of goniotomy with trabecular meshwork excision using KDB in severe and refractory glaucoma, with a postoperative follow-up of 12 months. The results demonstrate that good long-term IOP-lowering was achieved, with an average IOP reduction of 18.2% from the medicated baseline, in a high-risk cohort of treatment-resistant eyes. The IOP reduction was accompanied by a significant decrease (−32.0%) in antiglaucoma medications, independent of concomitant cataract extraction. We observed a relatively low rate of postoperative AEs. Moreover, there was an absence of serious sight-threatening complications, such as endophthalmitis, persistent hypotony or inflammation-related AEs, that can be relatively common following GDD or filtering surgery. Despite these encouraging results, however, this was accompanied with relatively high rates of eyes classified as failure due to a persistent ocular hypertension (17.5%). Yet, these results are within that reported by other groups following other types of surgery in refractory glaucoma [23,24,25]. Interestingly, most patients who were considered a success at 6 months [26] remained stable through 1 year, as evidenced by the surgical success rates between months 6 and 12 (92.3% vs 82.5%) using the 18 mmHg or less threshold. On the other hand, we observed that the rate of IOP reduction from baseline was higher at 6 months compared to 12 months (23.9% vs 18.2%), and that there was a sensible difference between the portion of eyes that achieved an IOP reduction greater than 20% from baseline at 6 months if compared to 1 year (57.7% vs 37.5%, respectively). Those results highlight the fact that the efficacy of the goniotomy with KDB in severe and refractory cases decreases with time. Those are important findings because scarce data exist beyond the 6-month timepoint for the KDB in this patient population, and the question of durability of success represents one of the greatest challenges to evaluating glaucoma surgical procedures.
To the best of our knowledge, no study has investigated the long-term effect of GTE with KDB exclusively in patients with severe and refractory glaucoma, therefore none is available for comparison. Yet, a small case series published by Miller et al. observed the outcomes of KDB goniotomy in 16 patients with uveitis-associated glaucoma. Despite the inclusion of complex cases, 62.5% (n = 10) maintained an IOP at or below their individual target through 9 months, and only a small percentage of patients needed more invasive glaucoma surgery [27]. In a larger study by Greenwood et al., phacoemulsification combined with GTE using KDB achieved an IOP reduction of 26% at 6 months. The mean baseline IOP decreased from 17.4 ± 5.2 mmHg to 12.8 ± 2.6 mmHg, and 58.3% of eyes had an IOP reduction greater than 20% from baseline [22]. The superior efficacy reported can certainly be attributed to the shorter follow-up and to the differences in patient selection, as only 35% of eyes were classified as suffering from severe glaucoma. Another study compared the 12 month outcomes of phacoemulsification combined either with goniotomy performed with the KDB or with single iStent trabecular bypass implantation in eyes with mild-to-moderate glaucoma. The authors showed that the mean IOP reduction was significantly greater in the phaco-KDB group compared to the phaco-iStent group (−5.0 ± 0.3 mmHg vs −2.3 ± 0.4 mmHg), and the proportion of patients achieving IOP reduction greater than 20% was higher in the goniotomy group (64.2% vs 41.6%) [28]. Yet, the difference in glaucoma severity and the effect of cataract extraction on the IOP, make comparison with our present results impossible.
Two recent studies evaluated the safety and efficacy of GTE with KDB at 12 and 18 months [29, 30]. Wakil et al. showed interesting results with postoperative IOPs of 14.4 ± 3.7 mmHg and 16.7 ± 7.6 mmHg for phaco-KDB group and standalone KDB, respectively at 18 months. The number of antiglaucoma medications after surgery was 1.3 ± 1.2 and 2.6 ± 1.2, respectively [29]. A postoperative IOP reduction of 19.3% at 12 months was observed by ElMallah et al., together with a reduction in antiglaucoma medications of 12.5% at the same time point [30]. The long-term results of both those studies in a cohort including patients ranging from mild to severe glaucoma matched our present results, despite the fact that our cohort was comprised solely of severe and refractory eyes [29, 30]. Moreover, ElMallah et al., observed a similar loss of efficacy between 6 and 12 months, with IOP reduction diminishing from 27.7% to 19.3%, confirming our observation and suggesting a similar postoperative progression in all glaucomatous eyes [30].
It has been shown that fewer medications are positively correlated with patient adherence and quality of life [31]. Furthermore, the reduction in chronic topical glaucoma medications might preserve the ocular surface from inflammation and its consequences [32]. In fact, patients with severe or refractory glaucoma often have scarred, thin, or inflamed conjunctiva. As goniotomy avoids the formation of a filtering bleb and its associated complications, it may constitute a safe alternative to subconjunctival procedures for these patients.
In the present study, the most common adverse event was perioperative blood reflux. It was observed in 32.5% of eyes, resolving spontaneously without complication. Those numbers compare well with other studies [22, 29, 30]. Also, blood reflux confirms the complete removal of TM and thus indicates correct execution of the procedure. Other adverse events, such as cystoid macular oedema, peripheral anterior synechia or tear in Descemet membrane, described in other studies using KDB were not observed in our study [22]. This study confirms the favourable safety profile of this procedure, which may support its use as a primary or adjunctive procedure in patients with severe or refractory glaucoma.
Study limitations
Despite its multicentric design and the heterogeneity of its cohort, the present study has several limitations. First, the retrospective nature of the study did not permit any randomisation or medication washout. Furthermore, the nature of the studied indication implies that only a relatively small number of patients met the inclusion criteria, leading to a potential size bias. Second, surgery was performed by 11 surgeons, which can be considered both a limitation or a strength. Even if this procedure is described as simple and fast, the difference of experience might influence the efficacy and safety of the procedure. Moreover, while the maximum treated area was between 90 and 110 degrees, the difference in treatment areas by different surgeons or in different patients, might affect the results. Finally, medication regimen and reinstatement protocols were not standardised, which might influence the number of post-operative antiglaucoma medications and, to a lesser extent, the surgical outcomes.
The present study demonstrates that excisional goniotomy with KDB achieves a statistically significant IOP and antiglaucoma medication reduction in severe or refractory glaucoma over a period of 12 months postoperatively. While its efficacy decreases with time, its very favourable safety profile makes it a potentially useful primary or adjunctive procedure in high-risk eyes, with thin, scarred and inflammatory conjunctiva. Further prospective and randomised studies are required to characterise long-term efficacy and safety of the dual-blade as a standalone procedure.
Summary
What was known before?
-
Excisional goniotomy with KDB have emerged in order to provide clinicians with a safe, effective, and minimally invasive alternative to traditional surgery.
-
Through recent years excisional goniotomy with KDB has demonstrated to be effective and safe in patients affected by mild-to-moderate glaucoma.
What this study adds?
-
Scare data exist on angle surgery in patients presenting sever or refractory glaucoma.
-
The present study demonstrates that excisional goniotomy with KDB achieves a statistically significant IOP and antiglaucoma medication reduction in severe or refractory glaucoma over a period of 12 months postoperatively.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. Jama. 2014;311:1901–11.
Mansouri K, Medeiros FA, Weinreb RN. Global rates of glaucoma surgery. Graefes Arch Clin Exp Ophthalmol. 2013;251:2609–15.
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–14.e1.
Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of various glaucoma surgeries and procedures in medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122:1615–24.
Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PloS One. 2017;12:e0183142.
Gillmann K, Mansouri K. Minimally invasive glaucoma surgery: where is the evidence? Asia Pac J Ophthalmol (Philos). 2020;9:203–14.
Gillmann K, Mansouri K, Ambresin A, Bravetti GE, Mermoud A. A prospective analysis of istent inject microstent implantation: surgical outcomes, endothelial cell density, and device position at 12 months. J Glaucoma. 2020;29:639–47.
Wellik SR, Dale EA. A review of the iStent(®) trabecular micro-bypass stent: safety and efficacy. Clin Ophthalmol. 2015;9:677–84.
Chaudhary A, Salinas L, Guidotti J, Mermoud A, Mansouri K. XEN Gel Implant: a new surgical approach in glaucoma. Expert Rev Med devices. 2018;15:47–59.
Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, et al. Efficacy, safety, and risk factors for failure of standalone Ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124:1579–88.
Mansouri K, Guidotti J, Rao HL, Ouabas A, D’Alessandro E, Roy S, et al. Prospective evaluation of standalone XEN gel implant and combined phacoemulsification-XEN gel implant surgery: 1-year results. J Glaucoma. 2018;27:140–7.
Mansouri K, Bravetti GE, Gillmann K, Rao HL, Ch’ng TW, Mermoud A. Two-year outcomes of XEN gel stent surgery in patients with open-angle glaucoma. Ophthalmol Glacoma. 2019;2:309–18.
Gillmann K, Bravetti GE, Rao HL, Mermoud A, Mansouri K. Combined and stand-alone XEN 45 gel stent implantation: 3-year outcomes and success predictors. Acta Ophthalmologica. 2020;99:e531–9.
Gillmann K, Bravetti GE, Rao HL, Mermoud A, Mansouri K. Impact of phacoemulsification combined with XEN gel stent implantation on corneal endothelial cell density: 2-year results. J Glaucoma. 2020;29:155–60.
Mansouri K, Gillmann K, Rao HL, Guidotti J, Mermoud A. Prospective evaluation of XEN gel implant in eyes with pseudoexfoliative glaucoma. J Glaucoma. 2018;27:869–73.
Gillmann K, Bravetti GE, Mermoud A, Rao HL, Mansouri K. XEN gel stent in pseudoexfoliative glaucoma: 2-year results of a prospective evaluation. J Glaucoma. 2019;28:676–84.
Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88:648–55.
Grant WM. Clinical measurements of aqueous outflow. Am J Ophthalmol. 1951;34:1603–5.
SooHoo JR, Seibold LK, Kahook MY. Ab interno trabeculectomy in the adult patient. Middle East Afr J Ophthalmol. 2015;22:25–9.
Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155:524–9.e2.
Parekh AS, Tafreshi A, Dorairaj SK, Weinreb RN. Clinical applicability of the International Classification of Disease and Related Health Problems (ICD-9) glaucoma staging codes to predict disease severity in patients with open-angle glaucoma. J Glaucoma. 2014;23:e18–22.
Greenwood MD, Seibold LK, Radcliffe NM, Dorairaj SK, Aref AA, Román JJ, et al. Goniotomy with a single-use dual blade: Short-term results. J Cataract Refract Surg. 2017;43:1197–201.
Dixon MW, Moulin TA, Margolis MS, Palko JR, Mortensen P, Conner IP, et al. Comparative outcomes of the molteno3 and baerveldt glaucoma implants. Ophthalmol Glaucoma. 2020;3:40–50.
Bravetti GE, Mansouri K, Gillmann K, Rao HL, Mermoud A. XEN-augmented Baerveldt drainage device implantation in refractory glaucoma: 1-year outcomes. Graefes Arch Clin Exp Ophthalmol. 2020;258:1787–94.
Arad T, Hoffmann EM, Prokosch-Willing V, Pfeiffer N, Grehn F. XEN-augmented baerveldt implantation for refractory childhood glaucoma: a retrospective case series. J Glaucoma. 2019;28:1015–8.
Salinas L, Chaudhary A, Berdahl JP, Lazcano-Gomez GS, Williamson BK, Dorairaj SK, et al. Goniotomy using the kahook dual blade in severe and refractory glaucoma: 6-month outcomes. J Glaucoma. 2018;27:849–55.
Miller VJ, Young CEC, SooHoo JR, Seibold LK, Kahook MY, Pecen PE, et al. Efficacy of goniotomy with kahook dual blade in patients with uveitis-associated ocular hypertension. J Glaucoma. 2019;28:744–8.
ElMallah MK, Seibold LK, Kahook MY, Williamson BK, Singh IP, Dorairaj SK. 12-month retrospective comparison of kahook dual blade excisional goniotomy with istent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv Ther. 2019;36:2515–27.
Wakil SM, Birnbaum F, Vu DM, McBurney-Lin S, ElMallah MK, Tseng H. Efficacy and safety of kahook dual blade goniotomy: 18-month results. J Cataract Refract Surg. 2020.
ElMallah MK, Berdahl JP, Williamson BK, Dorairaj SK, Kahook MY, Gallardo MJ, et al. Twelve-month outcomes of stand-alone excisional goniotomy in mild to severe glaucoma. Clin Ophthalmol. 2020;14:1891–7.
Sleath B, Blalock SJ, Carpenter DM, Sayner R, Muir KW, Slota C, et al. Ophthalmologist-patient communication, self-efficacy, and glaucoma medication adherence. Ophthalmology. 2015;122:748–54.
Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–23.
Acknowledgements
Supported in part by the Swiss Glaucoma Research Foundation, Lausanne, Switzerland.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by, Kaweh Mansouri, Kevin Gillmann and Giorgio Enrico Bravetti. The first draft of the manuscript was written by Giorgio Enrico Bravetti. Kaweh Mansouri and Kevin Gillmann commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
JPB: Alcon, Allergan, Bausch and Lomb, CorneaGen, Dakota Lions Eye Bank, Equinox, Expert Opinion, Glaucos, Gore, Imprimis/Harrow Health, JNJ, Kala, Kedalion, MELT Pharmaceuticals, MicroOptix, New World Medical, Ocular Surgical Data, Ocular Theraputix, Omega Ophthalmic, Orasis, Oyster Point, RxSight, Surface Inc., Tarsus, Tear Clear, Vittamed, Vance Thompson Vision, Verana Health (Digisight), Visionary Ventures, Zeiss. Lazcano-Gomez GSL-G: New World Medical. BKW: New World Medical. SKD: New World Medical, Iridex. LS: New World Medical. AAA: New World Medical, Aerie Pharmaceuticals, Bausch and Lomb. AM: Allergan, Bausch and Lomb, Iridex, New World Medical. KM: Santen, Sensimed, Topcon, Alcon, Allergan, Optovue, ImplantData. Others: no financial disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bravetti, G.E., Gillmann, K., Salinas, L. et al. Surgical outcomes of excisional goniotomy using the kahook dual blade in severe and refractory glaucoma: 12-month results. Eye 37, 1608–1613 (2023). https://doi.org/10.1038/s41433-022-02196-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02196-y
- Springer Nature Limited