Abstract
Introduction
This study aims to investigate the pattern of intraocular pressure (IOP) changes in different postures among patients with open-angle glaucoma (OAG).
Methods
A observational study was conducted on a total of 74 patients with OAG (148 eyes). IOP measurements were taken in a variety of positions, including supine, left lateral decubitus, right lateral decubitus, head tilted downwards position with immediate head-up (transient head tilted downwards), seated, seated with head tilted downwards, standing, and walking. Each position was held for 5 min before measurement. In all positions, the patient maintains both eyes looking forward and remains alert. In the head tilted downwards position, the angle of head tilt with respect to the sagittal plane was 30°.
Results
The overall trend of IOP changes showed a significant decrease with an increase in the position height (r = 0.037, p < 0.001). The IOP was significantly higher in the supine, left lateral decubitus, right lateral decubitus, and head tilted downwards positions than in the seated position (p < 0.001). Compared with the seated position with eyes at primary gaze, IOP decreased significantly when standing (p = 0.008) or walking (p < 0.001). The IOP in the left lateral decubitus and right lateral decubitus was significantly higher than in the supine position (p = 0.008, p = 0.001, respectively). The IOP decreased significantly during walking compared with standing (p < 0.001).
Conclusions
The magnitude of IOP strongly correlates with the body position during IOP measurement. The head tilted downwards, supine, left lateral decubitus, and right lateral decubitus positions result in a higher IOP than IOP at the seated position. Patients with OAG can potentially reduce IOP fluctuations by adjusting their daily postures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Open-angle glaucoma (OAG) is the most common type of glaucoma and lowering intraocular pressure (IOP) remains the most effective treatment method. |
The assessment of IOP is very important for patients with OAG. IOP fluctuations are an independent risk factor for the progression of visual field damage in patients with glaucoma. |
Posture is one of the factors most likely to cause fluctuations in IOP. The fluctuation of IOP in OAG in common daily life postures such as sleeping position, sitting, head tilted downwards, standing, and walking is an area worth researching. |
What was learned from the study? |
IOP decreases as the body position becomes more upright. The head tilted downwards, supine, left lateral decubitus, and right lateral decubitus positions result in a higher IOP than IOP at the seated position. IOP fluctuations in different postures may be unrelated to age. |
Patients with OAG can potentially reduce IOP fluctuations by adjusting their daily postures. |
Introduction
The concept of intraocular pressure (IOP) was first proposed back in 1626 [1]. High IOP is an undisputed risk factor for the occurrence and progression of glaucoma [2], and lowering IOP remains the only effective method for the treatment of glaucoma [3]. Although some patients have a well-controlled IOP during routine visits, they still experience glaucoma progression [4, 5]. In such cases, fluctuations in IOP may be the significant contributing factor [6, 7] and an independent risk factor for glaucoma injury [6, 8]. Many studies have shown that 24-h fluctuations in IOP can lead to the progression of glaucoma [2]. In addition, the short-term fluctuation of IOP caused by daily physical activities can lead to the progression of glaucoma [9]. Many daily activities, such as rubbing or massaging the eyes, assuming a face-down position, varying sleep positions, and wearing swim goggles, can lead to transient increases in IOP [10]. However, some of the temporary fluctuations in IOP during daily activities are difficult to capture, such as those during heavy lifting or running. According to the time of exposure to factors that can cause the fluctuation of IOP, the individual exposure to the fluctuation of IOP can be estimated [10]. The peak IOP at night is of great significance for patients with glaucoma [11]. Previous studies have shown that when transitioning from a supine position to a lateral position, IOP in the lower eye increases further [12]. Aerobic exercise can reduce IOP [13]. This means that there is a certain correlation between IOP and body position. As a result of varying lifestyle habits, different patients may spend extended periods in positions that favor an increase in IOP. This could potentially influence the prognosis of glaucoma. Avoiding the increase in IOP during fluctuation may improve the prognosis of patients with glaucoma [14]. The head tilted downwards position is a common posture in daily life, and there is currently limited research on the changes in IOP during such positions. There has also been little research on IOP fluctuations in the same patients with glaucoma who are in different body positions. IOP tends to decrease with increasing age [15], and sensitivity to IOP fluctuations may also increase with age [13]. Further research is needed to investigate the positional IOP fluctuations among different age groups.
In this study, we focused on patients with primary open-angle glaucoma (OAG) and measured IOP in eight different positions, including the head tilted downwards position. We investigated the patterns of IOP changes in various positions among patients with OAG.
Methods
Study Design
This prospective study included 74 patients (148 eyes) with OAG who visited the outpatient department of Beijing Tongren Hospital between January 2022 and March 2023. This was an observational study to examine the fluctuation of IOP under different postures. All participants signed an informed consent form before accepting the study. The study followed the tenants of the Declaration of Helsinki and has been approved by the Ethics Committee of Beijing Tongren Hospital (Beijing, China) affiliated to Capital Medical University (MR-11-22-009185).
Patients
The inclusion criteria for the patients were as follows: (1) diagnosis of primary open-angle glaucoma (POAG) or juvenile open-angle glaucoma (JOAG); (2) age between 18 and 80 years; (3) no history of ocular surgery within the past 3 months; (4) voluntary cooperation with the necessary IOP examinations, and provided informed consent.
The exclusion criteria were as follows: (1) angle-closure glaucoma or other types of OAG; (2) ocular conditions that prevent IOP measurement, such as corneal inflammation, corneal edema, and corneal scars; (3) other conditions apart from glaucoma that could affect IOP assessment, such as larger intraocular or orbital masses, and iris neovascularization; (4) history of ocular surgery within the past 3 months; (5) inability to provide informed consent for various reasons.
Diagnosis of POAG and JOAG
Patients were diagnosed with POAG if they had glaucomatous defects in the visual field (VF) test and IOP > 21 mmHg on at least two occasions, with open angles, supported by distinct glaucomatous optic neuropathy. When the fixed loss was below 20% and the false-positive and false-negative errors were less than 15%, the VF test was considered reliable [16]. The diagnosis of JOAG encompassed the signs of POAG in individuals younger than 40 years [17]. Categorized by visual field damage, all patients was divided into mild (mean deviation [MD] ≥ − 6 dB), moderate (MD between − 6 and − 12 dB), and severe glaucoma (MD worse than − 12 dB) [18, 19].
Assessments
Measurement Method of IOP Fluctuation
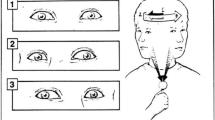
The IOP measurements were conducted in different positions and during walking for patients with OAG, with the aim to investigate the fluctuation patterns of IOP in various positions and their associated factors. The sequence of positions included supine, left lateral decubitus, right lateral decubitus, head tilted downwards position with immediate head-up, seated position, seated with head tilted downward, standing position, and walking. For all these positions, we ensured that both eyes were looking straight ahead. In the head tilted downwards position, the angle of head tilt relative to the sagittal plane was 30°. Each position was maintained for 5 min before measurement, and the participants remained awake with open eyes. Patients had taken a 5-min break between measuring IOP in different postures in this study. Eye pressure measurements were performed using the Icare tonometer (Icare PRO; Icare Finland Oy, Helsinki, Finland). All of the measurements were conducted between 9:00 AM and 11:00 AM. A total of six measurements were taken by the same examiner and averaged for each time point, with each measurement completed within 30 s, as shown in Fig. 1.
Statistical Analysis
SPSS26.0 (IBM, Chicago, USA) software was used to process data. The measurement data such as IOP values were tested for normality. The continuous variables conforming to the normal distribution were described by the mean and standard deviation. The comparison of IOP values among different body positions was based on a paired-sample t test, while the comparison of IOP changes among different groups was based on an independent-sample t test. One-way analysis of variance (ANOVA) was used for comparisons among multiple groups. Linear regression analysis was performed to examine the correlation between elevation of eye position and IOP. If the data did not conform to the normal distribution, they were described by the median and the upper and lower quartiles. The differences were statistically significant if p was lower than 0.05.
Results
Demographic and Baseline Characteristics
Among the 74 enrolled patients, there were 25 (33.78%) women and 49 (66.22%) men. In each subject, both eyes were examined, resulting in a total number of 148 examined eyes. A total of 38 (51.35%) patients were diagnosed with POAG, and 36 (48.65%) cases were diagnosed with JOAG. The mean IOP was 20.86 ± 6.59 mmHg, and number of IOP-lowering medications used was 3.26 ± 1.26. The study involved all patients receiving treatment. A total of 34 eyes (22.97%) had a history of previous glaucoma surgery, including trabeculectomy (16 eyes, 10.81%), minimally invasive glaucoma surgery (MIGS) (15 eyes, 10.14%), and glaucoma drainage implant (3 eyes, 2.03%). The rest received medication. Data regarding central corneal thickness, axial length, and anterior chamber depth for surgical subjects are presented in Table 1.
IOP Values and IOP Fluctuation
A total of 74 participants (148 eyes) were included in the measurement of IOP in different body positions. In all of the participants, the IOP measurements were conducted in eight different positions, including the supine position, left lateral decubitus position, right lateral decubitus position, seated position with immediate head elevation (transient head tilted downwards), seated position, seated position with head lowered then elevated, standing position, and walking. The average IOP for these eight positions was as follows: 23.05 ± 8.12 mmHg, 24.63 ± 7.88 mmHg, 25.29 ± 8.62 mmHg, 21.41 ± 6.86 mmHg, 20.86 ± 6.59 mmHg, 22.65 ± 8.52 mmHg, 19.82 ± 6.59 mmHg, and 19.20 ± 6.45 mmHg. Because routine clinical IOP measurements are taken in a seated position with eyes looking straight ahead, the variations in IOP in other positions were compared with the seated position with eyes straight ahead. Compared with the seated position with eyes looking straight ahead, the supine position, left lateral decubitus position, right lateral decubitus position, and head-lowered position were associated with a higher IOP, with statistical significance (p < 0.001). In contrast, compared with the seated position with eyes looking straight ahead, the standing and walking positions were associated with a lower IOP, with statistical significance (p = 0.008, p < 0.001, respectively). IOP was higher in the left lateral decubitus and right lateral decubitus positions than in the supine position, with statistical significance (p = 0.008, p = 0.001, respectively). IOP decreased in the walking position compared with the standing position, with statistical significance (p < 0.001). There was a negative correlation between the height of the eye position and the change in IOP, with statistical significance (r = 0.037, p < 0.001) as shown in Table 2 and Figs. 2 and 3.
Postural Changes in IOP in Different Age Groups
The participants were divided into three aged groups, namely 18–35 years, 36–53 years, and 54–71 years. The total number of cases (eyes) in the three groups was 29 (58), 22 (44), and 22 (46), respectively. There were no significant differences in seated IOP and MD values among the three groups (p = 0.081, p = 0.106, respectively). The difference between the IOP in various positions and seated IOP was calculated as the IOP variation for each position. There was no statistically significant difference in the changes in intraocular pressure between the three groups of subjects in the supine, left lateral decubitus, right lateral decubitus, head tilted downwards position with immediate head-up, seated with head tilted downward, standing position, and walking (p = 0.372, p = 0.116, p = 0.902, p = 0.230, p = 0.872, p = 0.377, p = 0.220) (Table 3 and Fig. 4).
Discussion
In this study, we measured IOP in various positions among patients with OAG. To the best of our knowledge, this is the first comprehensive investigation of positional IOP measurements specifically in patients with OAG, and it is also the first study investigating the changes in IOP in patients with OAG during head tilted downwards positioning.
In this study, we observed the pattern of changes in IOP among the patients with OAG in response to different body positions. Overall, we found that as the body position became more elevated, there was a trend of decreasing IOP. The IOP in three different sleeping positions and the IOP in the head tilted downwards position were higher than the IOP in the seated position. Additionally, the lateral sleeping position showed a higher IOP than the supine position. To minimize factors that could affect the accuracy of the results of this study, the IOP measurements in all of the positions were taken with the patients keeping their eyes open. Any change in posture leads to the redistribution of gravitational fluids [20]. As a result of the eccentric position of the eyes, when transitioning from a lying down position to standing, the eyes are exposed to particularly significant pressure changes [21]. Aqueous humor enters the aqueous veins through the anterior chamber and eventually returns to the bloodstream, circulating back to the heart [22]. Compared with the seated position, the decrease in the vertical distance between the eyes and the heart in three different sleeping postures and the head tilted downwards position could lead to an increase in IOP. The reduction in gravitational potential energy associated with the drainage of aqueous humor into the heart might contribute to the elevation of IOP. Compared to supine position, lateral position requires more gravity to overcome when aqueous humor flows out. This could be one of the main reasons for the elevation of IOP in these postures compared to the seated position. A study conducted in astronauts before and after spaceflight revealed that IOP increased by 1.3 mmHg under weightlessness conditions after flight, and acute exposure to lower body negative pressure of 25 mmHg led to a decrease in IOP relative to the preflight levels [23], which highlights the influence of gravity on IOP. In addition, the sympathetic nervous system and the parasympathetic nervous system are involved in the regulation of IOP [24]. Compared with the seated position, in these postures, enhanced sympathetic nerve stimulation leads to an increased secretion of epinephrine, enhanced atrial conduction, and an accelerated heart rate [25]. Local administration of epinephrine can lead to an increase in Schlemm’s canal diameter and trabecular meshwork width, thereby resulting in a reduction in IOP [26].
Two studies regarding the effects of tight neckties on IOP suggest that, in both healthy individuals and patients with glaucoma, IOP increases 3 min after placing a tight necktie [27, 28]. The jugular veins are typically positioned more superficially than the carotid arteries. The head tilted downwards position could potentially result in an increase in jugular venous pressure, leading to congestion of the choroid and increased choroidal blood volume [29], elevated IOP, and an increase in the episcleral venous pressure [30], thereby resulting in an increase in IOP.
Accurate 24-h IOP monitoring is crucial for patients with POAG. In the 24-h IOP measurements, some patients exhibit nocturnal IOP elevation. The present study showed that maintaining a supine position while awake with eyes open also led to an increase in IOP, indicating that body posture is one of the important factors contributing to the elevated IOP during sleep. However, in a 24-h IOP measurement, if the patient walks to the examination room before measurement or sits on the edge of the bed for a seated position measurement, the true IOP in the patient’s sleep position cannot be obtained. The most accurate method for measuring nighttime IOP in a 24-h measurement is to have the examiner positioned bedside while the patient maintains a supine position. As a result of the variations in IOP among the three sleeping positions, in order to obtain more accurate measurements of nighttime IOP, when monitoring the 24-h fluctuations of IOP at different time periods, all nighttime IOP measurements should be taken in the same sleeping posture. Our recommendation is that patients with OAG who rest in bed and stay awake may have lower IOP in the supine position compared to the lateral position. Multiple studies suggest that patients with well-controlled daytime IOP but elevated nighttime IOP, especially those who habitually sleep in lateral positions, could benefit from adjusting their sleeping posture to a supine position, so as to potentially reduce their nocturnal IOP. Apart from sleep, other factors such as lying on a sofa in a lateral position while watching TV at home could also elevate IOP in patients with OAG. Therefore, it is recommended that such patients minimize the duration of such positions.
The head tilted downwards posture is commonly encountered within the population. For individuals whose daily work involves prolonged seated computer use in an office setting, as well as other professions that require frequent sustained head tilted downwards postures, the cumulative time spent in a head tilted downwards position during working hours, along with the additional time spent using smartphones after work, might exceed 10 h per day. This fluctuation in IOP, due to its prolonged duration during daylight hours, extends beyond the scope of short-term IOP variations and may potentially contribute to visual field progression. Previous research has indicated that even with well-controlled IOP, significant fluctuations resulting from posture changes can lead to visual field deterioration [31]. Therefore, for patients with OAG, it is advisable to minimize the frequency of assuming a head tilted downwards position in daily activities. For example, when reading, one can use a book stand to adjust the height of the book, thereby maintaining an eye-level position while reading. Adjusting daily postures in this manner can help reduce the fluctuations in IOP.
In this study, we found that the IOP was lower in the standing and walking positions than in the sitting position. Additionally, the IOP was lower while walking than while standing. The act of standing upright or walking is associated with a temporary reduction in central blood volume, leading to a decrease in cardiac output and mean arterial pressure [32]. The decreases in cardiac output and mean arterial pressure can be compensated for by the arterial pressure reflex, which increases peripheral vascular resistance and heart rate [33]. When vascular resistance decreases and heart rate increases, the increased venous return to the heart may facilitate the drainage of aqueous humor into the aqueous veins, potentially leading to a reduction in IOP. Additionally, previous research has confirmed that aerobic exercise can result in a decrease in IOP [34]. Resistance exercise can lead to an increase in IOP [35], which positively correlates with the magnitude of resistance [36]. The heart rate and respiratory rate are higher in standing and walking postures than in the seated position. A study investigating the effect of respiration on IOP during resistance training in healthy individuals of different genders reported that IOP increased when the respiratory rate decreased and the residual volume increased, and there were no differences among the gender groups [36]. The aqueous humor drainage pattern is driven by the heart, and under the influence of the aqueous humor outflow pump system, it is pumped out of the eye at the same frequency as the heart rate [37]. Therefore, the enhancement of cardiac function accompanied by an increase in the heart rate, as well as the accelerated respiratory rate, may be reasons that contribute to the reduction of IOP. Therefore, there is valuable guidance for the daily postures of patients with POAG. It is recommended that patients increase their time spent standing and engaging in physical activity in their daily lives, as this may help maintain lower IOP. Additionally, the accuracy of IOP measurement is crucial for patients with glaucoma. We suggest that patients with glaucoma, when visiting the clinic for IOP measurement, should be instructed to maintain a calm seated position for at least 5 min before the measurement to obtain more accurate data. If the patient’s posture before measuring IOP is not standardized, such as when measuring seated IOP immediately after a long walk or measuring seated IOP immediately after prolonged standing, inaccurate seated IOP readings can be obtained. IOP measurements should adopt the same rules as blood pressure measurement, which requires maintaining a quiet state for at least 10 min before measurement.
In our study, there was no significant difference in IOP when comparing the IOP immediately after transitioning from sitting with a lowered head to sitting upright. The transition from a lowered head position to an upright position is a movement that we constantly perform in various aspects of life, such as during travel, work, and in the classroom. We chose to measure this position to observe the immediate changes in IOP upon transitioning from a lowered head position to an upright position. This suggests that the act of raising the head does not lead to an increase in IOP or may only cause a momentary change in IOP that is not measurable.
Since conventional IOP measurements are typically performed in a seated position, this study used the difference between IOP in various positions and seated IOP as the measure of IOP variation in different positions. There were no significant differences in the fluctuations of IOP in different postures among different age groups. Aging is associated with the progression of glaucoma, and one of the reasons for faster glaucoma progression at older ages is the initial intervention [38]. As age increases, a decrease in lung capacity may require deeper breaths during exercise, which may limit the range and intensity of physical performance [39]. Further research is needed to confirm the relationship between age and IOP fluctuations.
A limitation of this study is that, while investigating the positional changes in IOP, additional data such as pupil diameter, episcleral venous pressure, anterior chamber depth, and other relevant factors were not simultaneously collected. In future research, it would be beneficial to incorporate the measurement of more related factors and extend the duration and frequency of follow-up to observe the correlation between the positional fluctuations in IOP and the rate of visual field progression.
Conclusions
The observed variations in IOP with different body positions, as found in this study, offer valuable guidance for patients with glaucoma. By providing recommendations for patients’ sleeping postures and daily activities one can minimize fluctuations in IOP. Furthermore, the conclusions drawn from this study suggest that accurate nighttime IOP measurements should be obtained while patients are in their sleeping postures during 24-h IOP monitoring, rather than in a seated position. For the diagnosis and treatment of glaucoma, it is also advised that IOP measurements should be taken after the patient has maintained a seated level gaze position for at least 5 min, as this can yield more reliable IOP readings. The accuracy and reliability of IOP measurements are crucial for the diagnosis and treatment of glaucoma, and the conclusions drawn from this study can contribute to obtaining more dependable data in clinical settings. In the future, further research is needed to explore the factors that influence posture-related changes in IOP and their association with glaucoma.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Shaffer RN. The centennial history of glaucoma (1896–1996). American Academy of Ophthalmology. Ophthalmology. 1996;103(8 Suppl):S40–50. https://doi.org/10.1016/s0161-6420(96)30763-x.
Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9(2):134–42. https://doi.org/10.1097/00061198-200004000-00002.
Casson RJ, Chidlow G, Wood JP, Crowston JG, Goldberg I. Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol. 2012;40(4):341–9. https://doi.org/10.1111/j.1442-9071.2012.02773.x.
Collaer N, Zeyen T, Caprioli J. Sequential office pressure measurements in the management of glaucoma. J Glaucoma. 2005;14(3):196–200. https://doi.org/10.1097/01.ijg.0000159125.34241.79.
Varma R, Hwang LJ, Grunden JW, Bean GW. Inter-visit intraocular pressure range: an alternative parameter for assessing intraocular pressure control in clinical trials. Am J Ophthalmol. 2008;145(2):336–42. https://doi.org/10.1016/j.ajo.2007.10.002.
Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the advanced glaucoma intervention study. Ophthalmology. 2004;111(9):1627–35. https://doi.org/10.1016/j.ophtha.2004.02.017.
Mansouri K, Medeiros FA, Weinreb RN. Letter to the editor: 24-hour versus daytime intraocular pressure phasing in the management of patients with treated glaucoma. Br J Ophthalmol. 2011;95(4):594–5. https://doi.org/10.1136/bjo.2010.201327.
Singh K, Sit AJ. Intraocular pressure variability and glaucoma risk: complex and controversial. Arch Ophthalmol. 2011;129(8):1080–1. https://doi.org/10.1001/archophthalmol.2011.66.
Mansouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. Br J Ophthalmol. 2011;95(5):627–9. https://doi.org/10.1136/bjo.2010.192922.
McMonnies C. An examination of the hypothesis that intraocular pressure elevation episodes can have prognostic significance in glaucoma suspects. J Optom. 2015;8(4):223–31. https://doi.org/10.1016/j.optom.2014.07.008.
Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44(4):1586–90. https://doi.org/10.1167/iovs.02-0666.
Lee JY, Yoo C, Jung JH, Hwang YH, Kim YY. The effect of lateral decubitus position on intraocular pressure in healthy young subjects. Acta Ophthalmol. 2012;90(1):e68–72. https://doi.org/10.1111/j.1755-3768.2011.02208.x.
McMonnies CW. Intraocular pressure and glaucoma: is physical exercise beneficial or a risk? J Optom. 2016;9(3):139–47. https://doi.org/10.1016/j.optom.2015.12.001.
Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–13. https://doi.org/10.1001/archopht.120.6.701.
Liu X, Pan X, Ma Y, Jin C, Wang B, Ning Y. Variation in intraocular pressure by sex, age, and geographic location in China: a nationwide study of 284,937 adults. Front Endocrinol (Lausanne). 2022;25(13):949827. https://doi.org/10.3389/fendo.2022.949827.
Huo Y, Thomas R, Guo Y, et al. Superficial macular vessel density in eyes with mild, moderate, and severe primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2021;259(7):1955–63. https://doi.org/10.1007/s00417-021-05120-4.
Seibold LK, Wagner BD, Lynch AM, Kahook MY. The diurnal and nocturnal effects of pilocarpine on intraocular pressure in patients receiving prostaglandin analog monotherapy. J Ocul Pharmacol Ther. 2018;34(8):590–5. https://doi.org/10.1089/jop.2018.0050.
Bojikian KD, Nobrega P, Wen JC, et al. Macular vascular microcirculation in eyes with open-angle glaucoma using different visual field severity classification systems. J Glaucoma. 2019;28(9):790–6. https://doi.org/10.1097/IJG.0000000000001308.
Kita Y, Holló G, Saito T, et al. RETeval portable electroretinogram parameters in different severity stages of glaucoma. J Glaucoma. 2020;29(7):572–80. https://doi.org/10.1097/IJG.0000000000001509.
Suzuki Y. Influences of gravitational stress and total muscle mass on human cardiovascular adjustments during prolonged dynamic exercise. Ann Physiol Anthropol. 1990;9(2):139–51. https://doi.org/10.2114/ahs1983.9.139.
Petersen LG, Petersen JC, Andresen M, Secher NH, Juhler M. Postural influence on intracranial and cerebral perfusion pressure in ambulatory neurosurgical patients. Am J Physiol Regul Integr Comp Physiol. 2016;310(1):R100–4. https://doi.org/10.1152/ajpregu.00302.2015.
Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp Eye Res. 2004;78(3):625–31. https://doi.org/10.1016/j.exer.2003.09.021.
Greenwald SH, Macias BR, Lee SMC, et al. Intraocular pressure and choroidal thickness respond differently to lower body negative pressure during spaceflight. J Appl Physiol (1985). 2021;131(2):613–20. https://doi.org/10.1152/japplphysiol.01040.2020.
Wu F, Zhao Y, Zhang H. Ocular autonomic nervous system: an update from anatomy to physiological functions. Vision (Basel). 2022;6(1):6. https://doi.org/10.3390/vision6010006.
Piccirillo G, Moscucci F, Di Diego I, et al. Effect of head-up/-down tilt on ECG segments and myocardial temporal dispersion in healthy subjects. Biology (Basel). 2023;12(7):960. https://doi.org/10.3390/biology12070960.
Ye M, Chen Z, Li M, Chen W, Zhang H, Wang J. Effect of topical application of adrenaline on Schlemm canal, trabecular meshwork and intraocular pressure. Medicine (Baltimore). 2019;98(22):e15558. https://doi.org/10.1097/MD.0000000000015558.
Bozić M, Hentova Senćanin P, Branković A, Marjanović I, Dordević Jocić J, Senćanin I. Effect of a tight necktie on intraocular pressure. Med Pregl. 2012;65(1–2):13–7. https://doi.org/10.2298/mpns1202013b.
Teng C, Gurses-Ozden R, Liebmann JM, Tello C, Ritch R. Effect of a tight necktie on intraocular pressure. Br J Ophthalmol. 2003;87(8):946–8. https://doi.org/10.1136/bjo.87.8.946.
Waqar S, Fuller JR, Kersey T. Effect of dry-suit neck seals on intraocular pressure. Can J Ophthalmol. 2009;44(4):472–3. https://doi.org/10.3129/i09-088.
Foroozan R, Buono LM, Savino PJ, Sergott RC. Idiopathic dilated episcleral veins and increased intraocular pressure. Br J Ophthalmol. 2003;87(5):652–4. https://doi.org/10.1136/bjo.87.5.652.
Kim KN, Jeoung JW, Park KH, Lee DS, Kim DM. Effect of lateral decubitus position on intraocular pressure in glaucoma patients with asymmetric visual field loss. Ophthalmology. 2013;120(4):731–5. https://doi.org/10.1016/j.ophtha.2012.09.021.
Thomas KN, Cotter JD, Galvin SD, Williams MJ, Willie CK, Ainslie PN. Initial orthostatic hypotension is unrelated to orthostatic tolerance in healthy young subjects. J Appl Physiol (1985). 2009;107(2):506–17. https://doi.org/10.1152/japplphysiol.91650.2008.
Geinas JC, Marsden KR, Tzeng YC, et al. Influence of posture on the regulation of cerebral perfusion. Aviat Space Environ Med. 2012;83(8):751–7. https://doi.org/10.3357/asem.3269.2012.
Wylęgała A. The effects of physical exercises on ocular physiology: a review. J Glaucoma. 2016;25(10):e843–9. https://doi.org/10.1097/IJG.0000000000000454.
Zhu MM, Lai JSM, Choy BNK, et al. Physical exercise and glaucoma: a review on the roles of physical exercise on intraocular pressure control, ocular blood flow regulation, neuroprotection and glaucoma-related mental health. Acta Ophthalmol. 2018;96(6):e676–91. https://doi.org/10.1111/aos.13661.
Vera J, García-Ramos A, Jiménez R, Cárdenas D. The acute effect of strength exercises at different intensities on intraocular pressure. Graefes Arch Clin Exp Ophthalmol. 2017;255(11):2211–7. https://doi.org/10.1007/s00417-017-3735-5.
Johnstone M, Xin C, Tan J, Martin E, Wen J, Wang RK. Aqueous outflow regulation - 21st century concepts. Prog Retin Eye Res. 2021;83: 100917. https://doi.org/10.1016/j.preteyeres.2020.100917.
Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115(7):1123–1129.e3. https://doi.org/10.1016/j.ophtha.2007.10.031.
Fry DL, Ebert RV, Stead WW, Brown CC. The mechanics of pulmonary ventilation in normal subjects and in patients with emphysema. Am J Med. 1954;16(1):80–97. https://doi.org/10.1016/0002-9343(54)90325-3.
Acknowledgements
The authors thank Beijing Tongren Hospital, Capital Medical University for help and support.
The authors would also like to thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Qing Sang, and Ningli Wang: study concept and design and manuscript revision. Qing Sang, and Chen Xin: performed study. Qing Sang: drafted the manuscript. Diya Yang, Dapeng Mu, Ningli Wang: revised the manuscript. Qing Sang, and Diya Yang: statistical analysis. Ningli Wang: administrative, technical, material support, or study supervision. All authors participated in and provided help for the study.
Corresponding author
Ethics declarations
Conflict of Interest
Qing Sang, Chen Xin, Diya Yang, Dapeng Mu, and Ningli Wang declare no conflict of interest.
Ethical Approval
The study adhered to the tenets of the declaration of Helsinki and was approved by the Ethics Committee of Beijing Tongren Hospital (MR-11-22-009185).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sang, Q., Xin, C., Yang, D. et al. Effect of Different Postures on Intraocular Pressure in Open-Angle Glaucoma. Ophthalmol Ther 13, 149–160 (2024). https://doi.org/10.1007/s40123-023-00845-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00845-3