Abstract
Introduction
This study reports the long-term intraocular pressure (IOP)-lowering efficacy and safety of a single trabecular microbypass stent (iStent®; Glaukos Corp., San Clemente, CA, USA) for medically controlled open-angle glaucoma in Korean patients.
Methods
This retrospective observational study included 42 eyes of 35 patients with primary open-angle glaucoma (POAG). All subjects underwent single first-generation iStent® implantation with phacoemulsification by a single surgeon with 5 years follow-up. The primary outcomes were changes in IOP and the number of antiglaucoma medications compared to the preoperative values. The secondary outcome was the proportion of eyes with IOP ≤ 18 mmHg without medication, ≤ 15 mmHg without medication, and ≤ 18 mmHg with or without medication. Adverse events and need for secondary glaucoma surgery were also recorded.
Results
The mean IOP decreased from 15.8 ± 2.8 to 13.8 ± 1.7 mmHg and the mean number of medications was reduced from 2.24 ± 1.18 to 0.83 ± 1.12, respectively, at year 5. At 3 and 5 years, 80.6% and 78.6% of eyes, respectively, were receiving fewer medications than preoperative numbers. In contrast, only 50% of eyes on four preoperative medications showed medication reductions at 5 years. At years 3 and 5, 61.3% and 53.5% of eyes achieved IOP ≤ 18 mmHg without medication, whereas 90.3% and 89.3% of eyes achieved ≤ 18 mmHg regardless of medication use, respectively. Four eyes required additional glaucoma surgery (two Ahmed glaucoma valve implantations, one trabeculectomy, and one XEN 45 Gel Stent implantation), and all were receiving four preoperative antiglaucoma medications. Transient IOP elevation (14.3%) was the most common complication, followed by five hyphema, one stent obstruction, one stent malposition, and one severe anterior chamber reaction.
Conclusion
This study demonstrated a good safety profile and sustained IOP reduction after the implantation of a single trabecular microbypass stent (iStent®) with phacoemulsification in Korean patients. The majority of subjects with POAG showed a relatively good response; however, eyes receiving a higher number of medications preoperatively (especially four medications) had difficulty achieving a low target IOP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Many previous studies have demonstrated the short-term favorable efficacy of first-generation trabecular microbypass stenting (iStent®) in patients with high intraocular pressure (IOP). |
Few studies have investigated long-term outcomes after first-generation iStent® implantation. Moreover, there have been no published long-term follow-up studies on Korean patients. |
What was learned from the study? |
This study is the first to report the long-term effects of iStent® with phacoemulsification in Korean patients with medically controlled open-angle glaucoma in various disease severities in a real-world setting. |
At year 5, mean IOP was reduced from 15.8 ± 2.8 to 13.8 ± 1.7 mmHg, whereas the mean number of medications had decreased from 2.24 to 0.83. |
At the 5-year follow-up, 78.6% of eyes were receiving fewer medications (one or more) than preoperatively, and only 9.5% of eyes required additional glaucoma surgery. |
This study demonstrated an excellent safety profile and sustained IOP reduction after implantation of a single trabecular microbypass stent (iStent®) with phacoemulsification in Korean patients. |
Our results suggest that eyes receiving a higher number of medications (especially four medications) preoperatively may have difficulty achieving a low target IOP. |
Introduction
Glaucoma is one of the leading causes of blindness worldwide [1]. The goal of glaucoma treatment is to maintain visual function and quality of life at a sustainable cost [2]. The mainstay of glaucoma treatment remains to reduce intraocular pressure (IOP), which is the only treatment strategy proven to prevent progression [2, 3].
Canal-based minimally invasive glaucoma surgery (MIGS) provides advantages, such as quicker visual recovery and sparing of conjunctival tissue for future glaucoma surgeries [4]. The iStent® (Glaukos Corporation, Aliso Viejo, CA, USA) is one of the canal-based MIGS device. The first-generation single trabecular microbypass iStent®, with an internal diameter of 120 µm, and the second-generation iStent® inject or iStent® inject W, composed of two stents, each with an internal diameter of 80 µm, were designed to allow direct aqueous flow into Schlemm’s canal, bypassing the trabecular meshwork [4, 5].
Many previous studies have focused on the short-term safety and efficacy of first-generation iStent® implantations [6,7,8]. However, few long-term follow-up studies have been conducted over 5 years [9,10,11,12]. Moreover, no long-term follow-up studies have been conducted after iStent® implantation in Korean patients. This real-world study aimed to investigate the long-term IOP-lowering efficacy and safety of a single trabecular microbypass stent with phacoemulsification in Korean patients with primary open-angle glaucoma (POAG). To the best of our knowledge, this study is the first to investigate 5-year outcomes in Korean patients.
Methods
Patients
This single-center, retrospective case series was conducted at Daegu Veterans Health Service Medical Center. The study protocol was approved by the Institutional Review Board of Daegu Veterans Hospital (2020–2010). All participants provided signed informed consent and the study adhered to the tenets of the Declaration of Helsinki.
The study included eyes with visually significant cataracts and coexisting POAG that underwent combined cataract extraction with iStent® implantation. These patients often had poor compliance, drug allergy, or the need to reduce ocular hypotensive medications. The exclusion criteria were eyes undergoing another procedure during the operation; eyes with angle-closure glaucoma or secondary glaucoma, such as pseudoexfoliative glaucoma, pigmentary glaucoma, or neovascular glaucoma; and eyes with a history of previous glaucoma surgery.
Surgery
The surgical technique has been described in our previous studies [8, 13]. All combined iStent® implantation with phacoemulsification surgeries were performed by a single glaucoma specialist (S.-H.L.). All subjects underwent implantation of one iStent® device between January 2018 and June 2019. Each single-piece, heparin-coated, titanium stent had a length of 1.0 mm, height of 0.33 mm, and snorkel bore diameter of 120 μm [4]. Briefly, under topical anesthesia using a 0.5% proparacaine eye drop, uncomplicated phacoemulsification was completed through a temporal clear incision with a 2.4-mm keratome. A single-piece, hydrophobic acrylate intraocular lens was inserted. Ocular biometric parameters are summarized in Table 1.
An ophthalmic viscosurgical device was placed in the anterior chamber to deepen the angle and to visualize the trabecular meshwork. A single trabecular microbypass stent was inserted nasally into Schlemm’s canal (SC) (3–4 o’clock in the right eye vs. 8–9 o’clock in the left eye) using a Swan–Jacob gonioprism [8].
Postoperative Management and Follow-up
The details of management described in our previous reports were used [8, 13]. Postoperatively, topical antibiotics (0.5% gatifloxacin) and topical steroid (0.1% fluorometholone acetate) eye drops were prescribed four times daily for 1 week and then tapered according to the resolution of inflammation [8, 13]. In accordance with the World Glaucoma Association guidelines [14], each patient’s best-corrected visual acuity, IOP, glaucoma medication use, and complications were recorded at 1 day, 1 week, 1 month, 2 months, 3 months, 6 months, and then annually thereafter postoperatively. Postoperatively, glaucoma medications were added according to the surgeon’s opinion based on disease severity and target IOP, considering the European Glaucoma Society guidelines and the American Academy of Ophthalmology Preferred Practice Pattern [2, 3, 8, 13]. In general, glaucoma medication was usually initiated or escalated if the IOP exceeded 18 mmHg or concerning optic nerve or visual field changes based on the landmark study of the Advanced Glaucoma Intervention Study [15], which demonstrated delayed visual field progression when IOP was consistently maintained at less than 18 mmHg [8, 13, 15].
Data Analysis
Combination glaucoma medications (e.g., timolol–dorzolamide, timolol–brinzolamide, and timolol–brimonidine) were recorded as two medications in the dataset. Baseline IOP, measured using Goldmann applanation tonometry, was obtained during the visit immediately before surgery and was based on the average of the two measures.
Statistical analyses were performed using Statistical Package for the Social Sciences for Windows (version 22.0; IBM Corp., Armonk, NY, USA). Repeated-measures analysis of variance was used to compare IOP and number of medications used during the study period. A paired t test was used to determine the statistical significance for normally distributed values at the time point, whereas the Wilcoxon signed-rank test was used for non-parametric analysis. The cumulative probability of success was assessed using stratified Kaplan–Meier survival analysis. For Kaplan–Meier survival analysis, success was defined as achieving the target IOP with medication reduction (one or more). The secondary outcome was the proportion of patients with IOP ≤ 18 mmHg without medication, IOP ≤ 15 mmHg without medication, and IOP ≤ 18 mmHg with or without medication, respectively. P < 0.05 was defined as statistically significant.
Results
Baseline Characteristics
This study included 42 eyes of 35 patients. The mean age of the patients was 73.7 ± 4.2 years. All the eyes were diagnosed with POAG. Four patients (9.5%) had previously undergone laser trabeculoplasty (two selective laser [SLT] and two argon laser [ALT]). The mean follow-up duration after surgery was 57.6 ± 7.1 months. The longest follow-up duration was 68 months. Attrition was due to death in five (11.9%), further glaucoma surgery in four (9.5%), and transfer of care to another hospital due to patient preference in three (7.1%) cases. At years 3 and 5, 28 (66.7%) and 25 (59.5%) patients, respectively, were enrolled. Most death (four of five deaths) and transfer to another hospital occurred between the fourth and fifth years.
Table 1 summarizes the baseline demographic characteristics of the participants. Briefly, the mean preoperative ocular parameters were as follows: IOP, 15.8 ± 2.8 mmHg; mean number of antiglaucoma medications, 2.2 ± 1.2; vertical cup-to-disc ratio 0.77 ± 0.09; visual field mean deviation, − 9.21 ± 6.70 dB; and pattern standard deviation, 5.69 ± 3.47 dB, respectively.
IOP Reduction Through 5 years in the Intention-to-Treat Patients
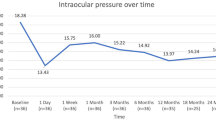
The mean IOP showed sustained reduction over the 5-year period (Fig. 1). During the follow-up period, the mean IOP at postoperative months 12, 24, 36, 48, and 60 was 14.1 ± 2.7, 14.2 ± 3.4, 14.4 ± 2.3, 14.0 ± 2.6, and 13.8 ± 1.7 mmHg, respectively (all P values < 0.05, paired t test). Similarly, the mean number of medications was reduced with a gradual trend of increasing mean medications. The mean numbers of medications at postoperative months 12, 24, 36, 48, and 60 were 0.66 ± 0.99, 0.75 ± 1.08, 0.75 ± 1.24, 0.96 ± 1.27, and 0.83 ± 1.13, respectively (Fig. 1; all P values < 0.05, paired t test).
IOP and number of glaucoma medication changes over 5 years in the intention-to-treat study participants. The mean IOP was reduced to 14.0 mmHg with 0.5 medications at the first postoperative month from 15.8 mmHg with 2.2 medications, peaking at 13.9 mmHg at 6 months. After year 2, the number of antiglaucoma medications gradually increased to 0.96 at year 4. At 60 months, the absolute reductions in the mean IOP and glaucoma medication use for survivors were 2.03 mmHg and 1.4 medications, respectively (both P < 0.001). IOP intraocular pressure
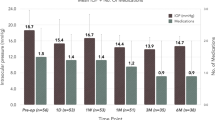
The proportion of eyes with one or more medication reductions was 97.6% at month 6 and gradually decreased over time to 78.6% at month 60 (Fig. 2; 94.4%, 86.6%, 80.6%, 78.6%, and 78.6% at year 1, 2, 3, 4, and 5, respectively). Success was defined as the achievement of the target IOP with one or more reductions in medication use. Kaplan–Meier survival estimates are summarized in Fig. 3a–c. Overall success in the cohort was 83.6% at year 5. Interestingly, eyes with greater preoperative medication use demonstrated poorer IOP control (1–2 vs. 3–4 medications, 95.0% vs. 68.8% at year 5 [P = 0.028]; 1–3 vs. 4 medications, 92.6% vs. 50.0% at year 5 [P < 0.001]; log-rank test).
Proportional analysis for medication reduction from preoperative values. Regarding medication burden, the proportions of eyes with one or more medication reductions compared to preoperative medication usage were 97.6% (6 months), 86.6% (24 months), 80.6% (36 months), 78.6% (48 months), and 78.6% (60 months)
Kaplan–Meier survival analysis estimates (A–C). The eyes with greater preoperative medication use demonstrated poorer IOP control (1–2 vs. 3–4 medications, 95.0% vs. 68.8% at year 5 [P = 0.028]; 1–3 vs. 4 medications, 92.6% vs. 50.0% at year 5 [P < 0.001]; log-rank test; B, C). IOP intraocular pressure
Figure 4 summarizes the qualified success and complete success according to the different criteria for achieving the target IOP. In brief, complete success (IOP ≤ 15 mmHg and IOP ≤ 18 mmHg without medication) rates were 54.7% and 57.1% at month 6 vs. 46.4% and 53.6% at month 60, respectively (P = 0.099 and 0.256, McNemar test) .Qualified success rate (IOP ≤ 18 mmHg with or without medication) was 96.2% at month 6 vs. 89.3% at month 60, respectively (P < 0.001, McNemar test).
Postoperative Complications
Transient IOP elevation (14.3%) was the most common complication, followed by five cases of hyphema, one with stent obstruction, one with stent malposition requiring repositioning, one with vitreous prolapse, one with cyclodialysis cleft, and one with a severe anterior chamber reaction. Vitreous prolapse was resolved following the application of a neodymium-doped yttrium aluminum garnet (YAG) laser. In one eye, stent obstruction resolved after YAG laser treatment.
Four eyes (9.5%) required additional glaucoma surgery (two with Ahmed glaucoma valve implantations, one with trabeculectomy, and one with XEN 45 Gel Stent implantation), and all were receiving four antiglaucoma medications preoperatively. The additional details are presented in Table 2. Among these four patients, two had proliferative diabetic retinopathy and two had a previous history of laser trabeculoplasty.
Discussion
The Korean National Insurance System (Health Insurance Review & Assessment Service) approved the use of trabecular microbypass stents in 2017. Many studies have been published on the short-term safety and efficacy of first-generation iStent® implantations [6,7,8]. However, there have been few long-term follow-up studies [9,10,11,12], and no studies with long-term follow-up after iStent® implantation in Korean patients. To the best of our knowledge, this study is the first to investigate 5-year outcomes in Korean patients.
This real-world retrospective study of single trabecular microbypass stent implantation combined with phacoemulsification demonstrated a statistically significant sustained IOP reduction and decreased medication burden among Korean patients with POAG over 5 years. Although the mean medication slightly increased after 2 years postoperatively, the mean medication at year 5 was 0.83 from 2.24 preoperative medications (P < 0.001, paired t test).
In our study, IOP was reduced from 15.8 mmHg with 2.24 medications to 13.8 mmHg with 0.83 medications at 5 years. The proportion of eyes achieving IOP ≤ 18 mmHg with or without medication was 94.4% and 89.3% of eyes, and 52.8% and 46.4% had IOP ≤ 15 mmHg without medication at years 1 and 5 in our study, respectively. A low mean preoperative IOP of 15.8 mmHg might lead to a high success rate; thus, we defined IOP ≤ 15 mmHg without medication as a strict success criterion.
Similar to our report, Fea et al. [16] showed that IOP was decreased from 17.8 mmHg with 1.9 medications to 15.9 mmHg with 0.5 medications at 4 years postoperatively. Ferguson et al. [17] reported outcomes after combination procedure, observing that the mean IOP decreased from 18.8 to 14.9 mmHg. The proportions of IOP ≤ 18 mmHg were 81.0% and 83.0% at 1 year and 6 years, and the proportions of IOP ≤ 15 mmHg were 53.0% and 53.0% at 1 year and 6 years in their study.
In this context, similar long-term outcomes after combined phacoemulsification with first-generation iStent® implantation have been reported. Arriola-Villalobos et al. reported long-term outcomes in mild-to-moderate open-angle glaucoma, with 11 of 20 patients completing 5 years of follow-up [11]. In their study, the mean IOP reduction at the final visit was 3.16 mmHg (19.42 preoperatively vs. 16.26 final), with a mean reduction of 0.48 (1.32 preoperatively vs. 0.84 final). Ansari [10] reported the 5-year outcomes in 35 eyes with moderately advanced glaucoma. In that study, the mean IOP was reduced from 18.5 mmHg with 2.3 medications to 14.7 mmHg with 2.7 medications at 5 years. Ziaei and Au [9] reported a mean IOP reduction of 4.87 mmHg with 0.59 medications in the Manchester iStent® study with 7 years of follow-up.
These differences in IOP reduction might have occurred because of different baseline characteristics, including preoperative IOP, etiology of glaucoma, homogeneity, ethnic differences, and decision-making by the surgeon. For example, our study included only POAG cases and a small percentage of previous trabeculoplasty cases (n = 2, 4.8%), whereas the Manchester iStent study [9] included patients with pseudoexfoliation syndrome and mixed-mechanism glaucoma, and another study from the UK included a relatively high number of patients with previous laser procedures (20%) and only moderately advanced glaucoma cases [10]. Moreover, in our study, the operator (S.-H.L.) prescribed additional medications considering the customized target IOP, with reference to the American preferred practice pattern [3] and European guidelines [2]. We did not prescribe medications if eyes with mild POAG achieved IOP ranging from 15 to 18 mmHg without medication. And combination medications were considered as two medications in our dataset. This preference may result in a greater reduction in the number of medications while achieving target IOP.
Regarding glaucoma severity, according to the Hodapp-Anderson criteria [2], our patients were heterogeneous (early, 33.3%; moderate, 33.3%; severe-end stage, 33.3%). In addition, 45.2% (19/42 eyes) of the eyes received three or more preoperative medications. These patients were considered high-risk cases as defined in the Manchester iStent study [9]. On the basis of our results, patients with more preoperative medications or more advanced stages of the disease might show a higher risk of surgical failure in the Kaplan–Meier survival analysis. The long-term survival rate (decrease in the medication number of one or more) was significantly lower in the higher medication group (≥ 3 preoperative medications, 68.8%; 4 preoperative medications, 50.0%) than in the lower medication group (1–2 preoperative medications, 95.0%; 1–3 preoperative medications, 92.6%) at year 5. In our previous study, we postulated reasons for the higher surgical failure rate in the higher medication group [8]; possible explanations include post-trabecular outflow resistance (distal outflow resistance), properties of the SC and lymphatics [18], and biomechanical changes in the SC and cytoskeleton-induced long-term use of glaucoma medication [8, 19].
Although our study did not include pigment dispersion syndrome and pseudoexfoliative glaucoma, previous studies have reported a relatively good IOP reduction [20, 21]. Secondary open-angle glaucoma, including pseudoexfoliation syndrome and pigment dispersion syndrome, obstruction, and consequent increased resistance to the outflow of aqueous humor through the conventional outflow pathway, causes increased IOP. Buchacra et al. reported that performing a bypass of the trabecular meshwork between the anterior chamber and Schlemm’s canal and restoring the physiologic aqueous outflow might be an effective treatment option for these types of glaucoma [21].
Four patients (9.5%) required additional glaucoma surgery; all had maximal antiglaucoma topical medication and were at advanced-stage disease. Indeed, they required a lower target IOP to avoid progression, according to the European Glaucoma Society guidelines [2]. Similarly, Ziaei and Au [9] reported that 12% of patients underwent further glaucoma surgeries over 7 years, and Salimi et al. [22] also reported a 10% reoperation rate over 8 years after two first-generation iStent® implantations. This low reoperation rate, along with sustained IOP reduction throughout the follow-up period, supports the long-term efficacy of iStent® in contrast to the short-term IOP reduction achieved by laser trabeculoplasty or cataract surgery [22,23,24,25].
In patients with greater preoperative medication use, more aggressive or traditional glaucoma surgery, such as trabeculectomy or implantation of glaucoma drainage devices, may yield more favorable results [8]. In terms of MIGS, combination approaches using different techniques and more stents are being explored, such as a combination of iAccess precision blade goniotomy [26] or Kahook dual blade [27], titrated IOP control using multiple iStent® implantations (two or three first-generation iStent® implants) as suggested by Katz et al. [12], two or three multiple mixed-generation trabecular stents (iStent inject® ± iStent®) [28], newest-generation three-stent, iStent infinite® [29], or other subconjunctival MIGS. Multiple stents could achieve a lower IOP, with previous results showing incrementally greater and more sustained reductions in multi-iStent® eyes [12] or more three-stent eyes (iStent inject® + iStent®) than in two-stent eyes achieving the target IOP [28]. Recently, multicenter prospective studies have reported that iStent infinite® achieved clinically significant IOP reduction in patients with medically uncontrolled OAG with prior therapy [29].
The main limitation of this study was the lack of control of phacoemulsification alone due to the retrospective case-series study design. These major limitations are commonly found in other MIGS studies [8, 9, 17, 30]. Many previous studies including ocular hypertension study have suggested that cataract surgery has clinical benefit to reduce IOP in eyes with glaucoma or ocular hypertension [24, 31, 32]. Thus, our results should be interpreted carefully in terms of magnitude of IOP reduction and number of glaucoma medications. There are some considerations for the interpretation of our dataset regarding the additive effect of cataract surgery on IOP control.
First, we tried to minimize these confounding factors and referenced our previous study that compared combined phaco-iStent® inject and phacoemulsification alone [13]. In our previous study, mean IOP decreased 14.3 ± 2.7 to 13.1 ± 2.1 mmHg after phacoemulsification in 100 Korean non-glaucomatous eyes with cataract (data not shown in this manuscript) [13]. Second, angle status and preoperative baseline IOP are important factors for IOP reduction after cataract surgery alone. The amount of IOP reduction after phacoemulsification in glaucomatous eye has been reported from 1.0 to 5.5 mmHg [24, 31, 33]. The single most-common significant factor associated with greater IOP reduction after phacoemulsification is higher IOP before phacoemulsification [31]. And, IOP reduction after cataract surgery is much more prominent in patients with closed-angle glaucoma than in those with open-angle glaucoma. In our study, we included controlled POAG only, with a baseline IOP of 15.8 mmHg, whereas previous studies mainly had the majority of eyes with high IOP and various glaucoma subtypes. Thus, we postulated that the IOP-lowering efficacy of phacoemulsification might be limited in our study participants. Our hypothesis was supported by the results of previous studies. In one Korean study [31] of a total 106 controlled patients with glaucoma who received phacoemulsification, IOP decreased from 14.25 ± 3.35 to 13.17 ± 2.90 (1 year), 13.89 ± 2.66 (2 years), and 14.17 ± 4.21 mmHg (3 years), respectively. Majstruk et al. [34] also reported that IOP decreased by a mean of 1.15 ± 3 mmHg and the number of glaucoma medications remained unchanged (17 ± 2.7 mmHg with 1.5 ± 0.8 medications, preoperatively). In this context, the authors thought that IOP reduction was minimal after phacoemulsification in eyes with IOP in the low- or mid-teens. However, there are controversies still abound. Poley et al. [24] reported a mean IOP decrease of 1.6 mmHg in patients with a preoperative IOP of 15–17 mmHg, a range closely corresponding to that in our study, whereas Baek et al. [31] reported that IOP changes are nearly zero in patients with a preoperative IOP of 15 or 16 mmHg. Third, all enrolled patients in our study underwent phacoemulsification with MIGS between 2018 and 2019, reflecting glaucoma treatment preference trends choosing combined phaco-MIGS surgery as the primary procedure. In American Glaucoma Society reports [35], phaco alone was the most preferred surgical approach in 44% of patients with POAG, phaco-trabeculectomy in 24%, with MIGS in 22%, and with glaucoma drainage device (GDD) in 9% in March 2016. In 2020 Japanese Glaucoma Society reports [36], the frequency of MIGS with phacoemulsification remarkably increased (79.0%) for non-operated eyes with mild open-angle glaucoma associated with cataract. Finally, we could get the beneficial effects for reducing medication burden and decreasing the risk of IOP spike during the early postoperative period. Previous studies have also shown IOP spikes above 30 mmHg in up to 27% of patients with POAG in the first operative days after phacoemulsification alone [31, 37]. A higher risk of IOP spikes in the early postoperative period exposes patients to additional optic nerve damage to their already compromised optic disc [38].
In summary, phacoemulsification itself may have strengthened the IOP-lowering effects observed in the Ocular Hypertension Treatment Study [32]. Despite these drawbacks, this is the first study to evaluate the long-term IOP-lowering efficacy and safety profiles of iStent® in Korean eyes, representing real-world clinical outcomes. The scope of detailed conclusions that may be drawn for specific patient groups provides valuable insights into the natural history of disease progression following this intervention in the real world [8, 9].
Our study has some other limitations. First, the number of participants was relatively small and heterogeneous, and the study was a retrospective design with a relatively high loss to follow-up. To overcome these major limitations, future comparative studies with larger sample sizes would be valuable in confirming our results. Second, the analysis included both eyes without adjustments or corrections. Although many previous studies included both eyes for analysis, the mean IOP in contralateral eyes decreased independently of whether contralateral eyes were undergoing topical ocular hypotensive therapy or not after trabeculectomy [39]. Thus, a careful interpretation is required. And we also that consider regression to the mean usually occurs in non-randomized studies [40]. Third, routine specular microscopy was not performed to check for corneal endothelial cell loss; however, clinically significant corneal edema or severe endothelial cell loss of greater than 30% [41] was not observed in our study.
Conclusions
Our real-world outcomes demonstrated a relatively good safety profile and sustained IOP and medication reduction following the implantation of a single trabecular microbypass stent (iStent®) with phacoemulsification in Korean patients. Overall, only 9.5% of patients required further glaucoma surgery, and all of them were on four preoperative medications. Our results suggest that eyes with a higher number of preoperative medications (especially four medications) had difficulty achieving a low target IOP.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–7.
European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th edn. Br J Ophthalmol. 2021;105(Suppl 1):1–169.
Gedde SJ, Vinod K, Wright MM, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology. 2021;128(1):P71–150.
Wagner IV, Ang B, Checo L, Simsek D, Draper C, Dorairaj S. Spotlight on Schlemm’s canal microstent injection in patients with glaucoma. Clin Ophthalmol. 2023;17:1557–64.
Guedes RAP, Gravina DM, Lake JC, Guedes VMP, Chaoubah A. Intermediate results of iStent or iStent inject implantation combined with cataract surgery in a real-world setting: a longitudinal retrospective study. Ophthalmol Ther. 2019;8(1):87–100.
Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–45.
Seibold LK, Gamett KM, Kennedy JB, et al. Outcomes after combined phacoemulsification and trabecular microbypass stent implantation in controlled open-angle glaucoma. J Cataract Refract Surg. 2016;42(9):1332–8.
Kim HJ, Lim SH. Clinical outcomes of trabecular microbypass stent (iStent) implantation in medically controlled open-angle glaucoma in the Korean population. Medicine (Baltimore). 2020;99(33):e21729.
Ziaei H, Au L. Manchester iStent study: long-term 7-year outcomes. Eye (London). 2021;35(8):2277–82.
Ansari E. 5-year outcomes of single iStent (G1) trabecular microbypass implantation with phacoemulsification in moderately advanced primary open angle glaucoma. PLoS ONE. 2021;16(9):e0257015.
Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Morales-Fernandez L, Fernandez-Perez C, Garcia-Feijoo J. Glaukos iStent inject® trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573.
Katz LJ, Erb C, Carceller Guillamet A, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–62.
Rho S, Lim SH. Clinical outcomes after second-generation trabecular microbypass stents (iStent inject®) with phacoemulsification in Korean patients. Ophthalmol Ther. 2021;10(4):1105–17.
Shaarawy T, Grehn F, Sherwood M, World Glaucoma Association. WGA Guidelines on design and reporting of glaucoma surgical trials (guidelines on design and reporting of glaucoma surgical trials). Amsterdam: Kugler.
The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–40.
Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015;2015:795357.
Ferguson TJ, Mechels KB, Dockter Z, et al. iStent trabecular microbypass stent implantation with phacoemulsification in patients with open-angle glaucoma: 6-year outcomes. Clin Ophthalmol. 2020;14:1859–66.
Hamanaka T, Matsuda A, Sakurai T, Kumasaka T. Morphological abnormalities of Schlemm’s canal in primary open-angle glaucoma from the aspect of aging. Invest Ophthalmol Vis Sci. 2016;57(2):692–706.
Andres-Guerrero V, Garcia-Feijoo J, Konstas AG. Targeting Schlemm’s canal in the medical therapy of glaucoma: current and future considerations. Adv Ther. 2017;34(5):1049–69.
Ferguson TJ, Swan R, Ibach M, Schweitzer J, Sudhagoni R, Berdahl JP. Trabecular microbypass stent implantation with cataract extraction in pseudoexfoliation glaucoma. J Cataract Refract Surg. 2017;43(5):622–6.
Buchacra O, Duch S, Milla E, Stirbu O. One-year analysis of the iStent trabecular microbypass in secondary glaucoma. Clin Ophthalmol. 2011;5:321–6.
Salimi A, Watt H, Harasymowycz P. Long-term outcomes of two first-generation trabecular micro-bypass stents (iStent) with phacoemulsification in primary open-angle glaucoma: eight-year results. Eye Vis (Lond). 2021;8(1):43.
Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505–16.
Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34(5):735–42.
Shingleton BJ, Gamell LS, O’Donoghue MW, Baylus SL, King R. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25(7):885–90.
Gallardo MJ, Porter M. Efficacy and safety of pairing iStent inject trabecular micro-bypass and iAccess precision blade goniotomy in patients with open-angle glaucoma. Ophthalmol Ther. 2023;12(4):1973–87.
Ahmed A. Istent Inject W and Kahook dual blade for treating mild-to-moderate glaucoma. Georg Med News. 2023;337:16–20.
Paletta Guedes RA, Gravina DM, Paletta Guedes VM, Chaoubah A. Standalone implantation of 2–3 trabecular micro-bypass stents (iStent inject +/- iStent) as an alternative to trabeculectomy for moderate-to-severe glaucoma. Ophthalmol Ther. 2022;11(1):271–92.
Sarkisian SR Jr, Grover DS, Gallardo MJ, et al. Effectiveness and safety of iStent infinite trabecular micro-bypass for uncontrolled glaucoma. J Glaucoma. 2023;32(1):9–18.
Neuhann R, Neuhann T. Second-generation trabecular micro-bypass stent implantation: retrospective analysis after 12- and 24-month follow-up. Eye Vis (Lond). 2020;7:1.
Baek SU, Kwon S, Park IW, Suh W. Effect of phacoemulsification on intraocular pressure in healthy subjects and glaucoma patients. J Korean Med Sci. 2019;34(6): e47.
Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology. 2012;119(9):1826–31.
Iancu R, Corbu C. Intraocular pressure after phacoemulsification in patients with uncontrolled primary open angle glaucoma. J Med Life. 2014;7(1):11–6.
Majstruk L, Leray B, Bouillot A, et al. Long term effect of phacoemulsification on intraocular pressure in patients with medically controlled primary open-angle glaucoma. BMC Ophthalmol. 2019;19(1):149.
Vinod K, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society. J Glaucoma. 2017;26(8):687–93.
Iwasaki K, Arimura S, Takamura Y, Inatani M. Clinical practice preferences for glaucoma surgery in Japan: a survey of Japan Glaucoma Society specialists. Jpn J Ophthalmol. 2020;64(4):385–91.
Chen PP, Lin SC, Junk AK, Radhakrishnan S, Singh K, Chen TC. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(7):1294–307.
Yasutani H, Hayashi K, Hayashi H, Hayashi F. Intraocular pressure rise after phacoemulsification surgery in glaucoma patients. J Cataract Refract Surg. 2004;30(6):1219–24.
Vysniauskiene I, Shaarawy T, Flammer J, Haefliger IO. Intraocular pressure changes in the contralateral eye after trabeculectomy with mitomycin C. Br J Ophthalmol. 2005;89(7):809–11.
Linden A. Assessing regression to the mean effects in health care initiatives. BMC Med Res Methodol. 2013;13:119.
Ahmed IIK, Sheybani A, De Francesco T, et al. Long-term endothelial safety profile with iStent inject in patients with open-angle glaucoma. Am J Ophthalmol. 2023;252:17–25.
Acknowledgements
The authors wish to thank eWorldEditing and the PaperPal PreFlight service for their language editing.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was supported by a VHS Medical Center research grant, Republic of Korea (grant number VHSMC 21003, recipient Su-Ho Lim). The funders had no role in the study design, data collection or analysis, or preparation of the manuscript. The journal’s Rapid Service Fees were funded by Glaukos Corporation.
Author information
Authors and Affiliations
Contributions
Conceptualization: Myungjin Kim, Seungsoo Rho and Su-Ho Lim; Data curation: Seungsoo Rho and Su-Ho Lim; Funding acquisition: Su-Ho Lim; Resources: Su-Ho Lim; Writing–original draft: Su-Ho Lim; Writing–review and editing: Myungjin Kim, Seungsoo Rho, and Su-Ho Lim.
Corresponding author
Ethics declarations
Conflict of Interest
Myungjin Kim, Seungsoo Rho and Su-Ho Lim declare that they have no conflict of interests.
Ethical Approval
The study protocol was approved by the institutional review board (IRB) of Daegu Veterans Health Service Medical Center (IRB no. 2020–10). All participants provided signed informed consent, and the study adhered to the tenets of the 1964 Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kim, M., Rho, S. & Lim, SH. Five-Year Outcomes of Single Trabecular Microbypass Stent (iStent®) Implantation with Phacoemulsification in Korean Patients. Ophthalmol Ther 12, 3281–3294 (2023). https://doi.org/10.1007/s40123-023-00824-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00824-8