Abstract
Introduction
The aim of this work is to evaluate the accuracy of the Barrett Universal II (BU II), Emmetropia verifying optical (EVO) 2.0, Haigis, Hoffer Q, Hoffer QST (Savini/Taroni) (HQST), Holladay 1, Kane, Ladas Super, Sanders–Retzlaff–Kraff/theoretical (SRK/T), and T2 intraocular lens (IOL) power formulas for calculating spherical equivalent (SE) of toric IOL.
Methods
This study enrolled consecutive patients who underwent phacoemulsification and toric IOL implantation at the Eye Hospital of Wenzhou Medical University in Hangzhou from 2015 to 2022. We compared the new-generation formulas with Gaussian optics-based standard formulas, and calculated the mean absolute error (MAE), median absolute error (MedAE), and percentage of eyes within ± 0.25 diopter (D), ± 0.50 D, ± 0.75 D and ± 1.00 D of the target refraction. Subgroup analyses were conducted based on the anterior chamber depth (ACD), keratometry (K), and toricity (T).
Results
A total of 207 eyes of 207 patients were included in this study. Overall, the Kane and EVO2.0 formulas demonstrated the lowest MedAEs. The EVO2.0 formula exhibited the highest percentage of eyes within ± 0.50 D, ± 0.75 D, ± 1.00 D. Moreover, the EVO2.0 formula showed the lowest MedAE for flat K subgroup, the highest percentage of eyes within ± 0.50 D, ± 1.00 D for shallow ACD subgroup, the highest percentage of eyes within ± 0.75 D for regular ACD, flat K, T2–T3, T4–T5 subgroups. The Kane and formula performed the lowest MedAE in the T4–T5 subgroup.
Conclusions
Application of the Kane and EVO2.0 formulas significantly improved the prediction of postoperative SE outcome for toric IOL compared to the other formulas.
Similar content being viewed by others
Why carry out this study? |
Previous studies focused on the intraocular lens (IOL) power calculation in predicting spherical equivalent (SE) for non-toric IOLs. |
This study aims to comprehensively assess and compare the overall accuracy of new-generation formulas against vergence-based formulas for calculating SE after toric IOL implantation. |
What was learned from the study? |
The Kane and Emmetropia verifying optical 2.0 formulas demonstrated high accuracy in predicting SE for toric IOLs. |
Three improved IOL calculation formulas, the Ladas Super, T2, and Hoffer QST(Savini/Taroni) formula, performed the superior outcome compared to traditional third-generation formulas for calculating SE after toric IOL implantation. |
Introduction
Cataract surgery is regarded as a combined rehabilitative and refractive procedure. As modern surgical techniques advance, patients’ expectations for optimal postoperative vision continue to rise. The precision of the preoperative intraocular lens (IOL) power formula stands out as a key factor influencing postoperative refractive outcomes.
Various formulas for IOL power calculation have evolved over time, aiming to enhance the accuracy of predicting refractive results. The third- and fourth-generation formulas, previously proposed, mostly employ vergence formulas based on Gaussian optics, including the Barrett Universal II (BU II) [1], Haigis [2], Holladay 1 [3], Sanders–Retzlaff–Kraff/theoretical (SRK/T) [4], and Hoffer Q [5] formulas. In contrast, the latest generation of IOL formulas use artificial intelligence (AI) and regression equations for predictions, demonstrating commendable performance. Notably, studies suggest that the Kane formula [6] seems to be one of the most accurate formulas available [7, 8]. The Emmetropia verifying optical (EVO)2.0 formulaC has also exhibited good performance, ranking among the most accurate formulas [8, 10]. Additionally, Sheard RM [11] and Taroni L [12] have enhanced the SRK/T and Hoffer Q formulas, resulting in the T2 [11] and Hoffer QST (Savini/Taroni) (HQST) [13] formulas, respectively, indicating improved accuracy for traditional formulas. Ladas JG [14] introduced a super surface, incorporating the optimal aspects from four out of the five third-generation formulas to generate a super formula [15].
Nonetheless, prior studies basically used non-toric IOLs to assess prediction accuracy. In fact, it is crucial to extend this evaluation to toric IOLs, comparing the precision of various formulas in predicting spherical equivalent (SE) outcomes so as to further improve the refractive results. Therefore, our study aims to comprehensively assess and compare the overall accuracy of new-generation formulas against vergence-based formulas for calculating SE after toric IOL implantation. Subgroup analyses will also be conducted for each anterior chamber depth (ACD), keratometry (K), and toricity (T) group. We hope the illustration of these formulas can guide the selection of IOL power formulas for patients with cataracts, promoting the attainment of improved postoperative refractive outcomes.
Methods
Patients
This is a retrospective study, including consecutive patients who underwent clear cornea temporal incision phacoemulsification and intracapsular implantation of the AcrySof SN6AT(2–9) IOL (Alcon Laboratories, Inc., Fort Worth, TX, USA) during the period 2015–2022, at the Eye Hospital of Wenzhou Medical University in Hangzhou. All patients were successfully measured preoperatively with the IOLMaster 500 or 700 (Carl Zeiss Meditec AG, Jena, Germany). Subjective refraction was performed 1 month post-operatively by three experienced optometrists. According to the recommendations of the editorials by Hoffer et al., [16, 17] only one eye from each patient was included at random, and only one single toric IOL type was used. Exclusion criteria were: (1) age below 18 or above 90; (2) axial length (AL) less than 22 mm and more than 26 mm; (3) preoperative corneal astigmatism greater than 4 diopters (D); (4) other previous ophthalmic diseases, previous ophthalmic trauma, previous ophthalmic surgeries; (5) any intraoperative or postoperative complication and (6) postoperative Snellen best-corrected distance visual acuity (BCVA) worse than 20/40. Subgroup analysis was performed based on preoperative ACD, K and T: shallow ACD group—ACD ≤ 3.00 mm, regular ACD group—3.00 mm < ACD < 3.50 mm, deep ACD group—ACD ≥ 3.50 mm; flat K group—K ≤ 43D, regular K group—43D < K < 45D, steep K group—K ≥ 45D; low-toricity group—T2–T3, medium-toricity group—T4–T5, high-toricity group—T6–T9. This study was performed in accordance with the Declaration of Helsinki of 1964 and its subsequent amendments. This study was approved by the Ethics Committee of Wenzhou Medical University (2021-036-K-29-01).
Formula Calculation and Constant Optimization
IOL constant optimization was carried out by varying the constant used by trial and error until the mean prediction error (ME) for each formula was 0.00 D, following Wang et al.'s recommendations [18]. Published IOL power formulas (e.g., the Haigis, Hoffer Q, Holladay 1, SRK/T, and T2 formulas) were optimized using Excel spreadsheets and validated against IOLMaster 500 or 700. We can achieve the optimized value of the predicted anterior chamber depth (pACD; Hoffer Q), surgeon factor (Holladay 1), and A constant (SRK/T and T2), and so forth. Outcomes for the single lens constant (a0) optimization with the Haigis formula were used to compare the formulas on a more equal basis [19]. The a1 and a2 constants we used were the already optimized values for the SN6AT(2–9) IOLs as listed on the User Group for Laser Interference Biometry (ULIB) website [20]. Unpublished formulas (e.g., the BU II, EVO2.0, Kane, Ladas Super and HQST formulas) were optimized using specific computer programming languages (e.g., Python Software Foundation, Wilmington, DE, USA) through respective online calculator.
Primary Outcomes
According to the previous protocol, the refractive prediction error (PE) for each eye was defined as the difference between the SE of the 1-month postoperative subjective refraction and the predicted postoperative refraction for each IOL power formula. A negative PE indicated a myopic result, while a positive PE represented a hyperopic outcome. The median absolute error (MedAE) and mean absolute error (MAE) were defined as the median absolute value and mean absolute value of the refractive PE, respectively. The percentage of eyes within different PE ranges (± 0.25 D, ± 0.50 D, ± 0.75 D and ± 1.00 D) were calculated. The impact of ACD, K and T on ME, MAE, MedAE and the percentage of eyes within different PE ranges, were also investigated.
Statistical Analysis
The data were presented using Microsoft Office Excel (Microsoft, Redmond, WA, USA), and statistical analysis was performed using SPSS (version 26.0, IBM, Armonk, NY, USA). The Shapiro–Wilk test was used to verify the normality of data distribution. The non-parametric Friedman M test (with Bonferroni correction for multiple comparisons) was used to compare the differences in absolute prediction error (AE) between formulas. The Cochran’s Q test was utilized to compare percentage of eyes between formulas within different PE groups. The one-sample t test was conducted to evaluate whether the ME of each formula in subgroups differed significantly from zero. A P value less than 0.05 was considered statistically significant. All reported P values were two-sided.
The sample size was calculated using PASS (version 15.0.5, NCSS LLC, Kaysville, UT, USA) based on MedAE, which was the primary parameter of interest in this study. We considered a two-sided 5% significance level and power of 95%. The calculation indicated that the total sample size should consist of 113 eyes.
Results
Baseline
The study included 207 eyes of 207 patients. Patients’ demographics and biometric data are presented at Table 1. Optimized IOL constants are shown in Table 2.
PE of the Overall Study Cohort
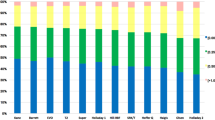
Table 3 and Fig. 1 summarize the MEs, standard deviation (SD)s, minimum (Min)s, maximum (Max)s, MAEs and MedAEs for the ten IOL formulas, as well as the percentage of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D of the target refraction for each formula.
Box plot graph of the absolute prediction errors and stacked bars comparing the percentage of eyes within a given diopter (D) range of predicted spherical equivalent (SE) refraction outcome. BU II Barrett Universal II, D diopters, EVO Emmetropia Verifying Optical, HQST Hoffer QST (Savini/Taroni), SRK/T Sanders–Retzlaff–Kraff/theoretica
The Kane and the EVO2.0 formulas were the most accurate formulas (P = 0.012, 0.016, respectively).
The Kane formula had the highest percentage of eyes within ± 0.25 D of the target refraction (52.7%), while the EVO2.0 formula had the highest percentage of eyes within ± 0.50 D, ± 0.75 D and ± 1.00 D (87.0, 98.1, 100.0%, respectively) (P = 0.025, 0.000, 0.021, respectively). In addition, the Ladas super formula also had the second highest values (97.1%) for the ± 0.75 D endpoint (P = 0.003). However, the BU II formula presented poor predictability (45.9%) in the percentage of eyes within ± 0.75 D, which was significantly different from the EVO2.0 formula (P = 0.048).
Subgroup Analysis with ACD
Table 4 and Figs. 2, 3, and 4 summarize the MEs, SDs, Mins, Maxs, MAEs, and MedAEs for the ten IOL formulas in ACD subgroups, as well as the percentage of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D for each formula.
Stacked bars comparing the percentage of cases within a given diopter (D) range of predicted spherical equivalent (SE) refraction outcome of each subgroup of anterior chamber depth (ACD). A Shallow ACD group, B Regular ACD group, C Deep ACD group. BU II Barrett Universal II, EVO Emmetropia Verifying Optical, HQST Hoffer QST (Savini/Taroni), SRK/T Sanders–Retzlaff–Kraff/theoretica
In the shallow ACD group, the formulas who had a ME significantly different from zero were the Hoffer Q and Holladay 1 formulas, with a myopic shift (P = 0.001, 0.034, respectively). No statistically significant difference existed in AE among formulas. The EVO2.0 formula had the highest percentage of eyes within ± 0.50 D and ± 1.00 D of the target refraction (92.5% and 100.0%, respectively) (P = 0.004 and 0.019, respectively).
In the regular ACD group, the formulas who had a ME significantly different from zero were the Hoffer Q, HQST, Holladay 1, Kane and T2 formulas, with a hyperopic shift (P = 0.003, 0.039, 0.026, 0.026, and 0.021, respectively). No statistically significant difference existed in AE among formulas. Both the EVO2.0 and Ladas super formulas had the highest percentage of eyes within ± 0.75 D of the target refraction (98.6% and 98.6%, respectively) (P = 0.005 and 0.004, respectively).
In the deep ACD group, the formulas who had a ME significantly different from zero were the Ladas super formula, with a myopic shift (P = 0.038). No statistically significant difference existed in AE as well as the percentage of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D among formulas.
Subgroup Analysis with K
Table 5 and Figs. 5, 6 and 7 summarizes the MEs, SDs, Mins, Maxs, MAEs and MedAEs for the ten IOL formulas in K subgroups, as well as the percentage of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D for each formula.
Stacked bars comparing the percentage of cases within a given diopter (D) range of predicted spherical equivalent (SE) refraction outcome of each subgroup of keratometry (K). A Flat K group, B Regular K group, C Steep K group. BU II Barrett Universal II, EVO Emmetropia Verifying Optical, HQST Hoffer QST (Savini/Taroni), SRK/T Sanders–Retzlaff–Kraff/theoretica
In the flat K group, the formulas who had a ME significantly different from zero were the BU II and SRK/T formulas, with a hyperopic shift (P = 0.037, 0.000, respectively). The EVO2.0 formula was the most accurate (P = 0.024), which also had the highest percentage of eyes within ± 0.75 D of the target refraction (100.0%) (P = 0.010).
In the regular K group, there was no significant difference between ME and zero in any formula. No statistically significant difference existed in AE as well as the percentage of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D among formulas.
In the steep K group, the formulas who had a ME significantly different from zero were the BU II, EVO2.0, SRK/T and T2 formulas, with a myopic shift (P = 0.023, 0.041, 0.000, 0.045, respectively). No statistically significant difference existed in AE as well as the percentage of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D among formulas.
Subgroup Analysis with T
Table 6 and Figs. 8, 9 and 10 summarizes the MEs, SDs, Mins, Maxs, MAEs and MedAEs for the ten IOL formulas in T subgroups, as well as the percentage of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D for each formula.
Stacked bars comparing the percentage of cases within a given diopter (D) range of predicted spherical equivalent (SE) refraction outcome of each subgroup of toricity (T). A Low-toricity group, B Medium-toricity group, C High-toricity group. BU II Barrett Universal II, EVO Emmetropia Verifying Optical, HQST Hoffer QST (Savini/Taroni), SRK/T Sanders–Retzlaff–Kraff/theoretica
In the low-toricity group, there was no significant difference between ME and zero in any formula. The EVO2.0 and Ladas super formulas both had the highest percentages of eyes within ± 0.75 D of the target refraction (96.3% and 96.3%, respectively) (P = 0.023, 0.023, respectively).
In the medium-toricity group, there was no significant difference between ME and zero in any formula. The Kane and HQST formulas were the most accurate formulas (P = 0.013 and 0.023, respectively). The EVO2.0 (99.2%) formula performed the highest percentage of eyes within ± 0.75 D (P = 0.006).
In the high-toricity group, there was no significant difference between ME and zero in any formula. No statistically significant difference existed in AE as well as the percentage of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D among formulas.
Discussion
In this study, we assessed the precision of diverse formulas in calculating the SE of toric IOLs. Our findings indicated that the Kane and EVO2.0 formulations exhibited superior outcomes. Notably, the EVO2.0 formula performed well across various ACD, K, and T subgroups. The Ladas super formula provided additional benefits within the normal ACD group.
Numerous scholars have emphasized the superiority of new-generation formulas over Gaussian optics-based standard formulas in predicting SE for non-toric IOLs [8]. Pereira et al. [21] asserted that the Kane, BU II, EVO, T2, and Ladas Super formulas were the most accurate preoperative refractive calculations for the overall cohort. In two large studies, Darcy et al. [7] and Melles et al. [8] concluded that the Kane formula performed the highest accuracy overall. Moreover, Cheng et al. [22] discovered that the EVO2.0 formula significantly further enhanced the accuracy compared to the previous version. It is noteworthy that a formula exhibiting high accuracy in predicting SE for non-toric IOLs may not be equally effective for toric IOLs. Given the distinct degrees on the two meridians of toric IOLs, the effective lens position (ELP)-related refractive effect for toric IOLs may differ from that of non-toric IOLs. As a result, formulas may inaccurately predict the ELP for toric IOLs, consequently affecting the overall SE outcome. Consequently, we selected a specific cohort of eyes implanted with toric IOLs to assess the predictive accuracy of both new-generation formulas and the standard formulas based on Gaussian optics, along with their improved version, in estimating SE in this investigation.
The Kane formula used multiple extensive datasets compiled by skilled high-volume surgeons, employing a blend of theoretical optics, regression analysis, and artificial intelligence (AI) components to make its predictions. An interesting facet of this calculation is the integration of patient sex as a variable into the equation [23]. Kane et al. [24] contended that the predictive efficacy of the Kane toric formula for astigmatism surpassed that of the Abulafia-Koch, Barrett, and EVO2.0 toric formulas. Our current findings further affirmed the Kane formula’s precision in predicting SE of toric IOL. Our study also found that the EVO2.0 formula also displayed a promising algorithm. Remarkably, it even had a higher percentage of eyes within ± 0.50 D, ± 0.75 D, and ± 1.00 D compared to the Kane formula, a phenomenon possibly linked to its emmetropization theory [25]. As a novel thick-lens formula, it calculates an "emmetropia factor" for each eye. Simultaneously, this formula considers both the optical dimensions of the eye and diverse IOL geometries [22].
Furthermore, our study also included an examination of three improved IOL calculation formulas – the Ladas Super, T2, and HQST formulas. The Ladas Super Formula integrates optimal components from the Hoffer Q, Holladay 1, Holladay 1 with Koch adjustment, and SRK/T formulas, employing a three-dimensional model to select the most suitable formula [14]. Additionally, this formula was revised in 2019 based on the postoperative data from over 4000 eyes and is now based on AI [26]. In our study, the Ladas Super Formula exhibited a significantly better performance than the SRK/T formula in the percentage of eyes within ± 0.75 D. The T2 formula, replacing the corneal height estimation steps in the SRK/T formula with a regression formula derived from extensive patient data [11], performed better than the SRK/T formula in our cohort. The HQST formula, retaining the Gaussian optic architecture while incorporating AI for enhanced ELP prediction and adjustments for eyes with AL > 25 mm, [12] demonstrated improved outcomes compared to the Hoffer Q formula. Despite these advancements, these three formulas still fell short of the predictive accuracy achieved by the Kane and EVO2.0 formulas, implying a potential need for enhancing.
In each of the ACD subgroups, the EVO2.0 formula provided outstanding outcomes, and the Ladas super formula also yielded good results in the regular ACD group. Hipólito-Fernandes et al. [27] investigated the impact of ACD in patients with normal ALs, concluding that the PEARL-DGS, Kane, EVO2.0, and BU II formulas were more accurate in the shallow ACD group. Although no significant differences were found with other formulas in our study, the EVO2.0 formula consistently once again showed a good predictive effect. In terms of ME, both the Hoffer Q and Holladay 1 formulas revealed myopic shifts in the shallow ACD group because of the absence of ACD [3, 5], which was similar to the other studies which implanted non-toric IOLs [19, 28]. Moreover, we discovered that the Ladas super formula yielded myopic outcomes in the deep ACD group, and the Hoffer Q, HQST, Holladay 1, Kane, and T2 formulas resulted a hyperopic outcome in the regular ACD group, suggesting that there is room for improvement for these formula.
In the analysis of K subgroups, the EVO2.0 formula emerged as the most effective in the flat K group. It is hard to discuss the specific factors contributing to its outstanding performance, as it has not been published; nevertheless, it still merits further attention. Besides, the BU II formula’s poor result is challenging to interpret due to its unknown principle, also possibly influenced by the small sample size in this subgroup, while the result of the SRK/T formula is easy to explain because of “cusp phenomenon” previously identified by scholars [29]. Reitblat et al. [29] reported myopic MEs for all formulas except the Haigis, Olsen, and Hoffer Q formulas. This aligned with our findings, indicating suboptimal prediction accuracy for each formula in the steep K group.
Additionally, we also investigated the SE predictive accuracy of each formula for toric IOL across various T subgroups. The EVO2.0 and Ladas super formulas demonstrated high performance in the low-toricity group, whereas the Kane, HQST, and EVO2.0 formulas performed well in the medium-toricity group. This revealed the superior predictability of new-generation and enhanced formulas in diverse T subgroups compared to traditional third-generation formulas.
We acknowledge certain limitations in our study. While our sample size calculation deemed it sufficiently large to detect significant differences in the overall MedAE, larger-scale studies can offer more substantial insights in subgroup analyses. In addition, the absence of lens thickness, central corneal thickness, and white-to-white measurements restricted our ability to draw comprehensive conclusions regarding the comparison between formulas and the application of some specific formulas. It is worth noting that these additional variables are optional in the aforementioned formulas, with none being mandatory. However, the fundamental requirements for IOL power calculation were fulfilled through the measurement of AL, ACD, and K [7, 21].
Conclusions
In summary, our findings indicated that the new-generation formulas, particularly the Kane and EVO2.0 formulas, have better performances in predicting SE for toric IOL in patients with normal ALs, and the EVO2.0 formula provided more accurate outcomes across various subgroups.
Data Availability
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Barrett GD. Barrett Universal II formula. Accessed February 14, 2024. https://calc.apacrs.org/barrett_universal2105/Default.aspx.
Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–73.
Holladay J, Prager T, Chandler T, Musgrove K, Lewis J, Ruiz R. A three-part system for refining intraocular lens power calculations. J Cataract Refract Surg. 1988;14:17–24.
Retzlaff J, Sanders D, Kraff M. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg. 1990;16:333–40.
Hoffer K. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19:700–12.
Kane JX. Kane formula. https://www.iolformula.com. Accessed 14 Feb 2024.
Darcy K, Gunn D, Tavassoli S, Sparrow J, Kane J. Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10 930 eyes from the UK National Health Service. J Cataract Refract Surg. 2020;46:2–7.
Melles R, Kane J, Olsen T, Chang W. Update on intraocular lens calculation formulas. Ophthalmology. 2019;126:1334–5.
Yeo TK. Emmetropia verifying optical (EVO) 2.0 formula. http://www.evoiolcalculator.com. Accessed 14 Feb 2024.
Hipólito-Fernandes D, Elisa Luís M, Gil P, et al. VRF-G, a new intraocular lens power calculation formula: a 13-formulas comparison study. Clin Ophthalmol (Auckland, NZ). 2020;14:4395–402.
Sheard R, Smith G, Cooke D. Improving the prediction accuracy of the SRK/T formula: the T2 formula. J Cataract Refract Surg. 2010;36:1829–34.
Taroni L, Hoffer K, Pellegrini M, Lupardi E, Savini G. Comparison of the new Hoffer QST with 4 modern accurate formulas. J Cataract Refract Surg. 2023;49:378–84.
Taroni L, Savini G. Hoffer QST (Savini/Taroni) (HQST) formula. https://hofferqst.com/. Accessed 14 Feb 2024.
Ladas J, Siddiqui A, Devgan U, Jun A. A 3-D “super surface” combining modern intraocular lens formulas to generate a “super formula” and maximize accuracy. JAMA Ophthalmol. 2015;133:1431–6.
Ladas JG. Ladas super formula. https://iolcalc.com/home. Accessed 14 Feb 2024.
Hoffer K, Savini G. Update on intraocular lens power calculation study protocols: the better way to design and report clinical trials. Ophthalmology. 2021;128:e115–20.
Hoffer K, Aramberri J, Haigis W, et al. Protocols for studies of intraocular lens formula accuracy. Am J Ophthalmol. 2015;160:403-405.e1.
Wang L, Koch D, Hill W, Abulafia A. Pursuing perfection in intraocular lens calculations: III. Criteria for analyzing outcomes. J Cataract Refract Surg. 2017;43:999–1002.
Melles R, Holladay J, Chang W. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125:169–78.
User Group for Laser Interference Biometry (ULIB). http://ocusoft.de/ulib. Accessed 14 Feb 2024.
Pereira A, Popovic M, Ahmed Y, et al. A comparative analysis of 12 intraocular lens power formulas. Int Ophthalmol. 2021;41:4137–50.
Cheng H, Kane J, Liu L, Li J, Cheng B, Wu M. Refractive predictability using the IOLMaster 700 and artificial intelligence-based IOL power formulas compared to standard formulas. J Refract Surg. 2020;36:466–72.
Connell B, Kane J. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019;4: e000251. https://doi.org/10.1136/bmjophth-2018-000251.
Pantanelli S, Sun A, Kansara N, Smits G. Comparison of Barrett and emmetropia verifying optical toric calculators. Clin Ophthalmol (Auckland, NZ). 2022;16:177–82.
Chen Y, Wei L, He W, Lu Y, Zhu X. Comparison of Kane, Hill-RBF 2.0, Barrett Universal II, and emmetropia verifying optical formulas in eyes with extreme myopia. J Refract Surg. 2021;37:680–5.
Kothari S, Reddy J. Recent developments in the intraocular lens formulae: an update. Semin Ophthalmol. 2023;38:143–50.
Hipólito-Fernandes D, Luís M, Serras-Pereira R, et al. Anterior chamber depth, lens thickness and intraocular lens calculation formula accuracy: nine formulas comparison. Br J Ophthalmol. 2022;106:349–55.
Eom Y, Kang S, Song J, Kim Y, Kim H. Comparison of Hoffer Q and Haigis formulae for intraocular lens power calculation according to the anterior chamber depth in short eyes. Am J Ophthalmol. 2014;157:818-824.e2.
Reitblat O, Levy A, Kleinmann G, Lerman T, Assia E. Intraocular lens power calculation for eyes with high and low average keratometry readings: comparison between various formulas. J Cataract Refract Surg. 2017;43:1149–56.
Medical Writing, Editorial, and Other Assistance
Hengli Lian, PhD, provided statistical assistance. Ruoqian Zhang, BD, provided coding assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study and the journal’s Rapid Service Fee was supported by the “Pioneer” and “Leading Goose” R&D Program of Zhejiang (Grant No. 2022C03070).
Author information
Authors and Affiliations
Contributions
Jingyi Shi: concept and design, data analysis, manuscript drafting, and statistical analysis; Zehui Zhu: concept and design, data analysis; Bin Hu: data acquisition; Shuyi Qian: data acquisition; Yalan Wang: data analysis; Pingjun Chang: concept and design, clinical revision; Yun-e Zhao: clinical revision, supervision, and final approval. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Jingyi Shi, Zehui Zhu, Bin Hu, Shuyi Qian, Yalan Wang, Pingjun Chang, Yun-e Zhao declare no competing interests.
Ethical approval
This study was performed in accordance with the Declaration of Helsinki of 1964 and its subsequent amendments. This study was approved by the Ethics Committee of Wenzhou Medical University (2021-036-K-29-01).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shi, J., Zhu, Z., Hu, B. et al. Accuracy of Ten Intraocular Lens Formulas in Spherical Equivalent of Toric Intraocular Lens Power Calculation. Ophthalmol Ther 13, 1321–1342 (2024). https://doi.org/10.1007/s40123-024-00926-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00926-x