Abstract
Introduction
This study aims to characterize ocular manifestations of juvenile Behçet’s disease (jBD).
Methods
This was a registry-based observational prospective study. All subjects with jBD from the Autoinflammatory Diseases Alliance (AIDA) Network BD Registry showing ocular manifestations before 18 years were enrolled.
Results
We included 27 of 1000 subjects enrolled in the registry (66.7% male patients, 45 affected eyes). The median (interquartile range [IQR]) age at ocular involvement was 14.2 (4.7) years. Uveitis affected 91.1% of eyes (anterior 11.1%, posterior 40.0%, panuveitis 40.0%), retinal vasculitis 37.8% and other manifestations 19.8%. Later onset (p = 0.01) and male predominance (p = 0.04) characterized posterior involvement. Ocular complications occurred in 51.1% of eyes. Patients with complications had earlier onset (p < 0.01), more relapses (p = 0.02) and more prolonged steroidal treatment (p = 0.02). The mean (standard deviation [SD]) central macular thickness (CMT) at the enrolment and last visit was 302.2 (58.4) and 293.3 (78.2) μm, respectively. Fluorescein angiography was pathological in 63.2% of procedures, with a mean (SD) Angiography Scoring for Uveitis Working Group (ASUWOG) of 17.9 (15.5). At the last visit, ocular damage according to the BD Overall Damage Index (BODI) was documented in 73.3% of eyes. The final mean (SD) best corrected visual acuity (BCVA) logMAR was 0.17 (0.47) and blindness (BCVA logMAR < 1.00 or central visual field ≤ 10°) occurred in 15.6% of eyes. At multivariate regression analysis, human leukocyte antigen (HLA)-B51 + independently predicted a + 0.35 change in the final BCVA logMAR (p = 0.01), while a higher BCVA logMAR at the first assessment (odds ratio [OR] 5.80; p = 0.02) independently predicted blindness.
Conclusions
The results of this study may be leveraged to guide clinical practice and future research on this rare sight-threatening condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Behçet’s disease (BD) is relatively uncommon in children, accounting for 15–20% of all cases, and the phenotypical characterization in the paediatric age group remains inadequately defined. |
The presence of uveitis, retinal vasculitis and related ocular complications is responsible for morbidity, damage accrual and disability. |
What was learned from the study? |
About 40% of children with juvenile (j)BD may have ocular inflammation, but it happens before 18 years for only 13%, explaining the inconsistency in the prevalence of ocular jBD reported in the literature. |
Age and sex appear to play a crucial role in determining both the onset time and anatomical site of ocular inflammation. Also, some of the findings of this study indicate the possible existence of a subgroup of children with early onset jBD who might face a worse global and ocular prognosis. |
The presence of HLA-B51 might independently predict a worse final visual acuity in children with jBD. |
Considering the rarity of this condition and the specific focus of the study, this in-depth analysis contributes significant insights to current literature, which may be used for potentially shaping clinical approaches and inspiring future research. |
Introduction
Ocular inflammation represents one of the three cardinal manifestations of Behçet’s disease (BD) along with recurrent oral and genital ulceration. The presence of uveitis, retinal vasculitis and related ocular complications is a significant contributor to morbidity, damage accrual and disability. The disease is relatively uncommon in the paediatric age, accounting for 15–20% of all cases [1,2,3,4]. In paediatric subjects, genetic predisposition appears to play a pivotal role, whereas in adults, the disease's onset might be more influenced by prolonged exposure to various environmental factors [5,6,7,8]. The clinical presentation of juvenile BD often evolves over time. Typically, the onset of symptoms is insidious, leading to a definitive diagnosis either within the adolescent years or extending into adulthood [1, 9,10,11,12]. Ocular inflammation associated with juvenile (j)BD predominantly includes uveitis (anterior, posterior or panuveitis) and retinal vasculitis, which are more commonly observed after the age of 10 [13]. There also appears to be a correlation between the type of ocular involvement and the child's age: anterior uveitis is reported more frequently before the age of 10, while panuveitis is more common during adolescence [13]. Other ocular symptoms associated with jBD include papilloedema, conjunctivitis and keratitis [14]. The prevalence of uveitis in children compared to adults remains a debated topic in the literature. Some studies suggest a lower incidence in the paediatric population [15,16,17,18,19,20]. However, factors like disease duration and inhomogeneous inclusion criteria might influence these findings, and long-term follow-up data do not confirm phenotypical differences between patients with paediatric- or adult-onset disease [21].
This study aims to characterize ocular manifestations of BD in the paediatric population through an in-depth analysis of data from a large international cohort, the Autoinflammatory Diseases Alliance (AIDA) Network BD registry (ClinicalTrials.gov ID NCT05200715) [22].
Methods
Objectives of the Study
The following objectives have been addressed: (1) to report the prevalence and demographic distribution of ocular involvement in jBD; (2) to provide a clinical description of ocular manifestations, including potential association with genetic markers; (3) to explore ocular complications, according to their frequency, risk factors and potential association with structural changes in the eye; (4) to explore the findings of instrumental exams; (5) to detail the treatments used in patients with jBD and ocular manifestations; (6) to describe the prognosis of children with ocular-jBD and identify risk factors predictive of systemic and ocular disease outcomes.
Study Design, Inclusion Criteria and Data Collection
This is a registry-based observational prospective study. Data were extracted from the AIDA Network BD registry. The data were locked on September 29, 2023. Records were then filtered based on the following inclusion criteria: (1) diagnosis of BD as per the International Study Group (ISG) criteria, International Criteria for BD (ICBD) or Pediatric (PED) BD criteria [15, 23, 24]; (2) initial BD manifestations observed before the age of 18 years; (3) ocular inflammatory manifestations observed before the age of 18 years and confirmed by an ophthalmologist experienced in uveitis as related to BD. Pseudonymized data extracted from the registry for the purpose of this study included demographic, genetic, clinical, instrumental, therapeutic, prognostic and clinimetric information. The data were organized into two distinct datasets tailored to patient- or eye-specific data, respectively.
Operative Definitions
When describing BD clinical phenotype, ocular disease, neurological manifestations (excluding isolated headache), vascular thrombosis/aneurysms and gastrointestinal lesions confirmed by endoscopic examination were classified as major organ involvement. All other BD features were classified as minor organ involvement. The anatomical classification of uveitis, ocular inflammation grading system and definition of ocular relapses were attributed by the treating ophthalmologist as per the Standardization of Uveitis Nomenclature (SUN) criteria [25]. The grading of vitreous haze was assigned according to Nussenblatt et al. [26]. The presence of cataract was defined as per the Lens Opacities Classification System III (LOCS III) [27]. The findings of fundus fluorescein angiography (FFA) were objectively assessed using the ASUWOG (Angiography Scoring for Uveitis Working Group) fluorescein angiographic score, developed by Tugal-Tutkun et al. (possible range 0–40) [28]. The optical coherence tomography (OCT) parameters were evaluated by the operator against the reference values of the specific population and instrument employed (Zeiss, Heidelberg Engineering or Topcon models). Visual acuity, expressed as Best Corrected Visual Acuity (BCVA) logMAR, was measured using age-appropriate Snellen charts and then converted to the logMAR system. Corticosteroid (CS) dosages were converted to their prednisone equivalent and expressed in mg/kg/day. The most recent body weight recorded before therapy initiation was used for this calculation. The total BD overall damage index (BODI) score was used to assess the global damage associated with BD [29]. In addition, the oculo-specific items of the BODI were used to assess ocular damage, including anterior segment changes, posterior segment changes, visual impairment and blindness, defined according to the glossary provided in Piga et al. [30].
Statistical Methods
Data were analyzed using the Jupyter Notebook via EDINA's Noteable platform, a cloud-based application providing access to coding environments (https://noteable.io). Descriptive statistics included counts and frequencies for categorical variables and mean and standard deviation (SD) or median and interquartile range (IQR) along with ranges for continuous variables. Shapiro-Wilk test was used to assess the normality distribution of data. Associations between categorical variables were analyzed using contingency tables with the chi-square test with Yates continuity correction. Differences in continuous data between independent groups were compared by the Mann-Whitney U test (2 groups) or Kruskal-Wallis H test with Dunn’s post-hoc test (more than 2 groups). Differences in continuous data between paired groups were compared by the paired-samples t-test. Relationships between continuous variables were evaluated using Pearson’s correlation for normally distributed data and Spearman’s correlation for non-normally distributed data. Univariate regression analysis was employed to predict outcomes, while multivariate regression analysis, utilizing multiple predictor variables simultaneously, was used to understand their combined effect on the outcome. For missing data, mode and median imputation were used, and results of the analysis conducted with and without imputation were provided. The threshold for statistical significance was set to p < 0.05, and all p-values were two-sided.
Regulatory Considerations
The study protocol adheres to the principles outlined in the Helsinki Declaration. The AIDA Network BD registry conforms to the General Data Protection Regulation (GDPR) ensuring compliance with legal requirements regarding the processing of personal data. For eligibility in the registry, patients (or their parents/legal guardians) must provide written consent, after receiving appropriate information. The protocol of the international AIDA Network BD registry was approved by the Ethics Committee of Siena University Hospital (Ref. 14,951) and by the ethics committees of all the participating investigation centers.
Results
The patient selection process for this study is described in Fig. 1. By September 29, 2023, 1000 subjects affected by BD were included in the registry. In 212 patients (21.2%), disease onset was observed before the age of 18 years. BD-related ocular manifestations were described in 93 of 212 (43.9%) at different time points during disease history. By stratification according to the age at onset of ocular manifestations, 27 subjects whose symptoms began before 18 years were identified.
Demographic Data
Demographic characteristics of the final study cohort are detailed in Table 1. Male (M) and female (F) patients had similar ages at disease onset (p = 0.77). There was a negative correlation between the age at BD onset and the delay of ocular involvement (Pearson’s r = – 0.4, p = 0.02).
Genetic Data
The human leukocyte antigen (HLA)-B51 haplotype was present in 14 subjects (51.9%), absent in 8 (29.6%) and unknown in 5 (18.5%). The characteristics of the subjects in relation to the presence or absence of HLA-B51 are provided as Supplementary Material (S1). In the HLA-B51 + group, the BCVA logMAR at the last follow-up visit was higher than in the HLA-B51- group (p = 0.05 calculated on native data; p = 0.03 after missing data imputation). The HLA-B27 haplotype was present in one subject (3.7%), absent in 11 (44.7%) and unknown in 15 (55.6%). One girl had a familial form of BD, also diagnosed in her sister. This patient was a carrier of both the HLA-B51 and HLA-Bw4 haplotypes with isoleucine at amino acid position 80 (HLA-Bw4-80I).
Clinical Data
Ocular inflammation was unilateral in 9/27 children (33.3%) and bilateral in 18/27 (66.7%), affecting a total of 45 eyes. Uveitis was found in 39 eyes (86.7%), retinal vasculitis in 17 (37.8%), retinitis in 6 (13.3%), retrobulbar optic neuritis in 2 (4.4%) and papillitis in 1 (2.2%). Beyond ocular involvement, there were other major organ manifestations in 4/27 subjects (14.8%), while 23/27 (85.2%) had only minor manifestations of BD, as detailed in Table 1.
Uveitis was classified anatomically as anterior in 5 eyes (11.1%), posterior in 18 (40.0%) and panuveitis in 18 (40.0%). Median age at BD onset was higher in subjects with posterior uveitis than in those with panuveitis or anterior uveitis (p = 0.01; Dunn’s post-hoc: p = 0.01 for panuveitis and p = 0.01 for anterior uveitis); median age at ocular involvement was higher in children with posterior uveitis than in those with anterior uveitis or panuveitis (p = 0.04; Dunn’s post-hoc: p = 0.02 for panuveitis and p = 0.09 for anterior uveitis) (Fig. 2a). Posterior uveitis was observed in 53.3% of male and 18.2% of female patients (p = 0.04), anterior uveitis showed a tendency towards female sex (27.3% versus 6.7%, p = 0.07), while panuveitis was represented in similar percentages of male (40.0%) and female (54.5%, p = 0.41) subjects (Fig. 2b).
a Visualization of the distribution of age at Behçet’s disease (BD) onset and age at ocular involvement according to the anatomical classification of uveitis. b Anatomical classification of uveitis was different in male and female patients, with a distinct prevalence of posterior uveitis in male patients
The mean (SD) BCVA logMAR measured at the time of enrolment in the registry was 0.30 (0.61), range – 0.30 to 2.00, without statistically significant differences according to sex (p = 0.80), age at disease onset (p = 0.06), presence of HLA-B51 (p = 0.12), different anatomical classes of uveitis (p = 0.80) and ethnic origin (p = 0.11). During the median follow-up period of 7.5 years (IQR 12.0), the mean number of relapses of ocular disease in children was 6.2 (SD 14.7, range 0.0–80.0).
Ocular complications occurred in 23 eyes (51.1%): cataract in 13 (28.9%), macular oedema in 9 (20.0%), posterior synechiae in 7 (15.6%), and macular epiretinal membrane, band keratopathy, pupillary seclusion, glaucoma and ghost vessels in 3 (6.7%) each; others were reported in < 6.7% of eyes (Supplementary Material S2). In the group with ocular complications, the patients had lower age at the onset of ocular manifestations [mean (SD) 10.8 (3.9) years vs. 14.9 (2.2) years, p < 0.01], higher number of ocular relapses over time [median (IQR) 2.0 (14.0) vs. 1.0 (1.0), p = 0.02] and more prolonged treatment with systemic CS [median (IQR) 30.0 (55.0) months vs. 9.0 (10.0) months, p = 0.02]. The duration of the ocular disease was similar between patients with or without ocular complications (p > 0.05). Patients with ocular complications occurring any time during disease history more frequently received biological (b) disease-modifying anti-rheumatic drugs (DMARDs) (p = 0.02) and specifically tumour necrosis factor (TNF)α inhibitors (p = 0.02).
Instrumental Data
Data on the findings of the fundus oculi examination were available for 35 procedures. The examination was normal in 17 cases (48.6%) and abnormal in 18 (51.4%). Findings reported in pathological fundus examinations are detailed as Supplementary Material (S3).
OCT findings were available for 17 procedures. The examination was normal in 13 cases (76.5%) and abnormal in 4 (23.5%). The following pathological findings were reported: diffuse intraretinal fluid in three eyes (17.6%), intraretinal cysts in two eyes (11.8%), spongy oedema in two eyes (11.8%) and tractional epiretinal membrane in one eye (5.9%). The mean (SD) CMT at the enrolment in the registry (measured in 10 eyes) was 302.2 (58.4; range 239–404) μm. The mean (SD) CMT at the last follow-up available (measured in 9 eyes) was 293.3 (78.2; range 180–404) μm.
Data on the findings of fundus fluorescein angiography (FFA) were available for 7 eyes undergoing 19 procedures. The examination identified signs of posterior uveitis and/or retinal vasculitis in 12/19 procedures (63.2%) while it resulted negative in 7 (36.8%). When applying the ASUWOG fluorescein angiographic scoring system on the earliest procedures performed for the seven eyes, the mean (SD) total score was 17.9 (15.5) (range 0–37). Angiographic signs detected in all the procedures performed over time are reported in Fig. 3 and detailed as Supplementary Material (S4).
The following findings at the slit-lamp evaluation were reported: active inflammation in the anterior chamber was found in 6/19 examinations (31.6%); fine keratic precipitates were found in 2/9 examinations (22.2%); vitreous inflammation was found in 7/18 examinations (38.9%); vitreous haze was graded in 7 eyes according to Nussemblatt grading system as grade I in 5 eyes, grade II in 1 eye and grade IV in 1 eye. Bulbar ultrasound was performed in four eyes, resulting in pathological findings in all cases: vitreous turbidity in three eyes and retinal detachment in one.
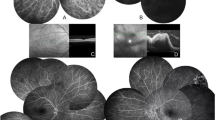
An iconographic collection of clinical and instrumental findings in ocular jBD is available as Supplementary Material (S5).
Therapeutic Data
Twenty children were treated with systemic CS (74.1%) by oral (17/20, 85%) or both oral and intravenous (3/20, 15%) route. The mean (SD) higher dosage of prednisone employed was 0.5 (0.3) mg/kg/day, range 0.1–1.0. The median (IQR) duration of CS therapy was 17.0 (23.5) months, range 1.0–137.0. Subconjunctival injections of prednisone and peribulbar injections of prednisolone were performed in two eyes each.
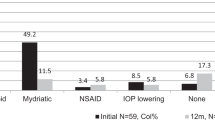
Table 2 shows the frequency of use of systemic CS, non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, conventional DMARDs and bDMARDs in the cohort. After stratification by the concomitant presence/absence of further major organ manifestations at disease onset, sex, ethnic origin or age at the onset of ocular involvement, the differences in use of each drug were not statistically significant (p > 0.05 for all). The frequency of employment of each drug was similar according to anatomical patterns of uveitis, except for methotrexate (MTX), which was more frequently used for anterior uveitis (60.0%) than posterior uveitis (5.6%) or panuveitis (33.3%) (p = 0.02). There were no statistically significant associations between the presence of retinal vasculitis and the use of any specific drug classes or single molecules.
Prognostic Data
A median (IQR) BODI score of 1.5 (3.3) (range 0–5) was calculated at the end of the follow-up, with a median disease duration of 7.5 (IQR 12.0) years [0.1–29.0]. According to linear correlation analysis, the total BODI score at the end of the follow-up inversely correlated with the age at onset of ocular involvement (Spearman’s r = – 0.5, p < 0.01) (Fig. 4A). Also, the total BODI score at the end of the follow-up was higher in case of bilateral ocular disease than unilateral (p = 0.02).
a Visualization of the inverse correlation between age at the onset of Behçet’s disease (BD)-associated ocular manifestations and total Behçet’s disease Overall Damage Index (BODI) score at the end of the follow-up (Spearman ρ = –0.57; p < 0.01); b age at onset of BD-associated ocular inflammation was lower in the group of children who developed permanent changes in the anterior segment during the disease course
At the end of the follow-up, ocular damage was observed in 33/45 eyes (73.3%): changes in the anterior segment in 17 (37.8%), changes in the posterior segment in 22 (48.9%), cataract in 13 (28.9%), visual impairment in 22 (48.9%) and blindness in 7 (15.6%). A lower age at the onset of ocular inflammation and the presence of complications were significantly associated with the development of permanent changes in the anterior segment (p = 0.01 and p < 0.01, respectively) (Fig. 4B). A younger age at the last follow-up visit and the presence of ocular complications were associated with the development of permanent changes in the posterior segment (p = 0.02 and p < 0.01, respectively). After running logistic regression analysis including the abovementioned factors, only the presence of ocular complications was a significant predictor of the development of permanent changes in the anterior and/or the posterior segments (coefficient = 2.5, OR = 11.9, p = 0.04 for the anterior segment and coefficient = 2.7, OR = 14.7, p < 0.01 for the posterior one, respectively).
A mean (SD) BCVA logMAR of 0.17 (0.47) (range – 0.30 to 2.00) was measured at the end of the follow-up. After median imputation of missing data, the BCVA logMAR at the end of the follow-up was found to be higher in the presence of HLA-B51 (p = 0.03), abnormal ocular fundus examination (p = 0.07), macular oedema (p = 0.05), cataract (p < 0.01) and posterior synechiae (p < 0.01). According to multivariate regression analysis, the presence of HLA-B51 could significantly predict a + 0.35 change in the BCVA logMAR at the last follow-up (p = 0.01), if the other variables analysed remain constant.
Blindness, defined as BCVA logMAR < 1.00 or central visual field ≤ 10°, was reported in seven eyes (unilateral in 5 children, bilateral in 1). Children who eventually experienced unilateral or bilateral blindness had a higher median BCVA logMAR at the initial evaluation recorded in the registry compared to those who did not develop blindness (p < 0.01). In addition, unilateral or bilateral blindness was associated with the presence of abnormal fundus examination (p = 0.03), macular oedema (p = 0.04) and posterior synechiae (p = 0.01). Multivariate regression analysis including BCVA logMAR at the initial evaluation, macular oedema, HLA-B51, sex, CS treatment duration, disease duration and posterior synechiae showed that BCVA logMAR at the initial evaluation (OR 5.80; p = 0.02) was an independent predictor of unilateral or bilateral blindness.
Discussion
This study aimed to provide a comprehensive characterization of ocular manifestations in children affected by BD by addressing demographic aspects, clinical observations, instrumental findings, therapeutic solutions and prognostic factors for unfavorable outcomes. In the AIDA Network BD registry, jBD accounted for 21.2% of the 1000 records, and ocular manifestations were observed in 43.9% of the 212 subjects with jBD at some point during their disease history. The prevalence is consistent with that resulting from a metanalysis conducted by Turk et al., where the overall frequency of ocular involvement in jBD was 45% [34–56%], similar to that of adult BD [30]. We may reasonably conclude that ocular disease is a frequent manifestation of jBD, although there are contrasting data in the literature, including a large Turkish study where ocular manifestations were found in 27.3% of jBD [18, 31].
The ocular symptoms typically manifested around the age of 14 years, with a potential range of 4 to 18 years. Similar mean ages at ocular involvement were reported by Citirik et al. and Friling et al., while in the PEDBD cohort a lower age was reported (10.9 years) [15, 32, 33]. In our cohort, ocular inflammation was among the initial signs of the disease for around 70% of children, similarly to what has been recently observed by Ostrovsky et al. [34]. Furthermore, there was an inverse relationship between the delay in ocular symptoms and the age at which BD began. Taken collectively, these observations may imply that adolescents might exhibit ocular inflammation at (or soon after) the onset of the disease while, in contrast, for younger children, the earlier the onset of BD, the more prolonged the anticipated delay in ocular manifestations. This hypothesis is consistent with the findings in a historical cohort of children from Turkey, where only 8 out of 62 (12.9%) with ocular BD were < 10 years [13].
In this study, a distinct male prevalence was observed among children with ocular BD. The precise male/female ratio in jBD remains undefined, and a hypothesis suggesting a sex-neutral balance in children has been put forth, mirroring findings in other immune-mediated diseases [35]. However, given the remarkable heterogeneity of this condition, it is plausible that specific symptom clusters could exhibit varying sex preferences. This was confirmed by a cluster analysis in 225 children that identified five clusters, among which the ocular and vascular ones had male preference and the mucocutaneous one had female preference [36]. Also, Shahram et al., in their description of one of the most extensive cohorts of jBD, observed a higher incidence of ocular inflammation in male patients, and similar findings were reported in the PEDBD cohort [15, 37].
As expected from the literature, the HLA-B51 haplotype was a significant genetic marker in our study cohort, with over half of the subjects carrying it. This haplotype appears to correlate with unfavorable clinical features; however, due to our limited sample size, many of these associations remain uncertain. Individuals with HLA-B51 + had a lower final visual acuity than the HLA-B51-, and this result was confirmed by multiple regression analysis showing that the presence of this genetic marker might independently predict a + 0.35 change of the final BCVA logMAR. HLA-B51 was confirmed as the principal genetic predisposing factor by genome-wide association study. A positive test increases the risk of developing BD by 5.79-fold and confers a higher probability of ocular involvement, with a correlation becoming stronger towards the East along the Silk Road [38,39,40,41]. However, the prognostic value of this allele has not been established yet. According to a monocentric study conducted by our research group on adult patients with BD, HLA-B51 may be a predictor of long-term structural complications and poor visual outcome [42]. Similar findings were reported by other studies: Zouboulis et al. showed that, in patients with a non-vascular phenotype at onset (recurrent oral aphthosis, genital ulcers or articular involvement), HLA-B51 positivity is a negative prognostic marker [43]; according to Kang et al., HLA-B51 could be associated with near total blindness in patients with posterior uveitis [44]. Contrarily, visual acuity was not influenced by the presence of HLA-B51 in a Korean cohort [45].
We also identified two patients with further predisposing genotypes. One of them, a girl with a moderate clinical phenotype, carried the HLA-Bw4 with isoleucine at amino acid position 80 (HLA-Bw4-80I) along with HLA-B51. In previous studies, the HLA-Bw4-80I was identified in HLA-B51- patients with BD from Germany and Turkey. These patients had a 2.4- and 2.2-fold increased risk of developing the disease, respectively, compared to healthy individuals [46]. The other patient carried the HLA-B27 allele, and his clinical picture was extremely severe, ending in multiple ocular complications and bilateral blindness. McGonagle et al. suggested that BD, psoriasis, psoriatic arthritis and spondyloarthropathies likely have a common aetiopathogenetic foundation, show clinical similarities and are linked with MHC class I alleles, including HLA-B51, HLA-B5, HLA-B27 and HLA-C0602 [47]. However, the strength of these associations varies among the diseases. As for the role of HLA-B27 as a predisposing factor for BD, it was assessed by a metanalysis of 3939 cases and 6077 controls, indicating that the risk of HLA-B27 for BD progression is overall increased by a factor of 1.55, but important differences exist according to geographical areas [48]. According to the analysis from Bettencourt et al., HLA-B27 may be a negative prognostic marker for disease severity, which could apply to our patient [49]. Interestingly, HLA-B27 positivity was a good prognostic factor in ocular BD according to another study, suggesting a complex influence of this allele on the disease course [50].
Moving to the ocular manifestations recorded in the 45 affected eyes, we found that uveitis was by far the most common, affecting 86.7% of them, with two-thirds having bilateral inflammation. The anatomical classification of uveitis revealed that posterior uveitis and panuveitis were the most common forms (40% each), while anterior uveitis was reported only in a minority of cases. Bilateral posterior uveitis was recognized as the most common type of uveitis in jBD by the metanalysis conducted by Turk et al. [30]. Interestingly, factors like age at disease onset and sex appeared to impact the type of uveitis observed. Male subjects and those who experienced a later disease onset (around the age of 14) were more likely to have posterior uveitis. Conversely, anterior uveitis was predominantly observed in those with an early systemic onset (around the age of 9) and consequently a longer delay before the appearance of ocular symptoms. The association of posterior uveitis with male sex was already reported in the PEDBD and Iranian cohorts [15, 37]. Similarly, Sungur et al. observed a greater prevalence of anterior uveitis in children under the age of 10 compared to older paediatric age groups, with anterior uveitis being the predominant type in the younger group [13].
Since the choroid is a vascular layer, variations in its thickness can serve as a reliable indicator to assess the response to inflammation. Balbaba et al. observed an increased subfoveal choroidal thickness in patients with jBD and ocular involvement, a finding already known from adult studies [51, 52]. Also, children with atrophic maculae and associated decrease in CMT have been described [51, 53]. Atrophy may be attributed to retinal ischaemia from repeated ocular flare-ups and also to a possible effect of subfoveal choroidal atrophy [53]. In our study, macular OCT examinations revealed several alterations, including diffuse intraretinal fluid, intraretinal cysts, spongy oedema and tractional epiretinal membranes, and showed a slight reduction of mean CMT from the enrolment in the registry up to the last follow-up available. Considering the follow-up duration of around 7 years and the concomitant improvement in visual acuity, this latter observation aligns more with a physiological reduction of the macular thickness during growth or a positive therapeutic outcome than with macular pathological alterations. We adopted the ASUWOG scoring system for a standardized description of FFA findings available for a small subset jBD children, an approach not seen in any previously published series to the best of our knowledge. The mean ASUWOG score at the first assessment available was 17.9 (15.5) (possible range 0 to 40), representing a moderate extent and severity of retinal inflammation, with macular oedema, retinal vascular staining or leakage in posterior pole arcades and optic disc hyperfluorescence being the most frequent findings, followed closely by peripheral retinal vascular staining or leakage. The abovementioned signs of retinal vasculitis and optic nerve inflammation were previously reported in adult patients with ocular BD [42].
Our findings indicate that a substantial portion of the morbidity associated with ocular jBD arises from its complications, which affected over half of the eyes. Indeed, the presence of complications in general and, in detail, macular oedema, cataract and/or posterior synechiae, were identified as risk factors linked to an impaired final visual acuity and permanent structural changes. In our cohort, individuals with frequent ocular relapses and prolonged systemic CS treatment exhibited more complications, potentially caused by the recurrent inflammatory insult and side effects of steroid therapy. Interestingly, children who experienced ocular symptoms at an early age faced a higher incidence of complications, regardless of disease duration. In this regard, in the study by Sungur et al., no significant differences were observed among paediatric age groups (< 10, 11–15 and 16–20 years) in the incidences of cataract and maculopathy; secondary glaucoma was markedly more frequent in the older group and band keratopathy in the younger one [13]. This warrants further validation with a more extensive cohort analysis, especially considering its potential significance in identifying patients who may benefit from more regular ophthalmological assessments.
Moving to the therapeutic information, our data underscore the complexity of treating BD-associated ocular inflammation in children. A substantial proportion of children (74.1%) received systemic CS treatment. Considering the relatively short duration of the ocular disease (median 7.5 years), this trend is less likely due to historical practices. Instead, it indicates that systemic CS continues to be a cornerstone in the current management of ocular jBD. We noticed a preference for the usage of low-to-moderate dosages of systemic CS for a long treatment period. Our findings align with several prior studies that confirm the ordinary use of systemic CS in 40–100% of children with ocular jBD, despite their well-recognized ocular and systemic side effects [13, 32, 34, 54]. Among the cDMARDs, azathioprine (AZA), cyclosporin A (CsA) and MTX were administered in 39%, 30% and 22% of cases, respectively, with other agents being seldom prescribed. While AZA and CsA are recommended for ocular jBD by the EULAR and French recommendations, the use of MTX is not encouraged as a monotherapy [55, 56]. Considering the prevalence of anterior uveitis in younger children in this study, as previously discussed, a potential explanation could be paediatricians' familiarity with MTX, derived from its application in juvenile idiopathic arthritis-associated uveitis. Regarding bDMARDs, the monoclonal TNFα inhibitor ADA was remarkably favoured, followed by IFX. The use of other molecules inhibiting TNFα or leveraging different mechanisms of action was anecdotal, in this case reflecting the international treatment recommendations [55, 56]. Finally, in our cohort, neither the presence of major organ involvement nor any specific systemic manifestations at disease onset independently influenced therapeutic decisions. Contrarily, the use of bDMARDs, and specifically TNFα inhibitors and ADA, was significantly associated with the detection of ocular complications, although our data did not allow us to further investigate the temporal relationship between the occurrence of each complication and the start of treatments. Nevertheless, it seems reasonable to infer that, in clinical decision-making, ocular manifestations are prioritized by physicians when formulating therapeutic strategies, as already observed by Batu et al. [54].
While comprehensive long-term prospective studies on jBD prognosis are lacking, ocular complications appear to be the predominant disability factor, along with vascular, neurological and intestinal manifestations [3]. In this study, the total BODI score at the last follow-up visit inversely correlated with the age at onset of ocular inflammation, and it was higher in children with bilateral ocular involvement, indirectly hinting at the significant impact of ocular disease on the overall prognosis in children. However, the influence of disease onset age on general prognosis remains unclear, given the inhomogeneous findings across studies, which could also be influenced by the disease relapsing-remitting course [7, 11, 21, 57,58,59]. Over 70% of eyes showed some degree of ocular damage by the end of the follow-up. Children with an earlier onset of ocular inflammation were more likely to develop permanent changes in both the anterior and the posterior segment, possibly because of multiple flare-ups, complications, prolonged CS therapy or a more severe clinical phenotype. In addition, HLA-B51 was an independent predictor of a worse final visual acuity, as previously discussed. Unilateral or bilateral blindness affected 22% of children (15% of eyes). Children who eventually became blind in at least one eye already had an impaired visual acuity at the first assessment available in the registry, indicating an aggressive ocular disease, similarly to what has been observed by Ostrovsky et al. [34]. In addition, we observed an association between unilateral or bilateral blindness and the presence of macular oedema and posterior synechiae. In a Japanese series of patients with ocular jBD presented by Kitaichi et al., the percentage of bilateral blindness was 7.4%, and the visual prognosis was significantly better in children compared to the adult series detailed in the same study [60]. Irreversible BCVA < 0.1 decimals was found in 10% and 6.1% of affected eyes according to Kramer et al. and Ostrovsky et al., respectively [32, 61].
Given the rarity of jBD and the specific focus of the research, this comprehensive analysis adds valuable information to the existing literature, which may be leveraged to potentially guide clinical practice and future research. However, we acknowledge some limitations which may potentially have influenced our findings. The study cohort was relatively small with imbalanced ethnicities (mostly of European descent and, to a lesser extent, Arab people), potentially limiting the generalizability of our findings to the broader population of children with ocular jBD. Regrettably, reliable information about growth and pubertal development was lacking. To overcome the issue of missing data, imputation was needed for a small number of analyses, potentially reducing the accuracy of the results. For prognostic and therapeutic outcomes, including a control group of children with jBD but without ocular involvement would have enhanced the robustness of our findings.
Conclusion
This study provides a thorough description of BD-associated ocular manifestations in the paediatric age group based on an international registry cohort. While a consistent proportion of children with jBD may incur ocular inflammation at some point in their life, it only happens before 18 in a few. Age and sex seem to be important players in the game but their role still has to be elucidated. Nevertheless, it seems reasonable to schedule routine ophthalmological screenings for paediatric patients with BD, especially during mid- to late adolescence. In this context, the use of standardized objective descriptions of the ophthalmological findings may help in establishing a functional partnership between the paediatric rheumatologist and the ophthalmologist with the common goal to enhance early detection and management of ocular manifestations. Regrettably, the therapeutic strategy for ocular jBD still relies heavily on CS, and a trend to use low-to-moderate oral dosages for extended treatment courses was noted. Robust research is necessary to endorse the use of more targeted therapies able to reduce steroid use while preventing disease relapses as the high number of relapses contributes to the development of complications and permanent structural changes.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Yildiz M, Haslak F, Adrovic A, et al. Pediatric Behçet’s disease. Front Med (Lausanne). 2021;8: 627192. https://doi.org/10.3389/fmed.2021.627192.
Hu YC, Chiang BL, Yang YH. Clinical manifestations and management of pediatric Behçet’s disease. Clin Rev Allergy Immunol. 2021;61(2):171–80. https://doi.org/10.1007/s12016-020-08809-2.
Pain CE. Juvenile-onset Behçet’s syndrome and mimics. Clin Immunol. 2020;214: 108381. https://doi.org/10.1016/j.clim.2020.108381.
Atmaca LS, Idil A, Batioĝlu F. A descriptive study on Behçet’s disease. Acta Ophthalmol Scand. 1996;74(4):403–6. https://doi.org/10.1111/j.1600-0420.1996.tb00718.x.
Mendes D, Correia M, Barbedo M, et al. Behçet’s disease—a contemporary review. J Autoimmun. 2009;32(3–4):178–88. https://doi.org/10.1016/j.jaut.2009.02.011.
Koné-Paut I, Geisler I, Wechsler B, et al. Familial aggregation in Behçet’s disease: high frequency in siblings and parents of pediatric probands. J Pediatr. 1999;135(1):89–93. https://doi.org/10.1016/s0022-3476(99)70333-1.
Borlu M, Ukşal U, Ferahbaş A, et al. Clinical features of Behçet’s disease in children. Int J Dermatol. 2006;45(6):713–6. https://doi.org/10.1111/j.1365-4632.2006.02754.x.
Atmaca L, Boyvat A, Yalçındağ FN, et al. Behçet disease in children. Ocul Immunol Inflamm. 2011;19(2):103–7. https://doi.org/10.3109/09273948.2011.555592.
Krause I, Uziel Y, Guedj D, et al. Childhood Behçet’s disease: clinical features and comparison with adult-onset disease. Rheumatology (Oxford). 1999;38(5):457–62. https://doi.org/10.1093/rheumatology/38.5.457.
Karincaoglu Y, Borlu M, Toker SC, et al. Demographic and clinical properties of juvenile-onset Behçet’s disease: a controlled multicenter study. J Am Acad Dermatol. 2008;58(4):579–84. https://doi.org/10.1016/j.jaad.2007.10.452.
Treudler R, Orfanos CE, Zouboulis CC. Twenty-Eight Cases of Juvenile-Onset Adamantiades-Behçet Disease in Germany. Dermatology. 1999;199(1):15–9. https://doi.org/10.1159/000018197.
Kötter I, Vonthein R, Müller CA, et al. Behçet’s disease in patients of German and Turkish origin living in Germany: a comparative analysis. J Rheumatol. 2004;31(1):133–9.
Sungur GK, Hazirolan D, Yalvac I, et al. Clinical and demographic evaluation of Behçet disease among different paediatric age groups. Br J Ophthalmol. 2009;93(1):83–7. https://doi.org/10.1136/bjo.2007.137141.
Koné-Paut I, Gorchakoff-Molinas A, Weschler B, et al. Paediatric Behçet’s disease in France. Ann Rheum Dis. 2002;61(7):655–6. https://doi.org/10.1136/ard.61.7.655.
Koné-Paut I, Shahram F, Darce-Bello M, et al. Consensus classification criteria for paediatric Behçet’s disease from a prospective observational cohort: PEDBD. Ann Rheum Dis. 2016;75(6):958–64. https://doi.org/10.1136/annrheumdis-2015-208491.
Pivetti-Pezzi P, Accorinti M, Abdulaziz MA, et al. Behçets disease in children. Jpn J Ophthalmol. 1995;39(3):309–14.
Kim DK, Chang SN, Bang D, et al. Clinical analysis of 40 cases of childhood-onset Behçet’s disease. Pediatr Dermatol. 1994;11(2):95–101. https://doi.org/10.1111/j.1525-1470.1994.tb00559.x.
Sarica R, Azizlerli G, Köse A, et al. Juvenile Behçet’s disease among 1784 Turkish Behçet’s patients. Int J Dermatol. 1996;35(2):109–11. https://doi.org/10.1111/j.1365-4362.1996.tb03272.x.
Lang BA, Laxer RM, Thorner P, et al. Pediatric onset of Behçet’s syndrome with myositis: case report and literature review illustrating unusual features. Arthritis Rheum. 1990;33(3):418–25. https://doi.org/10.1002/art.1780330317.
Allali F, Benomar A, Karim A, et al. Behçet’s disease in Moroccan children: a report of 12 cases. Scand J Rheumatol. 2004;33(5):362–3. https://doi.org/10.1080/03009740410005980.
Sota J, Rigante D, Lopalco G, et al. Clinical profile and evolution of patients with juvenile-onset Behçet’s syndrome over a 25-year period: insights from the AIDA network. Intern Emerg Med. 2021;16(8):2163–71. https://doi.org/10.1007/s11739-021-02725-9.
Vitale A, Della Casa F, Ragab G, et al. Development and implementation of the AIDA International Registry for patients with Behçet’s disease. Intern Emerg Med. 2022;17(7):1977–86. https://doi.org/10.1007/s11739-022-03038-1.
International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335(8697):1078–80. https://doi.org/10.1016/0140-6736(90)92643-V.
International Team for the Revision of the International Criteria for Behçet’s Disease (ITR-ICBD). The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–47. https://doi.org/10.1111/jdv.12107.
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First InternationalWorkshop. Am J Ophthalmol. 2005;140:509–16.
Nussenblatt RB, Palestine AG, Chan CC, et al. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–71.
Chylack LT Jr, Wolfe JK, Singer DM, et al. The lens opacities classification system III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–6.
Tugal-Tutkun I, Herbort CP, Khairallah M, Angiography Scoring for Uveitis Working Group (ASUWOG). Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis). Int Ophthalmol. 2010;30(5):539–52. https://doi.org/10.1007/s10792-008-9263-x.
Piga M, Floris A, Espinosa G, et al. Development and preliminary validation of the Behçet’s syndrome Overall Damage Index (BODI). RMD Open. 2020;6(2): e001192. https://doi.org/10.1136/rmdopen-2020-001192.
Turk MA, Hayworth JL, Nevskaya T, et al. Ocular manifestations of Behçet’s disease in children and adults: a systematic review and meta-analysis. Clin Exp Rheumatol. 2021;39 Suppl 132(5):94–101. https://doi.org/10.55563/clinexprheumatol/pt60bc.
Pain CE, Beresford MW, Fortune F, et al. Behçet’s syndrome in children and young people in the United Kingdom and the Republic of Ireland: a prospective epidemiological study. Rheumatology (Oxford). 2021;60(10):4728–36. https://doi.org/10.1093/rheumatology/keab084.
Citirik M, Berker N, Songur MS, et al. Ocular findings in childhood-onset Behçet disease. J AAPOS. 2009;13(4):391–5. https://doi.org/10.1016/j.jaapos.2009.04.016.
Friling R, Kramer M, Snir M, et al. Clinical course and outcome of uveitis in children. J AAPOS. 2005;9(4):379–82. https://doi.org/10.1016/j.jaapos.2005.04.005.
Ostrovsky M, Rosenblatt A, Iriqat S, et al. Ocular Behçet disease-clinical manifestations, treatments and outcomes according to age at disease onset. Biomedicines. 2023;11(2):624. https://doi.org/10.3390/biomedicines11020624.
Cattalini M, Soliani M, Caparello MC, et al. Sex differences in pediatric rheumatology. Clin Rev Allergy Immunol. 2019;56(3):293–307. https://doi.org/10.1007/s12016-017-8642-3.
Demir F, Sönmez HE, Bağlan E, et al. Cluster analysis of paediatric Behçet’s disease: data from the Pediatric Rheumatology Academy-Research Group. Mod Rheumatol. 2023;33(3):574–8. https://doi.org/10.1093/mr/roac044.
Shahram F, Nadji A, Akhlaghi M, et al. Paediatric Behçet’s disease in Iran: report of 204 cases. Clin Exp Rheumatol. 2018;36(6 Suppl 115):135–40.
de Menthon M, Lavalley MP, Maldini C, et al. HLA-B51/B5 and the risk of Behçet’s disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 2009;61(10):1287–96. https://doi.org/10.1002/art.24642.
Gül A. Genetics of Behçet’s disease: lessons learned from genomewide association studies. CUrr Opin Rheumatol. 2014;26(1):56–63. https://doi.org/10.1097/BOR.0000000000000003.
Maldini C, Lavalley MP, Cheminant M, et al. Relationships of HLA-B51 or B5 genotype with Behcet’s disease clinical characteristics: systematic review and meta-analyses of observational studies. Rheumatology (Oxford). 2012;51(5):887–900. https://doi.org/10.1093/rheumatology/ker428.
Horie Y, Meguro A, Ohta T, et al. HLA-B51 carriers are susceptible to ocular symptoms of Behçet disease and the association between the two becomes stronger towards the east along the silk road: a literature survey. Ocul Immunol Inflamm. 2017;25(1):37–40. https://doi.org/10.3109/09273948.2015.1136422.
Sota J, Cantarini L, Vitale A, et al. Long-term outcomes of Behçet’s syndrome-related uveitis: a monocentric italian experience. Mediators Inflamm. 2020;15(2020):6872402. https://doi.org/10.1155/2020/6872402.
Zouboulis CC, Turnbull JR, Martus P. Univariate and multivariate analyses comparing demographic, genetic, clinical, and serological risk factors for severe Adamantiades-Behçet’s disease. Adv Exp Med Biol. 2003;528:123–6. https://doi.org/10.1007/0-306-48382-3_24.
Kang EH, Park JW, Park C, et al. Genetic and non-genetic factors affecting the visual outcome of ocular Behcet’s disease. Hum Immunol. 2013;74(10):1363–7. https://doi.org/10.1016/j.humimm.2013.06.036.
Kim MS, Kim JH. Prognostic comparison of Behçet’s disease with or without HLA-Bw 51 antigen. Korean J Ophthalmol. 1989;3(2):85–9. https://doi.org/10.3341/kjo.1989.3.2.85.
Kuranov AB, Kötter I, Henes JC, et al. Behçet’s disease in HLA-B*51 negative Germans and Turks shows association with HLA-Bw4-80I. Arthritis Res Ther. 2014;16(3):R116. https://doi.org/10.1186/ar4569.
McGonagle D, Aydin SZ, Gül A, et al. ‘MHC-I-opathy’—unified concept for spondyloarthritis and Behçet disease. Nat Rev Rheumatol. 2015;11(12):731. https://doi.org/10.1038/nrrheum.2015.147.
Khabbazi A, Vahedi L, Ghojazadeh M, et al. Association of HLA-B27 and Behcet’s disease: a systematic review and meta-analysis. Auto Immun Highlights. 2019;10(1):2. https://doi.org/10.1186/s13317-019-0112-x.
Bettencourt A, Pereira C, Carvalho L, et al. New insights of HLA class I association to Behçet’s disease in Portuguese patients. Tissue Antigens. 2008;72(4):379–82. https://doi.org/10.1111/j.1399-0039.2008.01087.x.
Ahn JK, Park YG. Human leukocyte antigen B27 and B51 double-positive Behçet uveitis. Arch Ophthalmol. 2007;125(10):1375–80. https://doi.org/10.1001/archopht.125.10.1375.
Balbaba M, Ulaş F, Postacı SA, et al. Clinical and demographic features of pediatric-onset Behçet’s disease and evaluation of optical coherence tomography findings. Ocul Immunol Inflamm. 2020;28(4):606–12. https://doi.org/10.1080/09273948.2019.1611875.
Ishikawa S, Taguchi M, Muraoka T, et al. Changes in subfoveal choroidal thickness associated with uveitis activity in patients with Behçet’s disease. Br J Ophthalmol. 2014;98(11):1508–13. https://doi.org/10.1136/bjophthalmol-2014-305333.
Coskun E, Gurler B, Pehlivan Y, et al. Enhanced depth imaging optical coherence tomography findings in Behçet disease. Ocul Immunol Inflamm. 2013;21(6):440–5. https://doi.org/10.3109/09273948.2013.817591.
Batu ED, Sener S, Cam V, et al. Treatment with biologic drugs in pediatric Behçet’s disease: a comprehensive analysis of the published data. BioDrugs. 2023;37(6):813–28. https://doi.org/10.1007/s40259-023-00613-6.
Hatemi G, Christensen R, Bang D, et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis. 2018;77(6):808–18. https://doi.org/10.1136/annrheumdis-2018-213225.
Kone-Paut I, Barete S, Bodaghi B, et al. French recommendations for the management of Behçet’s disease. Orphanet J Rare Dis. 2021;16(Suppl 1):352. https://doi.org/10.1186/s13023-020-01620-4.
Gheita TA, El-Latif EA, El-Gazzar II, Egyptian College of Rheumatology-Behçet’s Disease Study Group (ECR-BDSG), et al. Behçet’s disease in Egypt: a multicenter nationwide study on 1526 adult patients and review of the literature. Clin Rheumatol. 2019;38(9):2565–75. https://doi.org/10.1007/s10067-019-04570-w.
Alpsoy E, Donmez L, Onder M, et al. Clinical features and natural course of Behçet’s disease in 661 cases: a multicentre study. Br J Dermatol. 2007;157:901–6. https://doi.org/10.1111/j.1365-2133.2007.08116.x.
Hamzaoui A, Jaziri F, Ben Salem T, et al. Comparison of clinical features of Behcet disease according to age in a Tunisian cohort. Acta Med Iran. 2014;52:748–51.
Kitaichi N, Miyazaki A, Stanford MR, et al. Low prevalence of juvenile-onset Behcet’s disease with uveitis in East/South Asian people. Br J Ophthalmol. 2009;93(11):1428–30. https://doi.org/10.1136/bjo.2008.154476.
Kramer M, Amer R, Mukamel M, et al. Uveitis in juvenile Behçet’s disease: clinical course and visual outcome compared with adult patients. Eye (Lond). 2009;23(11):2034–41. https://doi.org/10.1038/eye.2008.397.
Acknowledgements
Medical Writing/Editorial Assistance.
We acknowledge the use of ChatGPT [https://chat.openai.com/] at the final stage of preparing this manuscript. We entered the following prompt: “Improve my academic English writing style.” ChatGPT was also used as an interface to create graphic contents.
Funding
No funding or sponsorship was received for this study or publication of this article. This research is supported (not financially) by the European Reference Network (ERN) for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases (RITA). Thirteen of the authors of this publication [Carla Gaggiano, Jurgen Sota, Stefano Gentileschi, Petros P. Sfikakis, Maria Tarsia, Antonio Vitale, Valeria Caggiano, Gian Marco Tosi, Bruno Frediani, Tadej Avčin, José Hernández-Rodríguez, Claudia Fabiani, and Luca Cantarini] belong to institutes that are members of the ERN RITA [Azienda Ospedaliero-Universitaria Senese of Siena; Laiko General Hospital of Athens; University Medical Center of Ljubljana; Hospital Clínic of Barcelona].
Author information
Authors and Affiliations
Consortia
Contributions
Carla Gaggiano, Abdurrahman Tufan, Silvana Guerriero, Gaafar Ragab, Jurgen Sota, Stefano Gentileschi, Stefania Costi, Ibrahim A. Almaghlouth, Andrea Hinojosa-Azaola, Samar Tharwat, Petros P. Sfikakis, Giuseppe Lopalco, Matteo Piga, Giovanni Conti, Angela Mauro, Francesco La Torre, Ezgi Deniz Batu, Seza Ozen, Riza Can Kardaş, Maria Tarsia, Perla Ayumi Kawakami-Campos, George Fragoulis, Antonio Vitale, Valeria Caggiano, Gian Marco Tosi, Bruno Frediani, Tadej Avčin, José Hernández-Rodríguez, Claudia Fabiani, and Luca Cantarini substantially contributed to the conception or design of the work, the acquisition and interpretation of data and critically revised the paper. All the authors approved the final version and agreed to be responsible for all the aspects of the work. In addition, Carla Gaggiano wrote the first draft of the manuscript and performed data analysis and interpretation; Luca Cantarini, Claudia Fabiani, Tadej Avčin and José Hernández-Rodríguez took care of the final revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Carla Gaggiano, Abdurrahman Tufan, Silvana Guerriero, Gaafar Ragab, Jurgen Sota, Stefano Gentileschi, Stefania Costi, Ibrahim A. Almaghlouth, Andrea Hinojosa-Azaola, Samar Tharwat, Petros P. Sfikakis, Giuseppe Lopalco, Matteo Piga, Giovanni Conti, Angela Mauro, Francesco La Torre, Ezgi Deniz Batu, Seza Ozen, Riza Can Kardaş, Maria Tarsia, Perla Ayumi Kawakami-Campos, George Fragoulis, Antonio Vitale, Valeria Caggiano, Gian Marco Tosi, Bruno Frediani, Tadej Avčin, José Hernández-Rodríguez, and Luca Cantarini declare that they have no competing interests. Claudia Fabiani is an Editorial Board member of Ophthalmology and Therapy and was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
The study protocol adheres to the principles outlined in the Helsinki Declaration. The AIDA Network BD registry conforms to the General Data Protection Regulation (GDPR) ensuring compliance with legal requirements regarding the processing of personal data. For eligibility in the registry, patients (or their parents/legal guardians) must provide written consent, after receiving appropriate information. The protocol of the international AIDA Network BD registry was approved by the Ethics Committee of Siena University Hospital (Ref. 14951), and by the Ethics Committees of all the participating investigator centers.
Additional information
Prior Presentation: The results of this work were disclosed as a poster presentation at the 2023 Congress of the Italian Society of Pediatric Rheumatology (REUMAPED) held in Florence, Italy, on 16–18 November 2023. In addition, they were the subject of a PhD defense at the University of Siena, Italy, on 20 December 2023.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gaggiano, C., Tufan, A., Guerriero, S. et al. Ocular Manifestations in Juvenile Behçet’s Disease: A Registry-Based Analysis from the AIDA Network. Ophthalmol Ther 13, 1479–1498 (2024). https://doi.org/10.1007/s40123-024-00916-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00916-z