Abstract
Introduction
To characterize the response to antivascular endothelial growth factor (VEGF) treatment of macular neovascularization (MNV) secondary to age-related macular degeneration (AMD) with subclinical angioid streaks (AS) during a 2-year follow-up.
Methods
Retrospective, longitudinal, case-control, and multicentric study. Among a cohort of neovascular AMD population, we selected patients with subclinical AS and treatment-naïve MNV treated with anti-VEGF for a 2-year follow-up. An age- and sex-matched control group with treatment-naïve MNV secondary to AMD without subclinical AS was selected. Demographics and differences in treatment response (i.e., number of injections needed, anatomical and functional outcomes) between the two groups were analyzed.
Results
Among 102 eyes of 102 patients with neovascular AMD, 34 eyes of 34 patients (82 ± 6 years old) were included in the subclinical AS group, whereas 68 eyes of 68 patients (81 ± 6 years old, p = 0.342) in the control group. All eyes with subclinical AS presented RPD compared to 56% of eyes without subclinical AS (p < 0.001). During the 2-year follow-up, eyes with subclinical AS needed more injections (10.6 ± 3.2 vs 8.3 ± 3.1 injections for eyes with and without subclinical AS, respectively, p < 0.001). Visual acuity (VA) decreased during the treatment (from 0.53 ± 0.37 at the baseline to 0.69 ± 0.45 LogMAR at 2-year follow-up, p = 0.044) in eyes with subclinical AS; no VA changes were observed in the control group (p = 0.798). RPE atrophy at the end of the 2-year follow-up affected 74% of cases with subclinical AS and 29% of cases of the control group (p < 0.001).
Conclusions
MNVs secondary to AMD with subclinical AS are characterized by worse functional and anatomical outcomes after 2-year anti-VEGF treatment compared to MNV secondary to AMD without subclinical AS, supporting the different pathophysiological mechanisms involved in this recently described AMD phenotype.
Similar content being viewed by others
Why carry out this study? |
Subclinical age-related angioid streaks (AS) is a recently described new phenotype of age-related macular degeneration (AMD). |
To describe the response to antivascular endothelial growth factor (VEGF) treatment of macular neovascularization (MNV) secondary to AMD with subclinical AS during a 2-year follow-up. |
What was learned from the study: |
MNV secondary to AMD with subclinical AS showed a higher percentage of macular atrophy development and worse visual outcomes after 2 years of treatment, despite the greater number of injections needed. |
Our results suggested that age-related subclinical AS etiology is a negative predictor of functional outcomes in patients with neovascular AMD. |
Introduction
Age-related macular degeneration (AMD) is a complex disease with a multifactorial background involving genetic and environmental influences. AMD pathogenesis is not completely understood, even though three main structures are primarily involved: photoreceptors, retinal pigment epithelium (RPE) cells, and the choroidal circulation (especially the choriocapillaris) [1,2,3,4,5,6]. Recently, our group described a new AMD phenotype, namely subclinical angioid streaks (AS). Subclinical AS are characterized by structural OCT findings typical of AS [Bruch’s membrane (BM) undulations, BM breaks, and large dehiscences of BM] but with the absence of AS employing fundus examination (for this reason “subclinical”) in patients with landmarks of AMD [i.e., drusen and reticular pseudodrusen (RPD)] [7]. The primary involvement of the BM using structural OCT and the concomitant high prevalence of RPD suggested the predominant involvement of BM as a driving factor of this new phenotype.
The development of macular neovascularization (MNV) represents a landmark of advanced AMD development, characterizing the neovascular form of the disease [8]. However, the development of choroidal neovascularization (CNV) was reported in several other macular diseases, such as in angioid streaks [9, 10]. The main therapy of MNV/CNV is based on intravitreal antivascular endothelial growth factor (VEGF) injections. Nevertheless, the treatment response of neovessels (i.e., number of injections needed, treatment regimen, development of fibrosis and/or atrophy) is different based on the background disease [11,12,13]. The pathogenesis of subclinical AS phenotype showed several differences compared to other AMD phenotypes because of the greater involvement of the BM. For this reason, the response of MNV to the anti-VEGF treatment could be different in AMD patients with subclinical AS phenotype compared to MNV complicating other AMD phenotypes.
This study aimed to characterize the response to anti-VEGF treatment of treatment-naïve MNV secondary to AMD and subclinical AS phenotype during a 2-year follow-up. We compared their response to AMD eyes with treatment-naïve MNV but without AS treated with the same regimen.
Methods
Study Population
This is a retrospective, longitudinal, case-control, and multicentric study conducted at the Medical Retina and Imaging Unit, Department of Ophthalmology, IRCCS San Raffaele Hospital, University Vita-Salute San Raffaele in Milan, Italy, and at the IRCCS-Fondazione Bietti in Rome, Italy. Medical records of all consecutive patients with a diagnosis of AMD in at least one eye presenting between January and June 2021 and followed until June 2023 were reviewed. All included patients signed a written informed consent for the retrospective study that was approved by the local ethics committee for each involved center. Due to the retrospective nature of the study, the study did not require a specific Ethics Committee Approval in line with Italian laws. The study was conducted following the tenets outlined in the Declaration of Helsinki for research involving human subjects.
We included patients with the following features: (1) 50 years of age and older; (2) diagnosis of AMD; (3) presence of subclinical AS; (4) presence of treatment-naïve MNV at the baseline; (5) treatment with anti-VEGF injections (aflibercept as first choice) using a rigorous pro-re-nata (PRN) regimen; (6) 2-year follow-up. The presence of subclinical AS was defined according to the previously published criteria (i.e., BM breaks, and/or large dehiscences of BM using structural OCT and absence of AS employing fundus examination) [7]. For our clinical practice standards, all patients treated using a PRN regimen were treated with a loading phase of 3 monthly consecutive injections, and, after that, they were evaluated every 8 weeks (using aflibercept). In case of any sign of exudation [presence of intra/subretinal fluid (IRF/SRF) and/or subretinal hyperreflective material (SHRM) by means of structural OCT, and/or presence of hemorrhage using fundus examination], each patient was re-injected with no tolerance. All patients were followed by the same team using the same treatment strategy.
We excluded patients with the following features: (1) presence of any other macular disease; (2) previous macular treatment before the baseline (e.g., laser photocoagulation, photodynamic therapy, intravitreal injections); (3) relevant opacities of the optic media and/or inadequate fixation to permit high-quality imaging; (4) myopia > 6 diopters (D) of sphere or 3D of cylinder, and/or axial length > 25.5 mm; (5) no adherence to the previously reported rigorous PRN regimen; (6) presence of systemic diseases related to AS, including PXE, Paget’s disease, and hemoglobinopathies (sickle cell trait disease and thalassemia).
A control group was recruited including age- and gender-matched subjects with AMD but without subclinical AS. The sample of the control group was selected with a ratio of 1:2 because of the higher prevalence of AMD without subclinical AS. As in the subclinical AS group, also patients included in the control group were affected by treatment-naïve MNV at the baseline and were treated with the same rigorous PRN regimen during the 2-year follow-up.
If both eyes were includable in the subclinical AS or control group, only one eye for each patient was included. The included eye was randomly chosen flipping a coin.
All patients (in both subclinical and AMD groups) were evaluated at the baseline and at each control during the 2-year follow-up with a complete examination including assessment of distance best-corrected visual acuity (BCVA) using Snellen charts and converted to LogMAR for statistical evaluation, fundus examination, infrared reflectance (IR), fundus autofluorescence (FAF), and structural OCT. At the baseline, also fluorescein (FA) and indocyanine green angiographies (ICGA) and/or OCT-angiography (OCT-A) were performed to confirm the presence of MNV. Infrared reflectance, structural OCT, FA, and ICGA were acquired using HRA2 + OCT Spectralis (Heidelberg Engineering, Heidelberg, Germany) whereas OCT-A using PLEXElite 9000 (Car Zeiss, Meditec Inc. Dublin, CA, USA).
The following clinical findings were recorded: BCVA, central macular thickness (CMT), and subfoveal choroidal thickness (ChT) at the baseline and at 1- and 2-year follow-up; presence of drusen, RPD, or both; subtype of MNV; presence of macular RPE atrophy using structural OCT at the baseline and during the follow-up; fellow-eye status (intermediate AMD, neovascular AMD, or geographic atrophy); number of injections; switch to another anti-VEGF drug during the follow-up.
CMT was automatically assessed within a 1-mm ETDRS circle centered on the fovea by using the inbuild Spectralis OCT software. Subfoveal ChT was measured in structural EDI OCT with the inbuilt caliper in the foveal location.
Statistical Analyses
Statistical calculations were carried out using Statistical Package for the Social Sciences (SPSS) software (version 28.0.1.0; SPSS, Inc., Chicago, IL, USA). Categorical variables were reported as counts (percentages) whereas continuous variables as means ± standard deviation. The difference between the proportions of independent categorical variables has been analyzed with Pearson’s chi-square test. All continuous variables were tested for normal distributions using the Kolmogorov-Smirnov test. Measurement values between subclinical AS and control groups were compared using Student’s t-test for independent samples. Comparison of quantitative variables among the three different time points (i.e., baseline, 1-year follow-up, and 2-year follow-up) was performed using repeated measures analysis of variance (ANOVA) with Bonferroni post-hoc analysis.
The P-value cut-off point for statistical significance has been set to 0.05.
Results
Patients’ Demographics and Main Clinical Findings at Baseline
One hundred two eyes of 102 patients affected by treatment-naïve MNV secondary to neovascular AMD were enrolled. All patients were Caucasian. Among 102 eyes, 34 eyes of 34 patients (24 females and 10 males; mean age 82 ± 6 years old) were included in the subclinical AS group, whereas 68 eyes of 68 patients (46 females and 22 males; mean age 81 ± 6 years old) were enrolled in the control AMD group. No statistically significant difference in age and sex was disclosed between patients with subclinical AS and patients with other phenotypes of AMD (i.e., control group) (p = 0.342 and p = 0.763, respectively). Thirty-four eyes of the subclinical AS subgroup were included for the presence of BM breaks (32 out of 34 cases, 94%) and/or the presence of BM dehiscences (7 out of 34 cases, 21%) (Fig. 1). Fellow eyes of patients affected by subclinical AS were usually characterized by a more advanced stage of AMD (73% were affected by neovascular AMD and 18% by GA) compared to the fellow eyes of patients in the control group (45% were affected by neovascular AMD and 12% by GA) (p < 0.001) (Table 1).
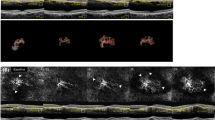
A representative case of a patient affected by neovascular age-related macular degeneration (AMD) with subclinical age-related angioid streaks in the right eye. A–C Baseline [i.e., diagnosis of treatment-naïve macular neovascularization (MNV)]. Fundus autofluorescence (A) and combined infrared reflectance (IR) with structural optical coherence tomography (OCT) B-scan passing through the optic nerve (B) showing the presence of peripapillary atrophy with petaloid aspect, Bruch’s membrane undulations (yellow asterisks), and breaks (red triangles) in the peripapillary area. Combined IR and structural OCT passing through the fovea showing exudation of a MNV with subretinal fluid, small intraretinal cystic spaces, and subretinal hyperreflective material. D–E Two-year follow-up (13 anti-VEGF injections). Fundus autofluorescence (D) shows the development of macular atrophy. Combined IR and structural OCT (E) show the absence of exudative signs in correspondence with the MNV
Analyzing the landmarks of AMD (presence of drusen, RPD, or both), all 34 eyes with subclinical AS presented RPD, in combination with drusen (15 out of 34 cases, 44%) or without (19 out of 34 cases, 56%). No cases showed only drusen without RPD. This distribution was significantly different (p < 0.001) compared to the control group (i.e., eyes with AMD but without subclinical AS) (Table 1). Indeed, in the control group, RPDs were presented in 56% of cases (38 out of 68 cases), in combination with drusen (32 out of 68 cases) or without (6 out of 68 cases). Thirty of 68 cases (44%) presented only drusen.
Analyzing the presence of macular atrophy, 5 of 34 eyes (15%) of the subclinical AS group and 5 of 68 eyes (7%) of the control group were affected by macular atrophy at the baseline (p = 0.239).
Among 34 eyes with subclinical AS, 73% (25 out of 34) displayed a type 1 MNV, 15% (5 eyes) a type 2 MNV, 9% (3 eyes) a mixed type 1 and 2 MNV, and 3% (1 eyes) a type 3 MNV. This distribution was significantly different (p = 0.012) compared to the control group (Table 1). Indeed, among 68 eyes with AMD but without subclinical AS, 68% (46 out of 68) displayed a type 1 MNV, 4% (3 eyes) a type 2 MNV, 3% (2 eyes) a mixed type 1 and 2 MNV, and 25% (17 eyes) a type 3 MNV. This different distribution in MNV subtypes was confirmed also analyzing only patients with RPD (all 34 subclinical AS eyes and 38 eyes of control group); indeed, among 38 RPD eyes of the control group, 60% (23 out of 38) displayed a type 1 MNV, 3% (1 eye) a type 2 MNV, 3% (1 eye) a mixed type 1 and 2 MNV, and 34% (13 eyes) a type 3 MNV.
At baseline, mean BCVA was about 20/63 Snellen equivalent in both groups (0.53 ± 0.37 LogMAR in the subclinical AS group, and 0.50 ± 0.43 LogMAR in the control group, p = 0.347). No significant difference was disclosed analyzing the CMT (p = 0.055), but subclinical AS eyes showed a significantly lower subfoveal ChT compared to the control group (149 \(\pm\) 69 µm vs 185 \(\pm\) 78 µm, respectively, p = 0.014) (Table 1).
Main Clinical Findings at 2-Year Follow-up
During the 2-year follow-up, 34 eyes affected by subclinical AS were treated with a mean of 10.6 ± 3.2 anti-VEGF injections, whereas 68 eyes of the control group were treated with a significantly lower number of anti-VEGF injections (8.3 ± 3.1 injections, p < 0.001). This difference was also evident when analyzing only the number of anti-VEGF injections in the first-year follow-up (6.9 ± 2.0 vs 5.3 ± 2.0 injections in the subclinical AS and control group, respectively, p < 0.001) (Figs. 1 and 2). The number of injections was significantly higher in subclinical AS group also when compared to only 38 eyes displaying RPD in the control group (10.6 ± 3.2 vs 8.7 ± 3.1 anti-VEGF injections, respectively, p = 0.007).
A representative case of a patient affected by neovascular age-related macular degeneration (AMD) without subclinical age-related angioid streaks in the right eye. A–B Baseline [i.e., diagnosis of treatment-naïve macular neovascularization (MNV)]. Fundus autofluorescence (A) and combined infrared reflectance (IR) with structural optical coherence tomography (OCT) B-scan passing through the fovea (B) showing the presence of a MNV with subretinal fluid (SRF) and sub-retinal pigment epithelium fluid but no alterations of Bruch’s membrane. D–E Two-year follow-up (9 anti-VEGF injections). Fundus autofluorescence D shows absence of macular atrophy. Combined IR and structural OCT E show the presence of persistent SRF
Seven out of 34 eyes in the subclinical AS group and 16 out of 68 eyes in the control group were switched to other anti-VEGF molecules during the follow-up because of no response to aflibercept.
Regarding BCVA outcomes, eyes affected by subclinical AS showed a significant decrease of BCVA during the 2-year follow-up (BCVA of 0.53 ± 0.37 LogMAR at the baseline and 0.69 ± 0.45 at 2-year follow-up, p = 0.016), whereas eyes affected by AMD without subclinical AS did not show significant difference of BCVA during the follow-up (p = 1) (Table 2). Furthermore, eyes affected by subclinical AS showed a significantly lower BCVA compared to eyes with AMD without subclinical AS at the end of 2-year follow-up (0.69 ± 0.45 and 0.50 ± 0.48 LogMAR, respectively, p = 0.032), despite the similar BCVA at the baseline (0.53 ± 0.37 LogMAR in the subclinical AS group, and 0.50 ± 0.43 LogMAR in the control group, p = 0.347). The difference in the 2-year BCVA was confirmed comparing subclinical AS eyes with only 38 eyes displaying RPD in the control group (0.69 ± 0.45 and 0.47 ± 0.46 LogMAR, respectively, p = 0.025).
From an anatomical point of view, CMT showed a significant decrease during the 2-year follow-up in both groups (p < 0.001 in both groups), and a significant reduction of subfoveal ChT was disclosed in both groups (p = 0.002 and p = 0.023 in the AMD with AS and AMD without AS groups, respectively) (Table 2).
Analyzing the presence of RPE atrophy at the end of the 2-year follow-up, 25 of 34 eyes (74%) of the subclinical AS group were affected by atrophy compared to 20 of 68 eyes (29%) of the control group (p < 0.001).
Discussion
In this study, we reported that treatment-naïve MNVs secondary to AMD with subclinical AS are characterized by a different response to anti-VEGF treatment (i.e., number of injections, anatomical and functional outcomes) during a 2-year follow-up compared to treatment-naïve MNV secondary to AMD without subclinical AS.
Subclinical AS is a recently reported phenotype of AMD that affects about 13.4% of patients with AMD [7]. This new phenotype is characterized by a predominant involvement of BM as a driving factor. Indeed, almost all cases displayed Bruch’s membrane alterations using structural OCT and the presence of RPD. These features are very close to pathophysiological mechanisms involved in the “typical” AS (i.e., visible on fundus examination). The development of CNV is a frequent scenario in patients affected by AS, facilitated by the locus minoris resistentiae of the BM interruption. However, CNVs secondary to AS are usually characterized by a worse prognosis due to the predominance of type 2 CNV, a high number of injections needed, and development of fibrosis and/or atrophy with worse visual outcomes [9,10,11,12,13]. Different molecules showed efficacy in stabilization of BCVA up to 4-year follow-up in CNV secondary to AS [11, 12, 14,15,16,17,18]. Recently, a few studies have shown promising results in terms of BCVA improvement and a low number of recurrences in CNVs secondary to AS treated by aflibercept [11, 18]. However, all studies were performed on small series (< 35 eyes), with variable follow-up, and all with PRN regimens. For this reason, a comparison between a PRN or proactive regimen (i.e., treat and extend) or between different molecules is not available.
Interestingly, in the present study, we found several differences in MNV secondary to AMD with subclinical AS and the control group (i.e., MNV secondary to AMD without AS), with several common features between CNV/MNV secondary to subclinical AS and typical AS. Indeed, in our series, MNV secondary to AMD with subclinical AS showed a greater proportion of type 2 MNV compared to MNV secondary to AMD but without subclinical AS (24% vs 7% of cases, p < 0.001). Furthermore, MNV secondary to AMD with subclinical AS needed more injections during the 2-year follow-up (10.6 ± 3.2 vs 8.3 ± 3.1 anti-VEGF injections in the AMD eyes with subclinical AS and without subclinical AS, respectively, p < 0.001). Despite the greater number of injections, MNV secondary to AMD with subclinical AS developed a higher rate of macular atrophy during the treatment, leading to worse visual outcomes. Indeed, MNV secondary to AMD with subclinical AS displayed a significant worsening of BCVA during the 2 years of treatment (p = 0.044), differently from the control group (i.e., MNV secondary to AMD without subclinical AS), which showed stable BCVA during the treatment. This could be due to the higher rate of atrophy development in the AMD with subclinical AS group (74% vs 29% in the AMD eyes with subclinical AS and without subclinical AS, respectively, p < 0.001). The development of macular atrophy is one of the worst events in the treatment of exudative MNV secondary to AMD [19,20,21,22]. The rate of atrophy development is very variable, depending also on the AMD phenotype. However, in our series, the higher percentage of macular atrophy development could also be associated with the PRN treatment. Indeed, it is well known that a greater fluctuation of the macular thickness is associated with higher development of macular atrophy during the treatment [23, 24]. In this way, a “proactive” treatment (like the treat and extend regimen) should reduce the fluctuation of macular thickness compared to a “reactive” treatment (as the PRN regimen). However, we need to keep in mind that both groups (i.e., MNV secondary to AMD with and without subclinical AS) were treated with the same anti-VEGF regimen, the same drug, and by the same teams. Therefore, we supported that the different percentage of macular atrophy development between the two groups was due to the different phenotypes of the disease, reflecting a different pathogenetic background. As previously reported, subclinical AS was supposed to be characterized by a predominant involvement of the Bruch’s membrane, in contrast to the supposed choriocapillaris and RPE/photoreceptor loss of the other phenotype of AMD [3,4,5,6,7, 25]. Furthermore, the group of AMD with subliclinical AS was characterized by the presence of RPD in almost all cases and a significantly thinner choroidal thickness compared to the control group. Both these two features are known to be significant risk factors for the development of atrophy [26,27,28].
One can argue that differences in MNV subtypes and responses to anti-VEGF were due to the greater prevalence of RPD in the subclinical AS group compared to the control group (100% vs 56%, respectively). However, all these differences were confirmed when we compared only the 38 eyes displaying RPD in the control group.
The present study has several limitations. First, even if we included 102 eyes, this is a relatively small sample size for patients with neovascular AMD. However, we selected only eyes treated by a rigorous PRN regimen and with the same drug in both groups. Another limitation could be due to the PRN regiment that was adopted in the treatment of MNV. However, the same regimen and same criteria of re-treatment were used in both groups, leading to results comparable between groups. Of course, prospective studies with larger sample sizes are needed to confirm our conclusions and to possibly gather more insights into the pathogenesis of these different AMD phenotypes.
Conclusion
In conclusion, we reported the characteristics of MNV secondary to AMD with subclinical AS, defining its treatment response to anti-VEGF therapy compared to MNV secondary to AMD without subclinical AS. We highlighted that MNV secondary to AMD with subclinical AS showed a higher percentage of macular atrophy development and worse visual outcomes after 2 years of treatment, despite the greater number of injections needed. This was probably due to the greater proportion of type 2 MNVs and the greater aggressivity of MNVs secondary to this AMD phenotype. All these features should be kept in mind during the treatment of MNV secondary to AMD for the different prognoses with different phenotypes and to better personalize the treatment of patients affected by this recently reported AMD phenotype.
Data Availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Ferris FL III, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51.
Friedman E. The pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2008;146:348–9.
Sacconi R, Corbelli E, Borrelli E, et al. Choriocapillaris flow impairment could predict the enlargement of geographic atrophy lesion. Br J Ophthalmol. 2021;105:97–102.
Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35:2562–73.
McLeod DS, Grebe R, Bhutto I, et al. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–91.
Sacconi R, Vella G, Battista M, et al. Choroidal vascularity index in different cohorts of dry age-related macular degeneration. Transl Vis Sci Technol. 2021;10(12):26.
Sacconi R, Tombolini B, Zucchiatti I, et al. Subclinical angioid streaks with pseudodrusen: a new phenotype of age-related macular degeneration. Ophthalmol Ther. 2023;12(5):2729–43.
Servillo A, Zucchiatti I, Sacconi R, et al. The state-of-the-art pharmacotherapeutic management of neovascular age-related macular degeneration. Expert Opin Pharmacother. 2023;24(2):197–206.
Corbelli E, Carnevali A, Marchese A, et al. Optical coherence tomography angiography features of angioid streaks. Retina. 2018;38:2128–36.
Rohart C, Le HM, Estrada-Walker J, Giocanti-Auregan A, Cohen SY. Long-term prognosis of choroidal neovascularization complicating angioid streaks. Retina. 2023;43(6):882–7.
Sekfali R, Mimoun G, Cohen SY, et al. Switching from ranibizumab to aflibercept in choroidal neovascularization secondary to angioid streaks. Eur J Ophthalmol. 2020;30(3):550–6.
Ramakrishnan T, Chandra S, Sivaprasad S. Long-term follow-up of management of choroidal neovascularisation secondary to angioid streaks with intravitreal anti-vascular endothelial growth factor. Eye (Lond). 2021;35(3):853–7.
Barresi C, Borrelli E, Fantaguzzi F, et al. Complications associated with worse visual outcomes in patients with exudative neovascular age-related macular degeneration. Ophthalmologica. 2021;244(6):512–22.
Neri P, Salvolini S, Mariotti C, Mercanti L, Celani S, Giovannini A. Long-term control of choroidal neovascularisation secondary to angioid streaks treated with intravitreal bevacizumab (Avastin). Br J Ophthalmol. 2009;93(2):155–8.
Sawa M, Gomi F, Tsujikawa M, Sakaguchi H, Tano Y. Long-term results of intravitreal bevacizumab injection for choroidal neovascularization secondary to angioid streaks. Am J Ophthalmol. 2009;148(4):584-590.e2.
Wiegand TW, Rogers AH, McCabe F, Reichel E, Duker JS. Intravitreal bevacizumab (Avastin) treatment of choroidal neovascularisation in patients with angioid streaks. Br J Ophthalmol. 2009;93(1):47–51.
Tilleul J, Mimoun G, Querques G, et al. Intravitreal ranibizumab for choroidal neovascularization in angioid streaks: four-year follow-up. Retina. 2016;36(3):483–91.
Parodi MB, Cicinelli MV, Marchese A, et al. Intravitreal aflibercept for management of choroidal neovascularization secondary to angioid streaks: the Italian EYLEA-STRIE study. Eur J Ophthalmol. 2021;31(3):1146–53.
Sacconi R, Brambati M, Miere A, et al. Characterisation of macular neovascularisation in geographic atrophy. Br J Ophthalmol. 2022;106(9):1282–7.
Sadda SR, Tuomi LL, Ding B, Fung AE, Hopkins JJ. Macular atrophy in the HARBOR study for neovascular age-related macular degeneration. Ophthalmology. 2018;125(6):878–86.
Querques L, Parravano M, Borrelli E, et al. Anatomical and functional changes in neovascular AMD in remission: comparison of fibrocellular and fibrovascular phenotypes. Br J Ophthalmol. 2020;104(1):47–52.
Evans RN, Reeves BC, Maguire MG, et al. Associations of variation in retinal thickness with visual acuity and anatomic outcomes in eyes with neovascular age-related macular degeneration lesions treated with anti-vascular endothelial growth factor agents. JAMA Ophthalmol. 2020;138(10):1043–51.
Schmidt-Erfurth U, Reiter GS, Riedl S, et al. AI-based monitoring of retinal fluid in disease activity and under therapy. Prog Retin Eye Res. 2022;86: 100972.
Sacconi R, Fragiotta S, Sarraf D, Sadda SR, Freund KB, Parravano M, Corradetti G, Cabral D, Capuano V, Miere A, Costanzo E, Bandello F, Souied E, Querques G. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog Retin Eye Res. 2023;92: 101113.
Sacconi R, Corbelli E, Carnevali A, Querques L, Bandello F, Querques G. Optical coherence tomography angiography in geographic atrophy. Retina. 2018;38:2350–5.
Vujosevic S, Alovisi C, Chakravarthy U. Epidemiology of geographic atrophy and its precursor features of intermediate age-related macular degeneration. Acta Ophthalmol. 2023;101(8):839–56.
Lad EM, Finger RP, Guymer R. Biomarkers for the progression of intermediate age-related macular degeneration. Ophthalmol Ther. 2023;12(6):2917–41.
Sacconi R, Battista M, Borrelli E, et al. Choroidal vascularity index is associated with geographic atrophy progression. Retina. 2022;42(2):381–7.
Funding
This work was supported by the Italian Ministry of Health and Fondazione Roma, Italy. The funding organization had no role in the design or conduct of this research. No funding or sponsorship was received for the publication of this article.
Author information
Authors and Affiliations
Contributions
Riccardo Sacconi, Francesco Bandello, Giuseppe Querques: concept and design. Riccardo Sacconi, Andrea Servillo, Federico Rissotto, Leonardo Bottazzi, Eliana Costanzo, Maria Sole Polito, Beatrice Tombolini, Mariacristina Parravano: drafting the manuscript. Riccardo Sacconi: statistical analysis. Andrea Servillo, Federico Rissotto, Leonardo Bottazzi, Eliana Costanzo, Maria Sole Polito, Beatrice Tombolini: collecting data.
Corresponding author
Ethics declarations
Conflict of Interest
Giuseppe Querques is an Editorial Board member of Opthalmology and Therapy. Giuseppe Querques was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Riccardo Sacconi is a consultant for: Allergan Inc (Irvine, California,USA), Bayer Shering-Pharma (Berlin, Germany), Carl Zeiss Meditec (Dublin, USA), Novartis (Basel, Switzerland), Roche (Basel, Switzerland). Andrea Servillo, Federico Rissotto, Leonardo Bottazzi, Eliana Costanzo, Maria Sole Polito Beatrice Tombolini: none. Mariacristina Parravano reports personal fees from Abbvie, Novartis, Bayer, Roche, Zeiss outside the submitted work. Francesco Bandello is a consultant for Alcon (Fort Worth,Texas,USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California,USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee,Belgium), Zeiss (Dublin, USA). Giuseppe Querques is a consultant for Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California,USA), Amgen (Thousand Oaks, USA), Heidelberg (Germany), KBH (Chengdu, China), LEH Pharma (London, UK), Lumithera (Poulsbo, USA), Novartis (Basel, Switzerland), Bayer Shering-Pharma (Berlin, Germany), Sandoz (Berlin, Germany), Sifi (Catania, Italy), Soof-Fidia (Albano, Italy), Zeiss (Dublin, USA).
Ethical Approval
This retrospective study adhered to the 1964 Helsinki declaration and its later amendments. All subjects signed an informed consent at the time of examination for retrospective studies. Due to the retrospective nature of the study, the study did not require a specific Ethics Committee Approval in line with Italian laws.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sacconi, R., Servillo, A., Rissotto, F. et al. Macular Neovascularization Secondary to Subclinical Angioid Streaks in Age-Related Macular Degeneration: Treatment Response to Anti-VEGF at 2-Year Follow-up. Ophthalmol Ther 13, 1211–1222 (2024). https://doi.org/10.1007/s40123-024-00918-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00918-x